Abstract
In renal transplant recipients (RTR), we recently found that low urinary excretion of homoarginine (hArg) is associated with mortality and graft failure. However, it is not known whether such prospective associations also hold true for plasma concentrations of hArg. In the present study, we therefore determined plasma concentrations of hArg in the same cohort, i.e. in 687 RTR (functioning graft ≥1 year), and in 140 healthy donors, before and after kidney donation. Plasma hArg concentrations were significantly lower in RTR compared to healthy controls [1.24 (0.95–1.63) µM vs. 1.58 (1.31–2.03) µM, P < 0.001], and kidney donation resulted in a decrease in plasma hArg concentration to 1.41 (1.10–1.81) µM (P < 0.001). In RTR, multivariable linear regression analysis revealed BMI (β = 0.124), heart rate (β = −0.091), pre-emptive transplantation (β = 0.078), antidiabetic medication (β = −0.091), eGFR (β = 0.272), plasma PTH (β = −0.098), uric acid (β = 0.137), alkaline phosphatase (β = −0.100), HDL (β = −0.111), NT-pro-BNP (β = −0.166), and urinary urea excretion (β = 0.139) as main determinants of plasma hArg (all P < 0.05). In RTR, plasma hArg concentration was inversely associated with all-cause [hazard ratio (HR) 0.59 (95% CI 0.50–0.70), P < 0.001] and cardiovascular mortality [HR 0.50 (0.39–0.66), P < 0.001], both expressed per standard deviation change in log-transformed hArg, independent of potential confounders. To conclude, our results suggest that the kidney is a major hArg production site and an important modulator of hArg homeostasis in the renal and cardiovascular systems. Moreover, low plasma hArg is independently associated with increased risk of cardiovascular mortality in RTR, which corroborates the cardiovascular importance of preserving kidney function after transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Homoarginine (hArg) is the non-proteinogenic methylene homologue of the amino acid arginine (Arg). While Arg is well known to play multiple physiological roles (Wu et al. 2009), our understanding of the biological significance of hArg is limited. Most recently, hArg has aroused the interest of scientists from different disciplines (Tsikas and Wu 2015). Similar to arginine albeit at lower concentrations, hArg circulates in blood and is excreted in the urine (Bernstein et al. 2015; Kayacelebi et al. 2014a, b; Marescau et al. 1997). In patients with impaired renal function, low circulating hArg concentrations emerged as a cardiovascular risk factor (Tomaschitz et al. 2014). Conversely, higher urinary hArg excretion is associated with lower rates of all-cause mortality and graft failure in renal transplant recipients (RTR) (Frenay et al. 2015a).
Both Arg and hArg are substrates for nitric oxide synthase (NOS) (Hecker et al. 1991; Moali et al. 1998; Bretscher et al. 2003; Alesutan et al. 2016), a family of isoenzymes generating nitric oxide (NO). NO exerts a multitude of biological activities including blood vessel dilatation and is of particular importance for the kidney and in the cardiovascular system (Moncada and Higgs 1993; Passauer et al. 2005; Zoccali 2006; O’Connor and Cowley 2010).
In consideration of the potential significance of circulating hArg in the renal and cardiovascular systems in general and in renal transplantation in particular, delineation of its origin and mechanism(s) of action would seem to be important for the health and wellbeing of transplant recipients. In previous studies, we found that RTR have lower hArg excretion rates than healthy controls (Frenay et al. 2015a). RTR patients also have higher concentrations of asymmetric dimethylarginine (ADMA) in blood than healthy controls (Frenay et al. 2015b), which is an important endogenous inhibitor of NOS activity (Tsikas et al. 2000). In the present study, we evaluated a possible role of circulating hArg in mortality outcome after kidney transplantation (KTx). In addition, plasma hArg concentrations were compared before and after donation of a kidney by a healthy subject.
Methods
Design and study population
This is a cross-sectional and longitudinal analysis of a single-center RTR cohort that has been previously described in detail (van den Berg et al. 2012a, 2014; Frenay et al. 2015a, b). A total of 707 RTR (KTx group) signed informed consent and participated in the present study. All transplantations were conducted at the University Medical Center Groningen (UMCG). Twenty RTR had missing biological samples, resulting in 687 RTR eligible for analysis of hArg in plasma samples. We also collected plasma samples from 140 healthy kidney donors before (DB group) and after (DA group) kidney donation. The Institutional Review Board approved the study protocol (METc 2008/186) in adherence to the Declaration of Helsinki. Baseline examination of a participant was performed between November 2008 and March 2011. The primary outcome measures of the study were all-cause mortality, death-censored graft failure and cardiovascular mortality. The latter was defined as death due to cerebrovascular disease, ischemic heart disease, heart failure, or sudden cardiac death, whereas graft failure was defined as necessity for return to dialysis or re-transplantation. Participants were followed up until the end of May 2013.
All RTR and healthy donors visited the out-patient clinic in the morning, after an overnight fast. A strict protocol (van den Berg et al. 2012b) was followed to measure both blood pressure (mmHg) and heart rate using a semi-automatic device (Dinamap® 1846, Critikon, Tampa, FL, USA). Clinical and biochemical analyses were performed by validated routine laboratory methods (Frenay et al. 2015a, b). eGFR was calculated using the CKD Epidemiology Collaboration (CKD-EPI) equation (Levey et al. 2009). Participants registered their dietary intake using a validated semi-quantitative FFQ as described elsewhere (van den Berg et al. 2013). Dietary data were converted into daily nutrient intake using the Dutch Food Composition Table of 2006 (The Hague 2006). All information on participants’ medical history, medication use and health status was obtained from patient records. Relevant transplant information was extracted from the UMCG renal transplant database. Information on smoking behavior was obtained by using a questionnaire, with classification as current, former or never smokers.
Measurement of plasma hArg
We measured the concentration of hArg in plasma samples collected during a previous study (Frenay et al. 2015a, b) of a large cohort of stable RTR and healthy donors before and after kidney donation. The concentration of hArg and Arg was determined in 10-µL aliquots of plasma ultrafiltrates by gas chromatography-mass spectrometry (GC–MS) as described elsewhere (Kayacelebi et al. 2014b). Plasma samples were analyzed in batches of 10, along with quality control (QC) samples analyzed in duplicate. A plasma sample of a healthy volunteer served as QC sample. QC1 samples were analyzed without addition of synthetic hArg. QC2 samples were spiked with 2 µM hArg. Considering the data from the ten runs, the hArg concentration in the QC1 and QC2 samples was determined to be (mean ± SD) 1.79 ± 0.06 and 3.75 ± 0.13 µM, respectively. The mean imprecision (relative standard deviation; RSD, %) was 3.4% (range 0–7.6%) in the QC1 samples and 3.5% (range 0–4.9%) in the QC2 samples. The concentration of 2 µM hArg added to the QC2 samples was determined with a mean accuracy (recovery, %) of 98% (range 87–108%). These QC data support the validity of the hArg concentrations measured in the plasma samples of RTR and donors.
Statistical analyses
Statistical analyses were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). Data are presented as mean ± SD or median [interquartile range (IQR)]. The distribution of all parameters was assessed by inspecting histograms and probability plots. Skewed data were normalized by logarithmic transformation, where appropriate.
Possible baseline differences between tertiles of plasma hArg in our RTR population were tested by using ANOVA for normally distributed, the Kruskal–Wallis test for non-normally distributed, and the Chi-square test for nominal data. Baseline associations with plasma hArg concentration and other parameters were explored by means of linear regression analysis. Possible crude associations of plasma hArg concentration with mortality and death-censored graft failure were investigated by Kaplan–Meier analyses, accompanied by log-rank tests. Changes in plasma hArg concentration in donors before and after donation were compared by paired t test. Plasma hArg concentrations in RTR and donors were compared by unpaired t-test.
Cox regression analysis was conducted for all-cause mortality and graft failure, and models were constructed with inclusion of all parameters that were significantly associated with plasma hArg concentration in multi-variable analysis (P < 0.05) analogous to the previously reported analysis for urinary hArg (Frenay et al. 2015b). These parameters were divided into two groups, i.e., one group for potential determinants and another group for potential effectors of plasma hArg. First, crude Cox analysis was performed (model 1), followed by adjustment for pre-emptive KTx, age and gender (model 2) and eGFR (model 3). Additionally, potential determinants of plasma hArg were added [BMI, heart rate, antidiabetic medication, alkaline phosphatase, and urea excretion (model 4). In the final model, parameters that were associated with plasma hArg, but were more likely to be in the causal pathway of plasma hArg rather than determine hArg, were added (model 5: HDL cholesterol, parathyroid hormone (PTH), uric acid, and NT-pro-BNP].
Finally, hazard ratio (HR) values are presented per standard deviation change of log-transformed plasma hArg concentration. Within all statistical analyses, a two-sided P value less than 0.05 was considered statistically significant.
Results
Cohort characteristics
The baseline RTR characteristics are summarized in Table 1. Plasma hArg concentration was 1.31 (1.06–1.75) µM in the male (57%) and 1.14 (0.91–1.52) µM in the female RTR. In the entire KTx cohort analyzed, plasma hArg concentration was 1.24 (0.95–1.63) µM. In the healthy donors, plasma hArg concentration was 1.58 (1.3–2.03) µM before and 1.41 (1.10–1.81) µM after kidney donation (P = 0.004). Plasma hArg concentration differed between RTR and healthy donors before (P < 0.0001) and after (P = 0.006) kidney donation.
In the tertiles as well as in the whole cohort, plasma hArg concentration was by a constant factor of about 1.15 higher in males compared to females. The highest plasma hArg concentrations were measured in the younger RTR who also had higher BMI and BSA values. PTH and hsCRP were lowest in the highest plasma hArg tertile. Urea and NT-pro-BNP were lowest in the highest plasma hArg tertile, whereas uric acid and urea excretion were highest in the highest plasma hArg tertile. With respect to renal function, serum creatinine and albuminuria were lowest in the highest plasma hArg tertile, while eGFR was highest.
RTR had a significantly higher systolic and diastolic blood pressure compared to controls. eGFR was significantly lower in RTR compared to controls. Urinary albumin excretion was significantly higher in RTR than in controls. NT-pro-BNP, HbA1c, triglycerides and hsCRP were all significantly higher in RTR compared to healthy controls. In the RTR cohort, we tested associations of plasma hArg concentrations with different clinical parameters by univariable and multivariable regression analyses. The results of these analyses are summarized in Table 2. BMI (β = 0.124), heart rate (β = −0.091), pre-emptive KTx (β = 0.078), antidiabetic medication (β = −0.091), eGFR (β = 0.272), plasma PTH (β = −0.098), uric acid (β = 0.137), alkaline phosphatase (β = −0.100), HDL (β = −0.111), NT-pro-BNP (β = 0.166), and urinary urea excretion (β = 0.139), showed the strongest independent associations with plasma hArg concentration (all P < 0.05).
Association of plasma hArg with mortality and graft failure
During a median follow-up period of 5.3 (4.5–6.0) years, 146 (21%) RTR died in total, of whom 58 (8%) were due to a cardiovascular cause. In the highest gender-stratified tertile of plasma hArg concentration 23 out of 228 patients (10%) died, in the middle tertile 52 out of 231 (23%) patients died, and in the lowest tertile 71 out of 228 (31%) patients died (log-rank test, P < 0.001, Fig. 1a). Corresponding frequencies for cardiovascular mortality were 10 (4%), 14 (6%), and 34 (15%) for the highest, middle, and lowest tertile (Fig. 1b). In total, 81 out of 687 (12%) RTR developed graft failure. In the highest plasma hArg tertile, the lowest frequency of graft failure was observed, i.e., 12 out of 228 (5%) RTR suffered from graft failure, while an intermediate risk of developing graft failure was observed in the middle tertile of plasma hArg, with 31 out of 231 (13%) RTR developing graft failure, and 38 out of 228 (17%) patients developing graft failure in the lowest tertile of plasma hArg (log-rank test, P < 0.001, Fig. 1c).
Cox regression analyses for the associations with all-cause and cardiovascular mortality, and graft failure are shown in Table 3. The crude Cox regression analysis (model 1) demonstrates that higher levels of plasma hArg are significantly associated with a lower risk of all-cause mortality (HR 0.59; 95% CI 0.50–0.70), P < 0.001). This significant association remained present after adjustment for age, gender, and pre-emptive KTx (model 2; HR 0.63; 95% CI 0.53–0.76, P < 0.001), as well as for eGFR (model 3; HR 0.72; 95% CI 0.60–0.86, P < 0.001). Upon additional consideration of BMI, heart rate, antidiabetic medication, alkaline phosphatase, and urea excretion, higher plasma hArg remained significantly associated with lower mortality (model 4; HR 0.78; 95% CI 0.64–0.95, P = 0.01). In the final model, after additionally adjusting for potential consequences of plasma hArg, i.e. HDL cholesterol, PTH, uric acid, and NT-pro-BNP, plasma hArg remained significantly associated with all-cause mortality (model 5; HR 0.75; 95% CI 0.62–0.93, P = 0.008). Analyses with cardiovascular mortality as end-point revealed lower point estimates, i.e. 0.50 (0.39–0.66) for the crude and 0.54 (0.38–0.75) for the fully adjusted association, compared to all-cause mortality.
Further, Cox regression analyses revealed that higher plasma hArg concentration is associated with a decreased risk of graft failure (crude model; HR 0.59; 95% CI 0.48–0.74, P < 0.001, Table 3). This association remained statistically significant after adjusting for age, gender, and pre-emptive KTx (model 2; HR 0.56; 95% CI 0.37–0.70, P < 0.001). Upon additional consideration of eGFR, the association of plasma hArg with graft survival lost significance (model 3; HR 0.82; 95% CI 0.65–1.03, P = 0.09).
In view of a potential interplay between hArg and PTH (Tomaschitz et al. 2015), we also looked for an interaction between plasma hArg and PTH levels for predicting mortality risk and risk of graft failure. We found no significant effect modification by PTH in the association for mortality (P interaction = 0.15) or graft failure (P interaction = 0.49).
Discussion
Clinical aspects
The most important finding of the present study is that high plasma hArg concentrations are associated with reduced all-cause and cardiovascular mortality in stable RTR. These associations remained present after adjustment for potential confounders and for factors that could share the causal pathway of plasma hArg. Another important finding of the study is that donation of one kidney by a healthy subject results in a modest decrease in plasma hArg concentration. The findings of the present study would seem to provide a reasonable explanation for why low circulating and low urinary hArg concentrations are crucial risk factors for renal, cardiovascular and cerebrovascular diseases and mortality (Atzler et al. 2013, 2014; Drechsler et al. 2011, 2013; Frenay et al. 2015a; Kayacelebi et al. 2014a; März et al. 2010; Pilz et al. 2011a, b, 2014, 2015; Haghikia et al. 2017).
Mean circulating hArg levels were found to be 1.5 µM in females and 2 µM in males in 33 healthy adults (Marescau et al. 1997). In 19 healthy subjects, we measured mean circulating hArg concentrations of 1.87 µM (Kayacelebi et al. 2014b). The plasma hArg levels measured in the healthy donors of the present study prior to kidney donation (median, 1.58 µM) are within reported intervals. After donation of a kidney, observed plasma hArg concentrations (median, 1.41 µM) are still within normal intervals, yet they are by about 11% lower than those measured in the same donors prior to the kidney donation. In the RTR, the plasma hArg concentration (median, 1.24 µM) was even by about 22% lower compared to healthy donors. Plasma hArg concentrations measured in our 687 RTR were considerably lower than those reported for 829 RTR in another study (Drechsler et al. 2015).
In patients with CKD, plasma levels of hArg are generally lower compared to those in healthy subjects and dependent on the degree of kidney failure, especially in patients with progressing CKD. It has been suggested that low circulating levels of hArg may be useful in the prediction of disease progression (Drechsler et al. 2013). In dialysis patients, lower plasma hArg levels were found to be associated with a significantly higher risk of dying compared to those patients with higher plasma hArg levels (Pilz et al. 2014). This group has also reported that serum hArg concentration is inversely associated with NT-pro-BNP levels in patients at cardiovascular risk with preserved left ventricular ejection fraction (Pilz et al. 2011a). This observation is supported by our results indicating an inverse association in the univariable analysis between plasma hArg and serum NT-pro-BNP concentrations in RTR. Moreover, in our RTR we found that plasma hArg concentration is inversely associated with serum hsCRP concentration, in agreement with the finding of RTR patients of another study (Drechsler et al. 2015).
Low serum hArg concentrations were found to be related to decreased eGFR, adverse cardiovascular events and death due to heart failure in a large cohort of patients with normal or slightly decreased eGFR who were hospitalized to undergo coronary angiography (Tomaschitz et al. 2014). These associations were more pronounced in patients with low serum hArg levels and an eGFR below 60 mL/min per 1.73 m2. Additionally, a significant association between low circulating levels of hArg and sudden cardiac death or death due to cardiac failure was found in patients on hemodialysis (Atzler et al. 2013). Similar associations were also found in patients with CKD (Ravani et al. 2013), with plasma hArg predicting the risk of progression to dialysis and death in CKD. Circulating hArg concentrations were also positively related to eGFR, independent of other relevant risk factors (Ravani et al. 2013). This relationship is strongly supported by our study and another recent study on RTR patients with anthropometric and clinical characteristics comparable to our RTR patients (Drechsler et al. 2015).
hArg has been associated with energy metabolism (Wyss and Kaddurah-Daouk 2000; Kleber et al. 2013). In our study, we found that plasma hArg concentrations are positively associated with serum albumin concentration, BSA and urea excretion. All these parameters are involved in protein balance and nutritional status (Kopple et al. 2000) and are important determinants of cardiovascular morbidity and mortality in pre- and post-renal transplantation (Rettkowski et al. 2007; Molnar et al. 2011).
A limitation of the present and other observational studies is that no conclusions can be drawn with respect to causal relationships of the detected associations. Further potential study limitations are that our cohort is single-centered and most participants were Caucasian, and the number of incident cases is relatively low for the adjustments made in the Cox models. Therefore, extrapolation of our findings to the general population is limited. On the other hand, appreciable strengths of our study are the long follow-up time and the large cohort size, as well as the comprehensive registration of the participants and no loss to follow-up. All these issues contribute to the robustness of our clinical results.
Biochemical aspects
We (Frenay et al. 2015a; Kayacelebi et al. 2014a; present study) and others (Atzler et al. 2013, 2014; Drechsler et al. 2011, 2013, 2015; März et al. 2010; Pilz et al. 2011a, b, 2014, 2015) found that low circulating and low urinary hArg concentrations are crucial risk factors for renal and cardiovascular diseases and mortality in adults. These observations are generally attributed to a diminished NO synthesis due to a relative deficit of hArg. Yet, while hArg is an alternative NOS substrate (Hecker et al. 1991) a recent study demonstrated that hArg can also attenuate the activity of recombinant NOS isoforms, most likely by competing with Arg (Alesutan et al. 2016). Because circulating hArg concentrations are up to 40-fold lower compared to those of Arg (Tsikas and Wu 2015), it is unlikely that hArg has a major impact on NO synthesis in humans (Atzler et al. 2016). NO is a potent vasodilatator and protects against cardiovascular disease (Xia and Vanhoutte 2011; Jones and Bolli 2006), while impaired NO synthesis leads to endothelial and myocardial dysfunction (Takahashi and Harris 2014; Yang and Ming 2006). Circulating hArg was found to be inversely correlated with markers of impaired endothelial function (Pilz et al. 2014). Yet, again, there is no strong evidence that the beneficial effects of hArg are indeed due to NO formation from hArg. In addition, the proposed prooxidative effects of hArg (Wyse et al. 2001; Sasso et al. 2015) are doubtful to be of relevance for physiological hArg concentrations (Hanff et al. 2016). Thus, hArg is unlikely to diminish NO bioavailability through elevation of oxidative stress. These considerations strongly suggest that the emergence of impaired hArg synthesis as a risk factor in the renal and cardiovascular systems is unrelated to NO and based on another mechanism yet to be unveiled. Whether the recently discovered anti-atherosclerotic effects whereby hArg augments phosphate-induced osteo-/chondrogenic transformation of vascular smooth muscle cells (Alesutan et al. 2016) are of any relevance in this context remains to be investigated.
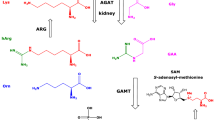
A genome-wide association study identified three genes which are involvement in the regulation of circulating hArg concentration (Kleber et al. 2013). In this study, the strongest association was observed for variants in the glycine amidinotransferase gene. This finding is of particular interest because l-arginine:l-glycine amidinotransferase (AGAT), also known as glycine amidinotransferase (GATM), is the enzyme that catalyzes the synthesis of hArg and guanidinoacetate from Arg (Tsikas and Wu 2015), and plays a major role in the energy metabolism in mitochondria. Thus, the protective effects exerted by hArg are more likely to be linked to energy metabolism rather than to NO synthesis (Kleber et al. 2013).
In conclusion, we here demonstrated that high plasma hArg concentrations are independently associated with reduced all-cause and cardiovascular mortality in stable renal transplant recipients. High plasma hArg concentrations are also associated with improved renal function. The kidney is an important source of hArg and the latter, therefore, of particular importance in RTR. Preservation of kidney function in RTR patients is crucial to preserve cardiovascular function. The molecular mechanism(s) by which hArg exerts its beneficial effects in the renal and cardiovascular systems remain to be explored.
Abbreviations
- ADMA:
-
Asymmetric dimethylarginine
- AGAT:
-
l-Arginine:l-glycine amidinotransferase
- BSA:
-
Body surface area
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- eGFR:
-
Estimated glomerular filtration rate
- GATM:
-
Glycine amidinotransferase
- hArg:
-
Homoarginine
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- KTx:
-
Kidney transplantation
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- NT-pro-BNP:
-
N-terminal pro-hormone of brain natriuretic peptide
- PTH:
-
Parathyroid hormone
- QC:
-
Quality control
- RTR:
-
Renal transplant recipients
References
Alesutan I, Feger M, Tuffaha R, Castor T, Musculus K, Buehling SS et al (2016) Augmentation of phosphate-induced osteo-/chondrogenic transformation of vascular smooth muscle cells by homoarginine. Cardiovasc Res 110:408–418
Atzler D, Rosenberg M, Anderssohn M et al (2013) Homoarginine—an independent marker of mortality in heart failure. Int J Cardiol 168:4907–4909
Atzler D, Gore MO, Ayers CR et al (2014) Homoarginine and cardiovascular outcome in the population-based Dallas Heart Study. Arterioscler Thromb Vasc Biol 34:2501–2507
Atzler D, Schönhoff M, Cordts K, Ortland I, Hoppe J, Hummel FC, Gerloff C, Jaehde U, Jagodzinski A, Böger RH, Choe CU, Schwedhelm E (2016) Oral supplementation with l-homoarginine in young volunteers. Br J Clin Pharmacol. doi:10.1111/bcp.13068
Bernstein HG, Jäger K, Dobrowolny H, Steiner J, Keilhoff G, Bogerts B, Laube G (2015) Possible sources and functions of L-homoarginine in the brain: review of the literature and own findings. Amino Acids 47:1729–1740
Bretscher LE, Li H, Poulos TL, Griffith OW (2003) Structural characterization and kinetics of nitric-oxide synthase inhibition by novel N5-(iminoalkyl)- and N5-(iminoalkenyl)-ornithines. J Biol Chem 278:46789–46797
Drechsler C, Meinitzer A, Pilz S et al (2011) Homoarginine, heart failure, and sudden cardiac death in haemodialysis patients. Eur J Heart Fail 13:852–859
Drechsler C, Kollerits B, Meinitzer A et al (2013) Homoarginine and progression of chronic kidney disease: results from the Mild to Moderate Kidney Disease Study. PLoS One 8:e63560
Drechsler C, Pihlstrøm H, Meinitzer A, Pilz S, Tomaschitz A, Abedini S, Fellstrom B, Jardine AG, Wanner C, März W, Holdaas H (2015) Homoarginine and clinical outcomes in renal transplant recipients: results from the assessment of lescol in renal transplantation study. Transplantation 99:1470–1476
Frenay AR, van den Berg E, de Borst MH, Beckmann B, Tsikas D, Feelisch M, Navis G, Bakker SJL, van Goor H (2015a) Plasma ADMA associates with all-cause mortality in renal transplant recipients. Amino Acids 47:1941–1949
Frenay AR, Kayacelebi AA, Beckmann B, Soedamah SS, de Borst MH, van den Berg E, van Goor H, Bakker SJL, Tsikas D (2015b) High urinary homoarginine excretion is associated with low rates of all-cause mortality in renal transplant recipients. Amino Acids 47:1827–1836
Haghikia A, Yanchev GR, Kayacelebi AA, Hanff E, Bledau N, Widera C, Sonnenschein K, Haghikia A, Weissenborn K, Bauersachs J, Bavendiek U, Tsikas D (2017) The role of l-arginine/l-homoarginine/nitric oxide pathway for aortic distensibility and intima-media thickness in stroke patients. Amino Acids. doi:10.1007/s00726-017-2409-2
Hanff E, Kayacelebi AA, Herrmann C, Obermann M, Das AM, Tsikas D (2016) Unaltered l-arginine/NO pathway in a MELAS patient: is mitochondrial NO synthase involved in the MELAS syndrome? Int J Cardiol 223:479–481
Hecker M, Walsh DT, Vane JR (1991) On the substrate specificity of nitric oxide synthase. FEBS Lett 294:221–224
Jones SP, Bolli R (2006) The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol 40:16–23
Kayacelebi AA, Nguyen TH, Neil C et al (2014a) Homoarginine and 3-nitrotyrosine in patients with takotsubo cardiomyopathy. Int J Cardiol 173:546–547
Kayacelebi AA, Beckmann B, Gutzki FM, Jordan J, Tsikas D (2014b) GC-MS and GC-MS/MS measurement of the cardiovascular risk factor homoarginine in biological samples. Amino Acids 46:2205–2217
Kleber ME, Seppala I, Pilz S, Hoffmann MM, Tomaschitz A, Oksala N, Raitoharju E, Lyytikainen LP, Makela KM, Laaksonen R, Kahonen M, Raitakari OT, Huang J, Kienreich K, Fahrleitner-Pammer A, Drechsler C, Krane V, Boehm BO, Koenig W, Wanner C, Lehtimaki T, Marz W, Meinitzer A (2013) Genome-wide association study identifies 3 genomic loci significantly associated with serum levels of homoarginine: the AtheroRemo Consortium. Circ Cardiovasc Genet 6:505–513
Kopple JD, Greene T, Chumlea WC et al (2000) Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney Int 57:1688–1703
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Marescau B, Nagels G, Possemiers I, De Broe ME, Becaus I, Billiouw JM, Lornoy W, De Deyn PP (1997) Guanidino compounds in serum and urine of nondialyzed patients with chronic renal insufficiency. Metabolism 46:1024–1031
März W, Meinitzer A, Drechsler C et al (2010) Homoarginine, cardiovascular risk, and mortality. Circulation 122:967–975
Moali C, Boucher JL, Sari MA et al (1998) Substrate specificity of NO synthases: detailed comparison of l-arginine, homo-l-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-l-arginine. Biochemistry 37:10453–10460
Molnar MZ, Kovesdy CP, Bunnapradist S et al (2011) Associations of pretransplant serum albumin with post-transplant outcomes in kidney transplant recipients. Am J Transplant 11:1006–1015
Moncada S, Higgs A (1993) The l-arginine-nitric oxide pathway. N Engl J Med 329:2002–2012
O’Connor PM, Cowley AW Jr (2010) Modulation of pressure-natriuresis by renal medullary reactive oxygen species and nitric oxide. Curr Hypertens Rep 12:86–92
Passauer J, Pistrosch F, Bussemaker E (2005) Nitric oxide in chronic renal failure. Kidney Int 67:1665–1667
Pilz S, Meinitzer A, Tomaschitz A et al (2011a) Low homoarginine concentration is a novel risk factor for heart disease. Heart 97:1222–1227
Pilz S, Tomaschitz A, Meinitzer A et al (2011b) Low serum homoarginine is a novel risk factor for fatal strokes in patients undergoing coronary angiography. Stroke 42:1132–1134
Pilz S, Edelmann F, Meinitzer A et al (2014) Associations of methylarginines and homoarginine with diastolic dysfunction and cardiovascular risk factors in patients with preserved left ventricular ejection fraction. J Card Fail 20:923–930
Pilz S, Meinitzer A, Gaksch M, Grübler M, Verheyen N, Drechsler C, Hartaigh BÓ, Lang F, Alesutan I, Voelkl J, März W, Tomaschitz A (2015) Homoarginine in the renal and cardiovascular systems. Amino Acids 47:1703–1713
Ravani P, Maas R, Malberti F et al (2013) Homoarginine and mortality in pre-dialysis chronic kidney disease (CKD) patients. PLoS One 8:e72694
Rettkowski O, Wienke A, Hamza A et al (2007) Low body mass index in kidney transplant recipients: risk or advantage for long-term graft function? Transplant Proc 39:1416–1420
Sasso S, Dalmedico L, Magro DD, Pereira EM, Wyse AT, de Lima DD (2015) Differential in vitro effects of homoarginine on oxidative stress in plasma, erythrocytes, kidney and liver of rats in the absence and in the presence alpha-tocopherol, ascorbic acid or L-NAME. Amino Acids 47:1931–1939
Takahashi T, Harris RC (2014) Role of endothelial nitric oxide synthase in diabetic nephropathy: lessons from diabetic eNOS knockout mice. J Diabetes Res 2014:590541
The Hague: Dutch food composition table (NEVO 2006) NEVO table 2006 (dutch nutrient databank: NEVO table 2006)
Tomaschitz A, Meinitzer A, Pilz S et al (2014) Homoarginine, kidney function and cardiovascular mortality risk. Nephrol Dial Transplant 29:663–671
Tomaschitz A, Verheyen N, Gaksch M, Meinitzer A, Pieske B, Kraigher-Krainer E, Colantonio C, März W, Schmidt A, Belyavskiy E, Rus-Machan J, van Ballegooijen AJ, Stiegler C, Amrein K, Ritz E, Fahrleitner-Pammer A, Pilz S (2015) Homoarginine in patients with primary hyperparathyroidism. Am J Med Sci 349:306–311
Tsikas D, Wu G (2015) Homoarginine, arginine, and relatives: analysis, metabolism, transport, physiology, and pathology. Amino Acids 47:1697–1702
Tsikas D, Böger RH, Sandmann J, Bode-Böger SM, Frölich JC (2000) Endogenous nitric oxide synthase inhibitors are responsible for the L-arginine paradox. FEBS Lett 478:1–3
van den Berg E, Engberink MF, Brink EJ et al (2012a) Dietary acid load and metabolic acidosis in renal transplant recipients. Clin J Am Soc Nephrol 7:1811–1818
van den Berg E, Geleijnse JM, Brink EJ et al (2012b) Sodium intake and blood pressure in renal transplant recipients. Nephrol Dial Transplant 27:3352–3359
van den Berg E, Engberink MF, Brink EJ et al (2013) Dietary protein, blood pressure and renal function in renal transplant recipients. Br J Nutr 109:1463–1470
van den Berg E, Pasch A, Westendorp WH et al (2014) Urinary sulfur metabolites associate with a favorable cardiovascular risk profile and survival benefit in renal transplant recipients. J Am Soc Nephrol 25:1303–1312
Wu G, Bazer FW, Davis TA et al (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Wyse AT, Bavaresco CS, Hagen ME, Delwing D, Wannmacher CM, Severo Dutra-Filho C et al (2001) In vitro stimulation of oxidative stress in cerebral cortex of rats by the guanidino compounds accumulating in hyperargininemia. Brain Res 923:50–57
Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80:1107–1213
Xia Z, Vanhoutte PM (2011) Nitric oxide and protection against cardiac ischemia. Curr Pharm Des 17:1774–1782
Yang Z, Ming XF (2006) Endothelial arginase: a new target in atherosclerosis. Curr Hypertens Rep 8:54–59
Zoccali C (2006) Traditional and emerging cardiovascular and renal risk factors: an epidemiologic perspective. Kidney Int 70:26–33
Acknowledgements
This work was supported by Grants from the Dutch Kidney Foundation (NSN C08-2254, P13-114), by COST Action BM1005: ENOG: European Network on Gasotransmitters (http://www.gasotransmitters.eu), and by the Top Institute Food and Nutrition (A-1003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The Institutional Review Board approved the study protocol (METc 2008/186), which was in adherence to the Declaration of Helsinki.
Conflict of interest
All authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kayacelebi, A.A., Minović, I., Hanff, E. et al. Low plasma homoarginine concentration is associated with high rates of all-cause mortality in renal transplant recipients. Amino Acids 49, 1193–1202 (2017). https://doi.org/10.1007/s00726-017-2420-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-017-2420-7