Abstract
Bisphosphonates are widely used in the clinical treatment of bone diseases with increased bone resorption. In terms of side effects, they are widely known to be associated with osteonecrosis of the jaw (BONJ). The objective of this study was to evaluate the effect of bisphosphonates on the gene expression of receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG) in vitro. Nitrogen-containing and non-nitrogen containing bisphosphonates have been compared. Human osteoblasts were stimulated with zoledronate and ibandronate at concentrations of 5 × 10−5 M, 5 × 10−6 M, and 5 × 10−7 M over the experimental period of 14 days. Furthermore, the hOB cell lines were stimulated by clodronate at concentrations of 5 × 10−3 M, 5 × 10−5 M, and 5 × 10−6 M. At each point in time, the gene expression levels of RANKL and OPG were quantified by real-time RT-PCR. The results showed a moderate enhancement of OPG gene expression whereas RANKL gene expression was strongly increased by nitrogen-containing bisphosphonates reaching a maximum after 14 days at high concentrations of 5 × 10−5 M. Lower concentrations did not enhance the RANKL and OPG expression considerably. The non-nitrogen-containing bisphosphonate clodronate, however, effected OPG and RANKL gene expression much less, even at higher concentrations of 5 × 10−3 M. The above-mentioned data suggest an enhanced RANKL/OPG gene expression after stimulation by bisphosphonates. Interestingly, clodronate might have little influence on osteoblast/osteoclast interaction with respect to OPG and RANKL gene expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bisphosphonates are widely used in the clinical treatment of bone diseases with increased bone resorption [1] such as Paget’s disease, osteoporosis, and malignant diseases like multiple myeloma or metastasis to the bone. As a serious side effect, these actions could cause an altered cell metabolism, which is considered to promote osteonecrosis (BONJ), which almost always occurs in the jaw [2]. The BONJ is typically associated with exposed bone, fistulae, and even pathological fractures [3, 4]. Especially after intravenous treatment with nitrogen-containing bisphosphonates, an incidence of 5–19% has been reported [5–7]. In addition to a direct effect on osteoclasts and osteoblasts, some authors suggest that a bisphosphonate-induced obliteration of the regional blood vessels could lead to an avascular osteonecrosis of the jaw [4, 8, 9]. The effect of bisphosphonates to increase bone mineral density has been attributed to a decreased bone turnover [10–15] by the inhibition of osteoclastic bone resorption.
Osteoclasts are known to differentiate from hematopoietic precursor cells. It was noted that the development of osteoclasts from their precursors usually requires the presence of osteoblasts through a mechanism that requires a trio of peptides, osteoprotegerin (OPG), receptor activator of nuclear factor-κB ligand (RANKL) and receptor activator nuclear factor-κB (RANK) [16]. RANKL is a member of the tumor necrosis factor (TNF) superfamily, produced and secreted by osteoblasts [17]. It stimulates osteoclasts through its known receptor RANK, which is a membrane-bound protein present on osteoclasts and their precursors. However, the interaction between RANKL and RANK can be competitively inhibited by the decoy receptor OPG, a soluble protein produced by osteoblasts [18–21].
The aim of this in vitro study was to demonstrate the impact of bisphosphonates on the RANKL and OPG gene expression in osteoblasts over a period of 14 days. The nitrogen-containing bisphosphonates zoledronate and ibandronate were compared to the non-nitrogen-containing bisphosphonate clodronate.
Material and method
Cell culture
Human osteoblasts (HOB-c, PromoCell, Heidelberg, Germany) between passages 5–9 were cultured at a density of 200,000 cells per well using 6-well plates. Their osteoblastic genuineness was proved by Osteocalcin gene expression though real-time reverse transcriptase polymerase chain reaction (RT-PCR; Fig. 1). The cells were allowed to attach for 2 days using an osteoblast-specific medium (10% FCS/DMEM Dulbecco modified medium; Invitrogen, Carlsbad, Ca/US) containing 1% l-glutamine, 1% penicillin/streptomycin/neomycin, 1% ascorbic acid, and 20 μg/ml dexamethasone. The cells were stimulated by osteoblast-specific medium containing zoledronate or ibandronate at a concentration of 5 × 10−5 M, 5 × 10−6 M, and 5 × 10−7 M. Clodronate was applied at higher concentrations of 5 × 10−3 M, 5 × 10−5 M, and 5 × 10−6 M. The osteoblast-specific cell culture medium without bisphosphonate addition was used as a control. The media and bisphosphonates were renewed every 4 days for a period of 14 days to guarantee a constant stimulation und nutrition supply over the experimental period. Two different osteoblast cell lines were stimulated by the 5 × 10−5-M bisphosphonate concentration.
Messenger RNA extraction and reverse transcriptase polymerase chain reaction
At the 1st, 2nd, 5th, 10th, and 14th day of cultivation, the osteoblasts were detached with 0.05% trypsin–EDTA solution (Invitrogen, Carlsbad, Ca, US) and individually harvested. With respect to the 5 × 10−3-M, 5 × 10−6-M, and 5 × 10−7-M bisphosphonate concentrations, the cells were detached at four points in time (2nd, 5th, 10th, and 14th day). Messenger RNA (mRNA) was extracted using a silicate gel technique that was provided by the Qiagen RNeasy extraction kit (Qiagen, Hilden, Germany). This process included a DNAse digestion step. The amount of extracted mRNA was measured by extinction at 260 nm; the contamination with proteins was determined with the 260/280 ratio.
To detect the mRNA of RANKL and OPG in osteoblasts, primers were designed using NCBI-nucleotide library and Primer3-design (Table 1). All primers had been matched to the mRNA sequences of the target genes (NCBI Blast software).
As housekeeping genes, human ribosomal protein (HuPO), actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and ribosomal Protein S18 (RPS18) were evaluated. We were able to show the most stable condition for the actin, GAPDH, and RPS18 genes by comparing the bisphosphonate-stimulated versus a non-stimulated cell culture using a specialized freeware called GeNorm.
As a quantitative RT-PCR, we used the SYBR Green real-time PCR (one-step RT-PCR, Bio-Rad, Hercules, CA/USA). This method ensures a reverse transcription using the individual primers immediately before PCR amplification and SYBR Green fluorescence measurement for quantification of gene expression. Samples were amplified in 96-well microplates in an IQ5-Cycler (Bio-Rad, Hercules, CA/USA) using an annealing temperature of 56°C and an elongation temperature of 71°C over 40 cycles. The background was determined during three to ten cycles and the threshold was set above this fluorescence, crossing the SYBR green fluorescence curve at the exponential part. This point was used to calculate the cycle number and CT value for quantitation. Furthermore, the CT values of actin, GAPDH, and RPS18 housekeeping genes and the individual primer efficacy were taken into consideration. Single product formation was confirmed by melting point analysis. For negative control, water samples instead of mRNA samples were used.
Statistical analysis
cDNA from individual cell experiments was analyzed in triplicate PCR. For statistical analysis of the CT values, the ΔΔCT method was applied [22, 23]. For each specific primer and real-time PCR, the replication efficiency was calculated on the basis of the SYBR Green fluorescence curves and by standard dilution series. The relative gene expression levels were standardized by those measured in the unstimulated control, which was set to 100%. Each point in time for relative mRNA is the mean ± standard deviation.
Results
Effect of bisphosphonates on RANKL gene expression
Time-course experiments were performed to determine the effects of zoledronate, ibandronate, and clodronate on RANKL gene expression. As shown in Fig. 2, treatment of hOB cells with 5 × 10−5-M ibandronate and zoledronate increased the gene expression of RANKL after 48 h, with ibandronate initially effecting a lower gene expression level. After 14 days, ibandronate led to the highest RANKL gene expression compared to the unstimulated control. Lower concentrations of 5 × 10−6 and 5 × 10−7 M rather decreased RANKL gene expression compared to control (Table 2).
Clodronate, a non-nitrogen-containing bisphosphonate, did not significantly enhance RANKL gene expression compared to an unstimulated osteoblast control. The gene expression levels even appeared to be decreased by clodronate during the first 10 days at concentrations of 5 × 10−6 and 5 × 10−7 M (Table 2). At the concentration of 5 × 10−3 M, an enhancement of gene expression was observed (Fig. 2).
Effect of bisphosphonates on OPG gene expression
At high concentrations of 5 × 10−5 M, the OPG gene expression was stimulated to the largest by zoledronate, reaching a maximum of 1.060% at day 2 compared to the non-stimulated control. Ibandronate also caused osteoblasts to increase their gene expression to a maximum level of 575% on day 10. Lower concentrations did not clearly effect the OPG gene expression. With concentrations of 5 × 10−7 M, zoledronate as well as ibandronate led to lower OPG gene expressions after 5 days compared to control. At 5 × 10−6 M, just ibandronate reached higher gene expression levels than the control group (Table 3).
The non-nitrogen-containing clodronate did not cause an OPG elevation on gene expression level (Table 3, Fig. 3).
Effect of bisphosphonates on RANKL/OPG ratio
With respect to the contrary effects on osteoclast proliferation and differentiation, the ratio of RANKL/OPG was taken in order to evaluate the resulting effect. The findings are presented in Table 4 and Fig. 4. Zoledronate and ibandronate increased the RANKL/OPG ratio at high concentrations of 5 × 10−5 M and to a lower extent at 5 × 10−6-M zoledronate and ibandronate. The highest values were reached after the first 5 days. Lower concentrations (5 × 10−7 M) of zoledronate and ibandronate led to a RANKL/OPG ratio of less than 1.
Clodronate showed a dose-dependent effect with increasing RANKL/OPG ratios till day 10 at a concentration of 5 × 10−3 M (Table 4, Fig. 4).
Discussion
Bisphosphonates interfere with bone remodeling processes that are controlled by mediators such as RANKL, RANK, and OPG.
Our results demonstrate that the RANKL gene expression was not much enhanced during the first 6 days of bisphosphonate stimulation and even inhibited by ibandronate stimulation. After 6 days, however, the gene expression level of RANKL was enhanced by zoledronate and ibandronate to a level of several hundred percent compared to the unstimulated control. The OPG gene expression was moderately enhanced by zoledronate and ibandronate stimulation. RANKL and OPG have a controversial impact on osteoclastogenesis and osteoclast differentiation: RANKL, a soluble paracrine-secreted protein, promotes the osteoclast differentiation, while OPG prevents through a decoy receptor binding. Therefore, the ratio of RANKL/OPG gene expression should allow a sufficient assessment of osteoblast-induced stimulus to osteoclasts. By a RANKL/OPG ratio >1, a stimulating influence of osteoblasts on osteoclastogenesis and differentiation could be assumed. In a dose-dependent manner, the RANKL/OPG ratio, elevated by zoledronate and ibandronate, could suggest an anabolic effect on osteoclasts via osteoblasts’ secretion after application of bisphosphonates at high concentrations. Lower bisphosphonate concentrations, however, seemed to cause an OPG gene expression in osteoblasts that exceeds the RANKL gene expression. Therefore, lower zoledronate and ibandronate concentrations might be sufficient to inhibit osteoclasts’ differentiation. Although our results demonstrated that nitrogen-containing bisphosphonates significantly affected RANKL and OPG gene expression during an experimental period of 14 days, a further modulation of RANKL and OPG gene expression on post-transcriptional level could not be ruled out.
Other studies focused on osteoblast RANKL and OPG gene expression during the first 2 or 6 days [18, 24]: Lin et al. found no significant influence on osteoblast RANKL and OPG gene expression during an experimental period of 48 h, investigating alendronate and pamidronate. In contrast, Mackie et al. found that the RANKL gene expression was inhibited and the OPG gene expression was not altered by stimulation of pamidronate in an osteosarcoma cell line for 6 days [24]. Although the discrepancy might be attributed to the use of different cell lines, human vs. rat and primary vs. cancer, the stimulation period as well as the applied concentrations are crucial points, as the RANKL gene expression was enhanced from day 6 to day 14 in our results mainly at high concentrations of 5 × 10−5 M.
A RANKL/OPG ratio >1, which was measured at high concentrations of nitrogen-containing bisphosphonates, seems to be contrary to the positive bone turnover seen clinically, which is considered to be caused by osteoclasts’ apoptotic cell death and consecutive positive bone turnover [25, 26]. Furthermore, there is data supporting the idea that osteoclasts’ resorption activity is disrupted by the loss of osteoclast cytoskeleton and loss of actin ring [27, 28]. These effects, however, require concentrations which are considered to be mainly reached in active osteoclasts during bone resorption, causing the liberation of mineral-bound bisphosphonates. Through a high RANKL/OPG ratio, osteoclast activity might be enhanced and could finally liberate mineral-bound bisphosphonates that reach concentrations, which are sufficient for osteoclast apoptosis. This theory would corroborate the clinical finding of a high bone density. Furthermore, histomorphological studies described increased numbers of osteoclasts expressing cysteine proteinase cathepsine K in patients with infected bisphosphonate-associated osteonecrosis compared to control [29]. Although the potential of actinomyces species is discussed to cause osteoclast recruitment, our data suggest a stimulatory effect through RANKL secretion by osteoblasts.
Clinical studies on the effect of zoledronate on RANKL and OPG serum levels in patients suffering from Paget’s disease of the bone did not show any effect compared to non-treated control group [30]. A high affinity of bisphosphonates to bone mineral could have caused the low blood concentrations that might not have been sufficient to effect the osteoblast RANKL and OPG expression. Therefore, these findings are in concordance with our results showing little influence of low bisphosphonate concentrations on RANKL and OPG gene expression.
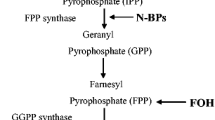
After osteoblast stimulation by clodronate, a non-nitrogen-containing bisphosphonate [31], little RANKL or OPG gene expression effect has been found. These findings are in agreement with previous results that support the different pharmacologic actions of nitrogen- and non-nitrogen-containing bisphosphonates in terms of preventing prenylation of small guanosine triphosphate (GTP)-binding proteins [32]. Nitrogen-containing bisphosphonates, including zoledronate and ibandronate, inhibit the production of isoprenoid lipids, such as isopentenyl pyrophosphate (IPP), farnesyl pyrophosphate (FPP), and geranylgeranyl pyrophosphate (GGPP), in the biosynthetic mevalonate pathway [1]. FPP is required for posttranslational modification of proteins, such as GTP-binding proteins, e.g. Ras, Rho, and Rac [32, 33]. These signaling molecules control regulator proteins that are possibly involved in RANKL and OPG gene expression.
Conclusion
Our data suggest a stimulating effect of bisphosphonates on osteoblast RANKL/OPG gene expression at high concentrations. This might accelerate the osteoclasts’ metabolism, promote mineral-bound bisphosphonate liberation, and therefore, enhance apoptotic cell death.
The RANKL and OPG gene expression was mainly increased by nitrogen-containing bisphosphonates; non-nitrogen-containing bisphosphonates had little influence on RANKL and OPG gene expression in osteoblasts.
References
Russell RG, Rogers MJ (1999) Bisphosphonates: from the laboratory to the clinic and back again. Bone 25:97–106
Rustemeyer J, Bremerich A (2010) Bisphosphonate-associated osteonecrosis of the jaw: what do we currently know? A survey of knowledge given in the recent literature. Clin Oral Investig 14:59–64
Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, Koutsoukou V, Gika D, Anagnostopoulos A, Papadimitriou C, Terpos E, Dimopoulos MA (2005) Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 23:8580–8587
Marx RE, Sawatari Y, Fortin M, Broumand V (2005) Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 63:1567–1575
Walter C, Al-Nawas B, du Bois A, Buch L, Harter P, Grotz KA (2009) Incidence of bisphosphonate-associated osteonecrosis of the jaws in breast cancer patients. Cancer 115:1631–1637
Walter C, Al-Nawas B, Grotz KA, Thomas C, Thuroff JW, Zinser V, Gamm H, Beck J, Wagner W (2008) Prevalence and risk factors of bisphosphonate-associated osteonecrosis of the jaw in prostate cancer patients with advanced disease treated with zoledronate. Eur Urol 54:1066–1072
Walter C, Grotz KA, Kunkel M, Al-Nawas B (2007) Prevalence of bisphosphonate associated osteonecrosis of the jaw within the field of osteonecrosis. Support Care Cancer 15:197–202
Abu-Id MH, Acil Y, Gottschalk J, Kreusch T (2006) Bisphosphonate-associated osteonecrosis of the jaw. Mund Kiefer Gesichtschir 10:73–81
Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL (2004) Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 62:527–534
Walter C, Klein MO, Pabst A, Al-Nawas B, Duschner H, Ziebart T (2010) Influence of bisphosphonates on endothelial cells, fibroblasts, and osteogenic cells. Clin Oral Investig 14:35–41
Glatt M, Pataki A, Evans GP, Hornby SB, Green JR (2004) Loss of vertebral bone and mechanical strength in estrogen-deficient rats is prevented by long-term administration of zoledronic acid. Osteoporos Int 15:707–715
Hornby SB, Evans GP, Hornby SL, Pataki A, Glatt M, Green JR (2003) Long-term zoledronic acid treatment increases bone structure and mechanical strength of long bones of ovariectomized adult rats. Calcif Tissue Int 72:519–527
Pataki A, Muller K, Green JR, Ma YF, Li QN, Jee WS (1997) Effects of short-term treatment with the bisphosphonates zoledronate and pamidronate on rat bone: a comparative histomorphometric study on the cancellous bone formed before, during, and after treatment. Anat Rec 249:458–468
Balena R, Toolan BC, Shea M, Markatos A, Myers ER, Lee SC, Opas EE, Seedor JG, Klein H, Frankenfield D et al (1993) The effects of 2-year treatment with the aminobisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strength in ovariectomized nonhuman primates. J Clin Invest 92:2577–2586
Elad S, Gomori MJ, Ben-Ami N, Friedlander-Barenboim S, Regev E, Lazarovici TS, Yarom N (2010) Bisphosphonate-related osteonecrosis of the jaw: clinical correlations with computerized tomography presentation. Clin Oral Investig 14:43–50
Bruzzaniti A, Baron R (2006) Molecular regulation of osteoclast activity. Rev Endocr Metab Disord 7:123–139
Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T (1990) Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA 87:7260–7264
Lin JM, Callon KE, Lin CQ, Bava U, Zheng MH, Reid IR, Cornish J (2007) Alteration of bone cell function by RANKL and OPG in different in vitro models. Eur J Clin Invest 37:407–415
Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E, Morinaga T, Higashio K (1998) Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139:1329–1337
Burgess TL, Qian Y, Kaufman S, Ring BD, Van G, Capparelli C, Kelley M, Hsu H, Boyle WJ, Dunstan CR, Hu S, Lacey DL (1999) The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol 145:527–538
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Mackie PS, Fisher JL, Zhou H, Choong PF (2001) Bisphosphonates regulate cell growth and gene expression in the UMR 106-01 clonal rat osteosarcoma cell line. Br J Cancer 84:951–958
Selander KS, Monkkonen J, Karhukorpi EK, Harkonen P, Hannuniemi R, Vaananen HK (1996) Characteristics of clodronate-induced apoptosis in osteoclasts and macrophages. Mol Pharmacol 50:1127–1138
Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF (1995) Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res 10:1478–1487
Hiroi-Furuya E, Kameda T, Hiura K, Mano H, Miyazawa K, Nakamaru Y, Watanabe-Mano M, Okuda N, Shimada J, Yamamoto Y, Hakeda Y, Kumegawa M (1999) Etidronate (EHDP) inhibits osteoclastic-bone resorption, promotes apoptosis and disrupts actin rings in isolate-mature osteoclasts. Calcif Tissue Int 64:219–223
Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA (1991) Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest 88:2095–2105
Hansen T, Kirkpatrick CJ, Walter C, Kunkel M (2006) Increased numbers of osteoclasts expressing cysteine proteinase cathepsin K in patients with infected osteoradionecrosis and bisphosphonate-associated osteonecrosis—a paradoxical observation? Virchows Arch 449:448–454
Polyzos SA, Anastasilakis AD, Efstathiadou Z, Kita M, Litsas I, Avramidis A, Arsos G, Moralidis E, Gerou S, Pavlidou V, Papatheodorou A, Terpos E (2009) The effect of zoledronic acid on serum dickkopf-1, osteoprotegerin, and RANKL in patients with Paget’s disease of bone. Horm Metab Res 41:846–850
Rogers MJ (2003) New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des 9:2643–2658
Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC (2000) Cellular and molecular mechanisms of action of bisphosphonates. Cancer 88:2961–2978
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koch, F.P., Merkel, C., Ziebart, T. et al. Influence of bisphosphonates on the osteoblast RANKL and OPG gene expression in vitro. Clin Oral Invest 16, 79–86 (2012). https://doi.org/10.1007/s00784-010-0477-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-010-0477-8