Abstract
Objectives
There is increasing evidence that inflammation and biomechanical loading can influence the effects of bisphosphonates (BP). The aim of this study was to investigate the influence of tensile strain application combined with IL-1ß and clodronate or zoledronate on human periodontal ligament fibroblasts (HPdLF) in vitro.
Materials and methods
HPdLF were cultured with 10 nM IL-1ß and 5 μM clodronate or zoledronate for 48 h. Cells were applied to cyclic tensile strain (CTS; 3% elongation) for 12 h in vitro. Cell number was analyzed directly after CTS by MTT assay. Gene expression of receptor activator of cyclooxygenase-2 (COX-2) was investigated using real-time PCR. MMP-8, TIMP-1, and PGE2 were measured by ELISA. Statistics were performed with SPSS (ANOVA, p < 0.05).
Results
Zoledronate reduced the cell number of HPdLF (60.3 vs. 100%), which was significant when combined with IL-1ß. Combined with 3% CTS, this effect was voided and cell number increased over the level of the control cells. IL-1ß led to a 10-fold increase of COX-2 gene expression. Combined with CTS and zoledronate, this increase was enhanced to a gene expression 70-fold that of control cells with related PGE2 synthesis. Clodronate neither reduce the cell number nor enhanced the COX-2 gene expression. CTS increased MMP-8 protein synthesis. Combined with BP, this increase was voided. TIMP-1 protein synthesis was increased at all conditions under CTS.
Conclusions
Mechanical loading might activate cell metabolism and abolish BP- and inflammation-induced reduction of viability. Combination of mechanical loading, inflammation, and nitrogen-containing bisphosphonates can cause pro-inflammatory effects.
Clinical relevance
Periodontal inflammation should be treated initially before BP intake to prevent decreased cell viability of the periodontium and increased inflammation, which might be enhanced by the addition of mastication forces.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2003, bisphosphonate-associated osteonecrosis of the jaw (BPONJ) was described for the first time [1]. Since then, the incidence of this complication has dramatically increased [2,3,4]. Bisphosphonates cause reduced bone formation and wound healing, which are further risk factors for developing an osteonecrosis. This mainly occurs after intake of highly potent nitrogen-containing bisphosphonates (BP) such as pamidronate or zoledronate [5, 6]. The pathogenesis of this disease is not yet completely known and seems to be a multifactorial disease.
Various studies have shown that nitrogen-containing BP not only affect the metabolism of osteoclasts to reduce bone resorption but also have a negative impact on the metabolism of different cell lines, such as osteoblasts, fibroblasts, keratinocytes, and endothelial cells. In particular, nitrogen-containing BP such as zoledronate have shown a toxic effect on different cell types dependent on the administered dose [7,8,9,10].
The periodontal ligament and the alveolar bone are exposed to a cyclic mechanical loading due to the process of mastication. The occlusal forces of the teeth are transmitted to the alveolar bone by the cells of the periodontal ligament, resulting in permanent turnover of the alveolar bone. Especially periodontal fibroblasts play an important role in the remodeling of the periodontal ligament and interaction with the surrounding cells of the alveolar bone. This mechanical loading is missing after extraction of a tooth and might reduce the bone turnover, especially in patients taking BP [11]. Patullo et al. described the hypofunction due to missing mastication as one of the risk factors for alveolar bone loss [12]. Mechanical loading caused by mastication or orthodontic tooth movement affects cell differentiation, synthesis of the extracellular matrix, and the synthesis of inflammatory cytokines of periodontal fibroblasts [13, 14].
Interleukin (IL)-1β plays a major role. It stimulates osteoclasts via the IL-1-receptor and enhances the synthesis of prostaglandin (PG) E2 and matrix-metallo-proteinases (MMP) [15,16,17]. Bisphosphonates also affect the production of inflammatory cytokines. Suzuki et al. observed an inhibiting effect of etidronate on the synthesis of cyclooxygenase (COX)-2 and PGE2 in different cell lines [18]. Zoledronate, however, showed a pro-inflammatory effect on neutrophil granulocytes and fibroblasts [19, 20]. Liu et al. showed that clodronate reduced the PGE2 synthesis caused by mechanical loading [21].
The present study analyzes the interaction between the bisphosphonates clodronate (non-nitrogen) and zoledronate (nitrogen-containing), the pro-inflammatory cytokine IL-1β, and mechanical loading regarding cell viability, COX-2 gene expression, PGE2 synthesis, and MMP-8/TIMP-1 synthesis of human periodontal ligament fibroblasts (HPdLF).
Material and methods
Cell culture
Cell cultures were prepared and maintained according to standard cell culture procedures. Commercially available HPdLF (Lonza, Basel, Switzerland) were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, USA) containing 10% FCS, 1% l-glutamine, and 1% penicillin/streptomycin/neomycin. Cell morphology was observed under light microscope for typical fibroblast morphology. Additionally, alkaline phosphatase was analyzed, which is characteristic for ligament fibroblasts, but not for gingival fibroblasts [22]. The cells were cultured in an incubator with 5% CO2 and 95% air at 37 °C. Cells were passaged at regular intervals depending on their growth characteristics using 0.05% Trypsin-EDTA solution (PAA, Pasching, Austria). Cells were used for experiments at passages four to six.
Mechanical strain devices and incubation with IL-1β and bisphosphonates
As an established experimental model of tensile strain, HPdLF were seeded at a density of 1.0 × 105 cells/well on flexible-bottomed six-well plates (Bioflex® Plates, Flexcell® International Corporation, Hillsborough, USA) and cultured in supplemented DMEM. After 2 days for cell attachment, spread, and growth to subconfluency, the medium was replaced by medium with 1% fetal calf serum (starvation medium) and with 10 nM IL-1β and or two different bisphosphonates (clodronate or zoledronate) of 5 μM for 48 h. A Flexercell Strain Unit (Modell FX 3000, Dunn Labortechnik Gmbh, Asbach, Germany), which is capable of controlling the magnitude, type, and frequency of cell deformation, was used to generate cyclic tensile strain (CTS) in HPdLF. Cells were subjected to CTS of 3% elongation (2 cN/mm2) in an incubator for 12 h. Experiments were repeated three times to confirm reproducibility, and three wells were used for each group.
MTT assay
Cell number of HPdLF was evaluated directly after application of CTS in the six-well plates with the MTT (3-(4,5-dimethylthiazol2-yl)-2,5-diphenyltetrazolium bromide) colorimetric assay (Sigma, München, Germany). Viable cells ferment tetrazolium bromide to formazan, which can be measured after cell lysis photometrically at 550 nm. The experiments were performed in triplicate.
Messenger RNA extraction and reverse transcriptase polymerase chain reaction
Cells were detached with 0.05% Trypsin-EDTA solution directly after stretching and were then individually harvested. Messenger RNA (mRNA) was isolated using the peqGOLD Total RNA KIT (peqLab Biotechnologie Gmbh, Erlangen, Germany). This included a DNAse digestion step. Both the quantity (260 nm) and quality (ratio 260/280 nm) of the RNA were determined by using a NanoDrop Spectrophotometer ND-100 (peqLab Biotechnologie Gmbh, Erlangen, Germany). Reverse transcription (RT) of RNA (100 ng) was performed by standard protocols with Gene Amp PCR System 2400 (Perkin Elmer, Massachusetts, USA) and iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, USA) in a total volume of 20 μl.
Primers (Eurofins MWG Operon, Ebersberg, Germany) were designed using the NCBI-nucleotide library and Primer3-design to detect the mRNA levels (Table 1). All primers had been matched to the mRNA sequences of the target genes (NCBI Blast software). As housekeeping genes, actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were evaluated. We were able to show the most stable expression for actin and GAPDH using specialized freeware called GeNorm.
Quantitative real-time PCR was performed with the IQ5-I-Cycler and IQ5 Optical System software version 2.0 (Bio-Rad Laboratories, Hercules, USA) according to the manufacturer’s instructions, which included an initial denaturation at 95 °C, annealing temperature of 56 °C, and an elongation temperature of 71 °C over 40 cycles. q-PCR amplification was conducted with a reaction mixture containing SYBR Green Supermix (Bio-Rad Laboratories, Hercules, USA), an appropriate amount of paired primers, and 2 μl template cDNA; the reason for this was to determine the threshold of the SYBR green fluorescence curve at the exponential part. This method was applied to calculate the cycle number and CT-value for quantitation.
Furthermore, the CT-values of the actin and GAPDH housekeeping genes and the individual primer efficacy were taken into account. Single product formation was confirmed by melting point analysis. Data were obtained from three individual experiments and normalized to the CT of actin and GAPDH. CDNA from individual cell experiments was analyzed in triplicate PCR. The relative expression levels of each mRNA were evaluated by using a modification of the ΔΔCT method [23].
Enzyme-linked immunosorbent assay
PGE2, MMP-8, and TIMP-1 were measured in supernatants by Quantikine Human Immunoassay (R&D Systems, Inc. Minneapolis, USA) according to the manufacturer’s instructions using a microplate reader (Metertech, Inc., Taipei, Taiwan). The assays were performed in triplicate.
Statistical analysis
SPSS 21.0 (IBM-SPSS, Ehningen, Germany) was used for statistical analyses. To detect the difference between the groups, one-way ANOVA was used with the post hoc Tukey test. A p value <0.05 was considered statistically significant. Box and whisker diagrams: The black bar in the middle of each box represents the median. The box includes all values between the 25th and 75th percentiles. Whiskers indicate values still within the 1.5 interquartile range (IQR). An asterisk (*) represents a significant difference compared to control cells.
Results
MTT assay
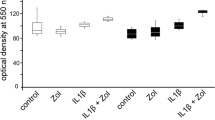
Zoledronate reduced the cell number of HPdLF compared to control cells (60.3 vs. 100%). Clodronate, IL-1β, and CTS had no influence on cell number (Fig. 1a). The combination of clodronate and IL-1β did not influence the cell number of HPdLF, whereas zoledronate combined with IL-1β reduced the cell number significantly under 60% compared to control cells (p < 0.05). The addition of CTS abolished this reduction and led to a significant increase of the cell number in all groups (Fig. 1b).
MTT assay. a Cell number test for human periodontal ligament fibroblasts (HPdLF) exposed to 5 μM clodronate, 5 μM zoledronate, and 10 nM IL-1β for 48 h or to 12 h of cyclic tensile strain at strengths (CTS) of 3% compared to control cells set to 100%. b Cell number test for HPdLF for different combinations of the single conditions. (X-axis = different strengths of SMS, Y-axis = cell viability compared to control group in percent). The assays were performed in triplicate, *p < 0.05. The whiskers at each value represent the standard deviation
COX-2 gene expression and PGE2 protein level
IL-1β led to a 10-fold increase of COX-2 gene expression. Clodronate and zoledronate did not increase COX-2 gene expression. Clodronate slightly reduced the increase induced by IL-1β, whereas zoledronate enhanced this increase. IL-1β in combination with CTS led to a 25-fold increase of COX-2 gene expression. Clodronate or zoledronate combined with mechanical loading did not increase COX-2. The combination of mechanical loading, IL-1β, and zoledronate increased COX-2 70-fold (Fig. 2).
Quantitative RT-PCR-results for cyclooxygenase (COX)-2 of human periodontal ligament fibroblasts (HPdLF) exposed to 10 nM IL-1β, 5 μM clodronate (clo.), 5 μM zoledronate (zole.), or combination of both for 48 and 12 h of cyclic tensile strain (CTS) at strengths of 3% compared to control cells (contr.). Data were obtained from three individual experiments (means ± SD, *p < 0.05)
The ELISA of PGE2-synthesis was done as an indirect proof of COX-2 at the protein level. Corresponding to the results at gene level, we observed significantly increased values of PGE2 after incubation of HPdLF with IL-1β. Zoledronate enhanced this increase nearly 4-fold. CTS alone did not increase PGE2 synthesis. Values for CTS combined with IL-1β were similar to IL-1β alone. The highest values of PGE2 were found after incubation with IL-1β and zoledronate combined with mechanical loading (Fig. 3).
Prostaglandin (PG) E2 secretion in supernatant liquor of human periodontal ligament ibroblasts (HPdLF) exposed to 10 nM IL-1β, 5 μM clodronate (clo.), 5 μM zoledronate (zole.), or combination of both for 48 and 12 h of cyclic tensile strain at strengths of 3%. Data given in picograms per milliliter relative to control cells
MMP-8 and TIMP-1 protein synthesis
IL-1β increased MMP-8 synthesis significantly. Clodronate and zoledronate did not alter MMP-8 synthesis, but reduced the IL-1β-induced MMP-8 synthesis slightly. Mechanical loading increased MMP-8, and when combined with IL-1β, this increase was enhanced. Both bisphosphonates erased the MMP-8 increase induced by mechanical loading (Fig. 4).
TIMP-1 values were not altered by IL-1β, clodronate, or zoledronate. The combination of IL-1β and zoledronate showed a slight reduction of TIMP-1. TIMP-1 synthesis was increased by mechanical loading in all groups especially in combination with IL-1β (Fig. 5).
Discussion
The present study analyzed the metabolism of HPdLF under mechanical loading, IL-1ß, and bisphosphonates. For the first time, we observed a positive interaction between mechanical loading and bisphosphonates regarding the cell number. The study also shows for the first time an additive pro-inflammatory effect between IL-1ß, mechanical loading, and zoledronate regarding COX-2 gene expression and PGE2 synthesis.
Zoledronate reduced the cell number of HPdLF, and IL-1ß enhanced this effect. Mechanical loading had a positive effect on the cell number and negated the decreased caused by zoledronate. Pabst et al. and Walter et al. observed this negative effect of zoledronate regarding the cell viability of different cell lines at the same concentration of 5 μM. Higher concentrations, such as 50 μM, enhanced the reduction of the viability [8, 24]. Agis et al. described a reduced cell viability and higher apoptosis rate of HPdLF by zoledronate at concentrations from 30 to 100 μM [25]; these results correspond to the present study, which additionally showed that IL-1ß enhanced this effect.
In a previous study, we combined zoledronate and clodronate with high levels of mechanical loading (10% elongation). We observed an additive negative effect of static mechanical loading regarding the reduction of cell viability [26]. In this study, we used a different protocol for mechanical loading entailing low forces and cyclic loading, which simulates mastication forces. The deviating results indicate that the type of mechanical loading might be a crucial effect modifier regarding the influence of bisphosphonates. Further studies are desirable to analyze this interaction more properly and maybe discover new insights into the pathomechanism of BPONJ.
IL-1ß alone and in combination with mechanical loading increased COX-2 gene expression and PGE2 synthesis. The combination IL-1ß, mechanical loading, and zoledronate led to the highest COX-2 gene expression and PGE2 synthesis. Diercke et al. could previously show that IL-1ß led to an increased COX-2 gene expression of human cementoblasts, which was enhanced by mechanical loading in the form of compression [27].
Previous studies have shown that mechanical loading might have pro-inflammatory effects, especially in an inflamed environment [28,29,30]. Nokhbehsaim et al. [30] could show that the effect of mechanical loading on the pro-inflammatory effect of IL-1ß is dependent on the duration of mechanical loading. They concluded that the pro-inflammatory effect of mechanical loading decreases under long-lasting mechanical loading. Long et al. [31] described an anti-inflammatory effect of mechanical loading. They observed reduced COX-2 gene expression and PGE2-synthesis after cyclic mechanical loading of 3–6% elongation. In contrast to our study, they used heterogenic cells of the periodontal ligament (PDL). PDL cells are composed of different cell types such as fibroblasts, osteoblasts, and cementoblasts. In a previous study, we could show that, for example, fibroblasts and osteoblast react differently to mechanical loading [32]. This might be an explanation for the deviating results.
Roemer et al. analyzed the effect of mechanical loading and the presence of bacteria on PDL cells. Mechanical loading and bacteria separately led to a 5-fold increase of COX-2 gene expression. The combination of both increased the COX-2 expression in the cells about 56-fold [29]. Similar observations were made by Nogueira et al. 2014 [28]. They could demonstrate that mechanical loading and the presence of Fusobacterium nucleatum led to a higher gene expression of COX-2 than both factors alone. Cyclic mechanical strain increased the pro-inflammatory effect of F. nucleatum. Both studies used different methods to simulate inflammatory conditions, but the results are in agreement with our study that there might be a synergistic effect between a pro-inflammatory stimulus and mechanical loading.
The combination IL-1ß, mechanical loading and zoledronate led to the highest COX-2 gene expression and PGE2 synthesis. This shows a pro-inflammatory effect of zoledronate in the presence of IL-1ß and mechanical loading for the first time. According to current knowledge, there is only one previous in vitro study analyzing the inflammatory effect of mechanical loading and a bisphosphonate. Liu et al. observed an anti-inflammatory effect of clodronate regarding increased COX-2 and PGE2 values after mechanical compression [21]. They concluded that clodronate might also decrease the COX-2-dependent RANKL synthesis and thus reduce bone resorption as well as orthodontic tooth movement. In our study, clodronate showed neither pro-inflammatory nor anti-inflammatory effects. Further studies analyzing the interaction of different types of BP with mechanical loading are urgently needed due to the important clinical context regarding inflammation and necrosis of the alveolar bone.
IL-1ß increased MMP-8 synthesis compared to the control cells. This is in concordance with the results of previous studies. Abe et al. showed a concentration-dependent increase of MMP-8 gene expression through stimulation with IL-1ß [15]. Mechanical loading increased both MMP-8 and TIMP-1 synthesis. The ratio between these two factors is important for the remodeling of the extracellular matrix in the PDL. In a previous study, we could show that the strength of mechanical loading is relevant for the changes of the MMP-8/TIMP-1 ratio [14]. This ratio is crucial for orthodontic tooth movement and mastication forces to avoid PDL destruction which might be caused by an isolated MMP-8 increase.
Both clodronate and zoledronate inhibited the MMP-8 synthesis, but not the TIMP-1 synthesis. This is in concordance with the results of Buduneli et al. They observed in an in vivo study an inhibiting effect of alendronate regarding the MMP-8 gene expression in rats. After injection of LPS to induce periodontitis, the animals showed an increase of MMP-8. After the injection of alendronate, a nitrogen-containing bisphosphonate, the MMP-8 gene expression was reduced [33]. Teronen et al. also described an inhibiting effect of clodronate and zoledronate on MMP-8 in vivo. Patients with clodronate intake showed a 50% reduction in MMP-8 in the gingival sulcus fluid around implants [33,34,35]. They concluded that bisphosphonates might be helpful to prevent implant loss caused by increased MMP-8 values due to periodontitis. Thus, both factors, mechanical loading and the bisphosphonate, might have a positive impact on the MMP-8/TIMP-1 ratio to prevent loss of the extracellular matrix.
Conclusion
For the first time, we were able to demonstrate that cyclic mechanical loading abolishes the negative effects of zoledronate and IL-1ß in terms of the viability of HPdLF in vitro. Mechanical loading might stimulate and reactivate the cells. However, the combination of mechanical loading, inflammation, and zoledronate increased the production of COX-2-dependent PGE2, and the combination of these factors should therefore be avoided for clinical reasons. Zoledronate and clodronate inhibited MMP-8 synthesis caused by IL-1ß or mechanical loading, which might prevent loss of the extracellular matrix.
Clinical relevance
Periodontal inflammation should be treated initially before BP intake to prevent decreased cell viability of the periodontium and increased inflammation, which might be enhanced by the addition of mastication forces.
References
Marx RE (2003) Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg : Off J Am Assoc Oral Maxillofac Surg 61(9):1115–1117
Landesberg R, Wilson T, Grbic JT (2006) Bisphosphonate-associated osteonecrosis of the jaw: conclusions based on an analysis of case series. Dent Today 25(8):52 54-57
Mortensen M, Lawson W, Montazem A (2007) Osteonecrosis of the jaw associated with bisphosphonate use: presentation of seven cases and literature review. Laryngoscope 117(1):30–34
Nastro E, Musolino C, Allegra A, Oteri G, Cicciu M, Alonci A, Quartarone E, Alati C, De Ponte FS (2007) Bisphosphonate-associated osteonecrosis of the jaw in patients with multiple myeloma and breast cancer. Acta Haematol 117(3):181–187. doi:10.1159/000097876
Huja SS, Fernandez SA, Phillips C, Li Y (2009) Zoledronic acid decreases bone formation without causing osteocyte death in mice. Arch Oral Biol 54(9):851–856
Pozzi S, Vallet S, Mukherjee S, Cirstea D, Vaghela N, Santo L, Rosen E, Ikeda H, Okawa Y, Kiziltepe T, Schoonmaker J, Xie W, Hideshima T, Weller E, Bouxsein ML, Munshi NC, Anderson KC, Raje N (2009) High-dose zoledronic acid impacts bone remodeling with effects on osteoblastic lineage and bone mechanical properties. Clin Cancer Res : Off J Am Assoc Cancer Res 15(18):5829–5839
McLeod NM, Moutasim KA, Brennan PA, Thomas G, Jenei V (2014) In vitro effect of bisphosphonates on oral keratinocytes and fibroblasts. J Oral Maxillofac Surg : Off J Am Assoc Oral Maxillofac Surg 72(3):503–509
Pabst AM, Ziebart T, Koch FP, Taylor KY, Al-Nawas B, Walter C (2012) The influence of bisphosphonates on viability, migration, and apoptosis of human oral keratinocytes—in vitro study. Clin Oral Investig 16(1):87–93
Walter C, Pabst A, Ziebart T, Klein M, Al-Nawas B (2011) Bisphosphonates affect migration ability and cell viability of HUVEC, fibroblasts and osteoblasts in vitro. Oral Dis 17(2):194–199
Ziebart T, Pabst A, Klein MO, Kammerer P, Gauss L, Brullmann D, Al-Nawas B, Walter C (2011) Bisphosphonates: restrictions for vasculogenesis and angiogenesis: inhibition of cell function of endothelial progenitor cells and mature endothelial cells in vitro. Clin Oral Investig 15(1):105–111
Niver EL, Leong N, Greene J, Curtis D, Ryder MI, Ho SP (2011) Reduced functional loads alter the physical characteristics of the bone-periodontal ligament-cementum complex. J Periodontal Res 46(6):730–741
Patullo IM, Takayama L, Patullo RF, Jorgetti V, Pereira RM (2009) Influence of ovariectomy and masticatory hypofunction on mandibular bone remodeling. Oral Dis 15(8):580–586
Jacobs C, Grimm S, Ziebart T, Walter C, Wehrbein H (2013) Osteogenic differentiation of periodontal fibroblasts is dependent on the strength of mechanical strain. Arch Oral Biol 58(7):896–904
Jacobs C, Walter C, Ziebart T, Grimm S, Meila D, Krieger E, Wehrbein H (2014) Induction of IL-6 and MMP-8 in human periodontal fibroblasts by static tensile strain. Clin Oral Investig 18(3):901–908
Abe M, Kawamoto K, Okamoto H, Horiuchi N (2001) Induction of collagenase-2 (matrix metalloproteinase-8) gene expression by interleukin-1beta in human gingival fibroblasts. J Periodontal Res 36(3):153–159
Ngan P, Saito S, Saito M, Lanese R, Shanfeld J, Davidovitch Z (1990) The interactive effects of mechanical stress and interleukin-1 beta on prostaglandin E and cyclic AMP production in human periodontal ligament fibroblasts in vitro: comparison with cloned osteoblastic cells of mouse (MC3T3-E1). Arch Oral Biol 35(9):717–725
Saito M, Saito S, Ngan PW, Shanfeld J, Davidovitch Z (1991) Interleukin 1 beta and prostaglandin E are involved in the response of periodontal cells to mechanical stress in vivo and in vitro. Am J Orthod Dentofac Orthop : Off Publ Am Assoc Orthod Constituent Soc Am Board Orthod 99(3):226–240
Suzuki Y, Nishiyama T, Hasuda K, Fujishiro T, Niikura T, Hayashi S, Hashimoto S, Kurosaka M (2007) Effect of etidronate on COX-2 expression and PGE(2) production in macrophage-like RAW 264.7 cells stimulated by titanium particles. J Orthop Sci : Off J Jpn Orthop Assoc 12(6):568–577
De Colli M, Zara S, di Giacomo V, Patruno A, Marconi GD, Gallorini M, Zizzari VL, Tete G, Cataldi A (2015) Nitric oxide-mediated cytotoxic effect induced by zoledronic acid treatment on human gingival fibroblasts. Clin Oral Investig 19(6):1269–1277
Hagelauer N, Pabst AM, Ziebart T, Ulbrich H, Walter C (2015) In vitro effects of bisphosphonates on chemotaxis, phagocytosis, and oxidative burst of neutrophil granulocytes. Clin Oral Investig 19(1):139–148
Liu L, Igarashi K, Kanzaki H, Chiba M, Shinoda H, Mitani H (2006) Clodronate inhibits PGE(2) production in compressed periodontal ligament cells. J Dent Res 85(8):757–760
Giannopoulou C, Cimasoni G (1996) Functional characteristics of gingival and periodontal ligament fibroblasts. J Dent Res 75(3):895–902
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25(4):402–408
Walter C, Klein MO, Pabst A, Al-Nawas B, Duschner H, Ziebart T (2010) Influence of bisphosphonates on endothelial cells, fibroblasts, and osteogenic cells. Clin Oral Investig 14(1):35–41
Agis H, Blei J, Watzek G, Gruber R (2010) Is zoledronate toxic to human periodontal fibroblasts? J Dent Res 89(1):40–45
Jacobs C, Walter C, Ziebart T, Dirks I, Schramm S, Grimm S, Krieger E, Wehrbein H (2015) Mechanical loading influences the effects of bisphosphonates on human periodontal ligament fibroblasts. Clin Oral Investig 19(3):699–708
Diercke K, Kohl A, Lux CJ, Erber R (2012) IL-1beta and compressive forces lead to a significant induction of RANKL-expression in primary human cementoblasts. J Orofac Orthop = Fortschr Kieferorthop : Organ/Off J Dtsch Ges Kieferorthop 73(5):397–412
Nogueira AV, Nokhbehsaim M, Eick S, Bourauel C, Jäger A, Jepsen S, Rossa C, Deschner J, Cirelli JA (2014) Biomechanical loading modulates proinflammatory and bone resorptive mediators in bacterial-stimulated PDL. Cells 2014:425421
Romer P, Kostler J, Koretsi V, Proff P (2013) Endotoxins potentiate COX-2 and RANKL expression in compressed PDL cells. Clin Oral Investig 17(9):2041–2048
Nokhbehsaim M, Deschner B, Winter J, Reimann S, Bourauel C, Jepsen S, Jager A, Deschner J (2010) Contribution of orthodontic load to inflammation-mediated periodontal destruction. J Orofac Orthop = Fortschr Kieferorthop : Organ/Off J Dtsch Ges Kieferorthop 71(6):390–402
Long P, Liu F, Piesco NP, Kapur R, Agarwal S (2002) Signaling by mechanical strain involves transcriptional regulation of proinflammatory genes in human periodontal ligament cells in vitro. Bone 30(4):547–552
Nettelhoff L, Grimm S, Jacobs C, Walter C, Pabst AM, Goldschmitt J, Wehrbein H (2016) Influence of mechanical compression on human periodontal ligament fibroblasts and osteoblasts. Clin Oral Investig 20(3):621–629
Buduneli E, Vardar-Sengul S, Buduneli N, Atilla G, Wahlgren J, Sorsa T (2007) Matrix metalloproteinases, tissue inhibitor of matrix metalloproteinase-1, and laminin-5 gamma2 chain immunolocalization in gingival tissue of endotoxin-induced periodontitis in rats: effects of low-dose doxycycline and alendronate. J Periodontol 78(1):127–134
Teronen O, Heikkila P, Konttinen YT, Laitinen M, Salo T, Hanemaaijer R, Teronen A, Maisi P, Sorsa T (1999) MMP inhibition and downregulation by bisphosphonates. Ann N Y Acad Sci 878:453–465
Teronen O, Konttinen YT, Lindqvist C, Salo T, Ingman T, Lauhio A, Ding Y, Santavirta S, Sorsa T (1997) Human neutrophil collagenase MMP-8 in peri-implant sulcus fluid and its inhibition by clodronate. J Dent Res 76(9):1529–1537
Acknowledgements
We thank the Osteology Foundation for funding this research project. Many thanks to Dr. Jutta Goldschmidt, Jutta Bühler, Ute Zerfass, and Lotte Groothusen for their assistance in the laboratory and Katherine Taylor for the orthographic correction of the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Funding
The work was supported by the Osteology Foundation (Lucerne, Switzerland).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Jacobs, C., Schramm, S., Dirks, I. et al. Mechanical loading increases pro-inflammatory effects of nitrogen-containing bisphosphonate in human periodontal fibroblasts. Clin Oral Invest 22, 901–907 (2018). https://doi.org/10.1007/s00784-017-2168-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2168-1