Abstract
Carnosinase 1 (CN1) contributes to diabetic nephropathy by cleaving histidine-dipeptides which scavenge reactive oxygen and carbonyl species and increase nitric oxide (NO) production. In diabetic mice renal CN1 activity is increased, the regulatory mechanisms are unknown. We therefore analysed the in vitro and in vivo regulation of CN1 activity using recombinant and human CN1, and the db/db mouse model of diabetes. Glucose, leptin and insulin did not modify recombinant and human CN1 activity in vitro, glucose did not alter renal CN1 activity of WT or db/db mice ex vivo. Reactive metabolite methylglyoxal and Fenton reagent carbonylated recombinant CN1 and doubled CN1 efficiency. NO S-nitrosylated CN1 and decreased CN1 efficiency for carnosine by 70 % (p < 0.01), but not for anserine. Both CN1 cysteine residues were nitrosylated, the cysteine at position 102 but not at position 229 regulated CN1 activities. In db/db mice, renal CN1 mRNA and protein levels were similar as in non-diabetic controls, CN1 efficiency 1.9 and 1.6 fold higher for carnosine and anserine. Renal carbonyl stress was strongly increased and NO production halved, CN1 highly carbonylated and less S-nitrosylated compared to WT mice. GSH and NO2/3 concentrations were reduced and inversely related with carnosine degradation rate (r = −0.82/−0.85). Thus, reactive metabolites of diabetes upregulate CN1 activity by post-translational modifications, and thus decrease the availability of reactive metabolite-scavenging histidine dipeptides in the kidney in a positive feedback loop. Interference with this vicious circle may represent a new therapeutic target for mitigation of DN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
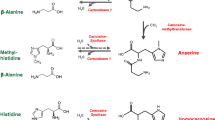
Carnosinase (CN1, EC 3.4.13.20) plays an important role in the development of nephropathy in diabetic patients. Susceptibility to diabetic nephropathy (DN) is strongly associated with a leucine repeat polymorphism in the CNDP1 gene encoding the CN1 protein. The shortest allelic form is associated with lower CN1 activities and a reduced probability of developing DN (Freedman et al. 2007; Janssen et al. 2005; Mooyaart et al. 2010). CN1 belongs to the M20 family of metalloproteases (MEROPS database; Rawlings ND 2004) and cleaves the histidine-dipeptides (HDP) carnosine (β-alanine-l-histidine) and anserine (β-alanyl-1-1-methylhistidine) (Boldyrev et al. 2013; Budzen and Rymaszewska 2013; Peters et al. 2010, 2011; Teufel et al. 2003). Both peptides have cytoprotective properties. Carnosine scavenges carbonyls (Barski et al. 2013; Negre-Salvayre et al. 2008; Vistoli et al. 2009), inhibits glycation (Alhamdani et al. 2007) and acts as ACE inhibitor (Hou et al. 2003; Nakagawa et al. 2006). Its function as antioxidant is debated (Babizhayev et al. 2013; Decker et al. 2000; Hipkiss 2011; Mozdan et al. 2005; Velez et al. 2008). Anserine has been shown to scavenge carbonyls (Aldini et al. 2005), to act as antioxidant (Kohen et al. 1988), and to reduce renal sympathetic nerve activity and blood pressure (Tanida et al. 2010). Anserine and carnosine have distinct actions; carnosine, but not anserine, reduces ischemic infarct size and preserves neurological function in a mouse model of permanent focal cerebral ischemia (Min et al. 2008). In diabetic mice, carnosine supplementation mitigates DN, reduces renal vasculopathy, normalizes vascular permeability (Peters et al. 2012), and improves wound-healing (Ansurudeen et al. 2012). In streptozotocin-induced diabetic rats, carnosine treatment prevents apoptosis of glomerular cells, podocyte loss (Peters et al. 2014; Riedl et al. 2011), and vascular damage (Pfister et al. 2011).
Diabetes mellitus is characterized by increased blood glucose, insulin and leptin levels (Somineni et al. 2014) and by impaired nitric oxide (NO) homeostasis with reduced availability of NO (Baylis 2012; Masha et al. 2011; Pollock and Pollock 2011), resulting in endothelial dysfunction, a critical mediator of diabetic vasculopathy (Sena et al. 2013). NO is required for S-nitrosylation, i.e. the covalent binding of NO to sulphur containing cysteines. This post-translational modification controls protein function (Foster et al. 2009; Hartmanova et al. 2013). Excessive production of reactive carbonyl species (RCS) and oxygen species (ROS) is another important mechanism underlying the pathogenesis of diabetic complication (Voziyan et al. 2014) such as cardiovascular and renal sequelae (Cheang et al. 2011; Forstermann and Sessa 2012). ROS such as hydrogen peroxide (H2O2) are known to lead to post-translational modifications by oxidizing amino acids in proteins. All amino acids can be modified by ROS but cysteine residues are most susceptible to oxidative changes due to the highly reactive thiol group (Gould et al. 2013). RCS react preferentially with arginine residues. The current paradigm of RCS-derived protein damage places the focus on methylglyoxal (MG), a spontaneous decomposition product of dihydroxyacetone phosphate and gylderaldehyde-3-phosphate, which contributes to extracellular matrix damage in diabetes (Voziyan et al. 2014).
ROS and RCS accumulate in diabetes mellitus, NO concentrations are low. We now tested whether reactive metabolites and NO modify CN1 activity by post-translational modification and whether this modification results in increased CN1 activity. This reduces the availability of reactive metabolite-scavenging histidine dipeptides anserine and carnosine in the kidney, which in turn should increase post-translational modification of CN1 in a positive feedback loop.
Materials and methods
CN1 activity
CN1 activity was assayed according to the method described by Teufel et al. (2003). Briefly, the reaction was initiated by addition of carnosine to renal tissue homogenate at a pH of 7. The reaction was stopped after defined periods by adding 1 % trichloracetic acid (final concentration in the test 0.3 %). Liberated histidine was derivatized by adding o-pthaldialdehyde (OPA) and fluorescence was read using a MicroTek plate reader (λ Exc 360 nm; λ Em 460 nm). V max values were obtained from Dixon plots using a linear regression program from at least three different assays. The effect of the S-nitrosylation on CN1 activity was measured after incubation with either 3-morpholinosydnonimine (SIN-1) or NaNO3 in different concentrations (between 0.2 and 2 mM) for 1 h at 30 °C. The effect of carbonylation was measured after incubation with H2O2 (with concentrations between 0.1 and 2 mM) and FeSO4 (between 50 and 500 µM) and following incubation with MG (between 0.1 and 2 mM) for 1 h at 30 °C. The kinetic parameters were determined by using various concentrations of substrates, and data fitting was performed according to the Michaelis–Menten equation.
Site-directed mutagenesis and overexpression of recombinant CN1 enzyme
We substituted cysteine with serine in CN1 (Mut1C102S and Mut2C229S). Recombinant FLAG-tagged proteins have been purified from CHO supernatant. The CDS of WT CN1 was cloned into the p3XFLAG-CM-13 Expression vector (Sigma-Aldrich). After sequence confirmation, site-directed mutagenesis (Site directed mutagenesis kit, Agilent technologies) was used to substitute the cysteine residues, respectively. Primer sequences were Mut1 sense CACGAAAGGCACCGTGTCCTTCTACGGCCACTTGG and Mut1 antisense CCAAGTGGCCGTAGAAGGACACGGTGCCTTTCGTG, Mut2 sense CATGGTGGAGGTGAAATCCAGAGACCAGGATTTTC and Mut2 antisense GAAAATCCTGGTCTCTGGATTTCACCTCCACCATG (sense TGC → TCC, antisense GCA → GGA, respectively). CHO cells were transfected with the expression vectors for CN1 wild type, Mut1 and Mut2. Cells were grown in the presence of geneticin (500 µg/ml, G418, Biochrom) in order to get a stable expression of CN1 protein and mutants. CHO cells were grown in protein- and serum-free media supplemented with 10 % l-glutamate in an incubator with 8 % CO2 at 37 °C. FLAG-tagged CN1 was secreted into the supernatant. After 72 h supernatant was collected and an aliquot was used for Anti-FLAG Western Blot (Monoclonal mouse Anti-FLAG M2 antibody, Sigma-Aldrich) for verification of CN1 expression. CHO supernatant was concentrated to 5 ml using Vivaspin 20 concentrator (Sartorius Stedim). The samples were loaded onto Anti-FLAG M2 affinity gel (Sigma-Aldrich) and washed with TBS. 3xFLAG peptide (100 µg/ml, Sigma-Aldrich) was used for competitive elution. Purity of recombinant proteins was checked by silver staining.
Diabetic mice (db/db mice)
Male C57BL/KsJm/Leptdb (db/db) mice (Stock 000662) and their normoglycemic heterozygous littermates were obtained from Charles River (Sulzfeld, Germany). The animals were housed in a 12-h light/dark cycle at 22 °C. Standard laboratory food and water was provided ad libidum. The experimental procedure was approved by the North Stockholm Ethical Committee for Care and Use of Laboratory Animals (for details see Peters et al. 2012).
Preparation of total protein extracts
25-week-old animals were euthanized by carbon-dioxide. The kidneys were removed, immediately homogenized in cold buffer containing 20 mM HEPES, 1 mM ethylene glycol-tetraacetic acid (EGTA), 210 mM mannitol and 70 mM sucrose per gram tissue, pH 7.2. The homogenate was centrifuged at 1500g for 5 min at 4 °C, and the supernatant was kept at −80 °C until analysis (see also Peters et al. 2012). Protein concentration was determined by Bradford assay.
mRNA quantification PCR
RT-PCR was performed according to a previously described protocol (Janssen et al. 2005). Equal loading was confirmed by RT-PCR for β-actin.
Plasma assays
Blood was collected from all animals from the tail tip and glucose was determined using an OneTouch Ultra Blood Glucose meter (LifeScan, Milpitas, CA, USA). Insulin was determined by using an Ultra-Sensitive Mouse Insulin ELISA kit (Crystal Chem, 130 Downers Grove, IL). Leptin was determined by using a Mouse Leptin ELISA Kit (Crystal Chem, 131 Downers Grove, IL).
GSH concentrations
GSH concentrations were measured using a fluorescence assay from BioAssay Systems, Hayward, USA.
NO production
NO2 and NO3 renal tissue concentrations, which reflect total local NO production, were measured with Cayman’s Nitrate/Nitrite colorimetric assay kit according to the manufacturer’s protocol (Cayman Chemical Company, MI, USA).
Detection of N-glycosylation
For detection of carnosinase in renal tissue, gel electrophoreses and subsequent Western blotting was performed according to the method described by Riedl et al. (2010). Samples were deglycosylated by PNGase F (New England Biolabs, Frankfurt, Germany) treatment according to the manufacturer’s recommendations.
Detection of S-nitrosylation
S-nitrosylation was detected by the Biotin Switch Technique (Forrester et al. 2009) from Cayman’s (S-nitrosylated Detection Assay). Briefly, free SH groups are blocked by S-methylthiolation. Subsequent cleavage of the S–NO bonds in the sample and biotinylation of the newly formed SH groups provides the basis for visualization using streptavidin-based colorimetric visualization of S-nitrosylated proteins.
CN1 carbonylation
For determination of protein carbonylation the reaction of carbonyl groups with 2,4-dinitrophenylhydrazine (DNPH) to form protein-bound 2,4-dinitrophenylhydrazones was used. Hydrazones were detected immunochemically with anti-dinitrophenyl antibodies using the OxyBlot™ protein detection kit (Chemicon/Millipore, Germany). In brief, 1 µg recombinant human CNDP1 (2489-ZN; R&D Systems, Germany) was incubated with Fenton reagent (70 µM FeSO4 plus 1 mM H2O2) and MG (1 mM), respectively, for 30 min at 37 °C followed by derivatization with 2,4-dinitrophenylhydrazine or a derivatization-control solution following manufacturer’s recommendations. SDS-PAGE and Western immunoblotting analysis of the carbonylated CN1 was performed using a standard protocol as published previously (Wagner et al. 2011) using the following antibodies: rabbit anti-CN1 (1:1000; HPA008933, Sigma-Aldrich, Germany) and rabbit anti-dinitrophenyl (1:2500; A6430, Invitrogen- Life Technologies, Germany). Secondary antibodies were purchased from Dianova, Germany.
Immunoprecipitation
Protein was isolated from whole kidney tissue homogenates of diabetic db/db and wild-type mice as described above. Protein (50 µg) was precipitated with 2 µg of a rabbit polyclonal serum carnosinase antibody (sc-98739, Santa Cruz Biotechnology, Germany) or rabbit control IgG (#2729, Cell Signaling Technology, Germany) at 4 °C overnight. Samples were then incubated with Dynabeads Protein G (10004D, novex through life technologies, Darmstadt, Germany) for 30 min at room temperature, centrifuged, and washed. Precipitates were eluted in 2× standard protein sample buffer in 10 min at 70 °C and analysed for CN1 (sc-98739, Santa Cruz Biotechnology) and CN1 carbonylation using the anti-dinitrophenyl antibody (Invitrogen-Life Technologies, Germany).
Statistical analysis
A minimum of three independent experiments were performed in duplicates and more. Data are given as mean ± SD. For comparison of three or more groups a one-way analysis of variance was performed, followed by post hoc analyses using Tukey’s test. Differences were considered significant at p < 0.05.
Results
Influence of glucose, insulin and leptin on recombinant CN1 activity
To analyse CN1 activity, recombinant CN1 (rCN1) was produced in CHO cells, purified and characterized for carnosine and anserine degradation. rCN1 was more effective in degrading carnosine than anserine (Table 1). Similar findings were obtained using human serum CN1. V max for human carnosine was 4.9 ± 0.6 and for anserine 0.9 ± 0.1 µmol/mg/h (K m for carnosine 0.9 mM, for anserine 1.2 mM). Incubation of recombinant and human CN1 with glucose (1–25 mM), insulin or leptin (each 0.01–1 mM) did not affect enzyme activity (Table 1). Ex vivo incubation of renal tissue slides of db/db and WT mice with glucose (1–25 mM) also did not increase CN1 activity.
Influence of recombinant metabolites on recombinant CN1 activity
Methylglyoxal (MG) dose-dependently increased carnosine cleavage activity of rCN1. Already at a concentration of 0.3 mM MG, rCN1 showed significantly increased cleavage of 1 mM carnosine (4.6 ± 0.4 vs. 3.8 ± 0.5 µmol/mg/h; p < 0.05, Fig. 1a) and of anserine (0.8 ± 0.1 vs. 0.6 ± 0.1 µmol/mg/h; p < 0.05). At 0.5 mM MG, cleavage activity of rCN1 was increased by 75 % for carnosine and by 30 % for anserine (Table 1). Incubation with Fenton reagent also dose-dependently increased histidine dipeptide cleavage by rCN1. 0.25 mM Fenton reagent increased turnover of 1 mM carnosine to 4.8 ± 0.8 as compared to 3.8 ± 0.8 µmol/mg/h in Fenton free medium (p < 0.05). At a Fenton reagent concentration of 0.5 mmol/l, carnosine and anserine cleavage efficiency of rCN1 was increased by 130 and 70 %, respectively (Table 1). The addition of 3-morpholinosyndomine (SIN-1), an NO donor, caused a dose-dependent reduction of the rCN1 catalytic rate for carnosine (Fig. 1b). This was already significant at 0.2 mM SIN-1, (2.6 ± 0.5 vs. 3.8 ± 0.5 µmol carnosine/mg/h; p < 0.05). At a concentration of 1 mM SIN-1, carnosine cleavage rate was less than 10 % compared to the NO free control (0.3 ± 0.1 vs. 3.8 ± 0.5 µmol/mg/h; p < 0.001). 0.5 mM SIN-1 reduced CN1 cleavage activity for carnosine to 30 % and for anserine to 85 % of control (p < 0.005/0.05; Table 1). Coincubation of SIN-1 with Fenton reagent and MG, respectively, reduced the CN1 activity suppressing action of SIN-1 dose-dependently. Incubation of Mut2C229S with 0.5 mM SIN-1 reduced efficiency for carnosine and anserine turnover to 42 and 85 % (2.9 ± 0.4 and 0.6 ± 0.05; p < 0.005/0.05 vs. untreated controls).
Post-translational modifications of rCN1
To determine whether the change in CN1 activity is caused by carbonylation and S-nitrosylation, rCN1 was incubated with methylglyoxal (MG), Fenton reagent (H2O2 + FeSO4) and SIN-1. Introduction of carbonyl groups by oxidative reactions was demonstrated by OxyBlot analysis of rCN1 (Fig. 2a). Biotin-Switch assay showed that both rCN1 cysteines are S-nitrosylated by 3-morpholinosyndomine (SIN-1), an NO donor (Fig. 2b).
Post-translational modifications of CN1. a Incubation of recombinant CN1 with Fenton reagent (70 µM FeSO4 + 1 mM H2O2) or methylglyoxal (MG, 1 mM) causes substantial protein carbonylation as shown by OxyBlot™. Carbonylated CN1 was identified by SDS-PAGE and Western immunoblotting with anti-dinitrophenyl (a-DNP). b Recombinant CN1 S-nitrosylation after incubation with the NO donor 3-morpholinosydnonimine (SIN-1, 0.5 mM) was measured using the Biotin-Switch assay. Both cysteine residues at position 102 and 229 of CN1 were nitrosylated. Western blot served as loading control
Role of cysteine residues for CN1 activity
To investigate the role of the two cysteine residues on rCN1 activity, the cysteines were substituted by serine (Mut1C102S and Mut2C229S). Mut1C102S largely abolished enzyme activity for carnosine and anserine (V max/K m for carnosine 0.9 ± 0.7; for anserine 0.1 ± 0.05 µmol/mg/h/mM), whereas substitution of cysteine at position 229 (Mut2C229S) did not significantly affect cleavage function compared to unsubstituted rCN1 (V max/K m for carnosine 6.5 ± 0.8, for anserine 0.6 ± 0.1 µmol/mg/h/mM). Incubation of Mut2C229S with MG and with Fenton reagent doubled carnosine and anserine turnover compared to untreated Mut2C229S (V max/K m for carnosine/anserine with MG: 11.5 ± 1.4/1.1 ± 0.09; with 0.5 mM Fenton reagent: 14.1 ± 2.1/1.2 ± 0.15; untreated Mut2C229S 6.5 ± 0.8/0.6 ± 0.1 µmol/mg/h/mM; p < 0.005 vs. untreated Mut2C229S). Activity of Mut1C102S was low and unchanged when incubated with MG or Fenton reagent (data not shown).
Activity and post-translational modifications of CN1 diabetic mice
In db/db mice renal carnosine degrading activity was 175 % (0.51 ± 0.13) of WT controls (0.29 ± 0.17 µmol/mg/h; p < 0.001). V max for carnosine and anserine was increased, substrate affinity higher as shown by a lowered K m value, and thus efficiency (V max/K m) of the renal CN1 for carnosine and anserine cleavage 88 and 61 % higher than in WT (Table 2). Proteinuria, blood glucose, leptin and insulin were higher in db/db mice compared to WT controls (Fig. 3a–d; 99 ± 36 vs. 2.2 ± 1.4 µg albumin/mg creatinine, 23 ± 2 vs. 8 ± 1 mmol/l; 72 ± 8.7 vs. 5.3 ± 0.9 ng/ml; each p < 0.005 and 3.5 ± 1.6 vs. 1.1 ng/ml; p < 0.01). Carbonylation of renal proteins was increased, as demonstrated by oxyblot (Fig. 3e). Renal tissue GSH and NO2/NO3 concentrations were reduced (700 ± 99 vs. 932 ± 108 µM in WT, and 9.2 ± 6.6 vs. 18.1 ± 5.3 µM in WT, both p < 0.01) and inversely correlated with the carnosine degradation rate of CN1 (r = −0.82 and −0.85; both p < 0.01). Renal CN1 protein concentrations were similar in db/db mice as in WT controls. All three glycosylation sites of CN1 were glycosylated in db/db and in WT animals, demonstrating that in diabetic milieu CN1 activity is not increased by direct glycation. Western immunoblotting detected only one band corresponding to the fully N-glycosylated CN1 protein, deglycosylation by PNGase treatment reduced its molecular weight to 61 kDa (data not shown). Renal CN1 in db/db mice was more carbonylated (as demonstrated by immunoprecipitation) and less S-nitrosylated (as demonstrated by Biotin-Switch assay, Fig. 4a, b) than in WT controls. Renal tissue carnosine and anserine concentrations were lower in diabetic mice than in WT controls and correlated with renal tissue CN1 activity (r = −0.7 and −0.85 in db/db and −0.65 and −0.8 in WT mice).
Comparison of WT and db/db mice. a Proteinuria (µg Albumin/mg creatinine), b blood glucose (mmol), c blood Leptin (ng/ml), d blood Insulin (ng/ml) and e renal carbonyl stress, demonstrated by Oxblot, were increased, f renal GSH and g renal nitrate/nitrite levels were decreased in db/db mice compared to controls
CN1 carbonylation and S-nitrosylation in db/db mice compared to WT. a Immunoprecipitation of mice kidney tissue lysates. Protein was precipitated with CN1 antibody (a-CN1) or control (IgG). CN1 was demonstrated in db/db and wild-type mice by a-CN1 (left panel), CN1 carbonylation was detected by using the anti-dinitrophenyl antibody (a-DNP) (right panel). The presence of the high molecular band demonstrates that CN1 was present also in its dimeric form, as previously reported (Chang et al. 2010). b S-nitrosylation of renal tissue CN1 of control and db/db mice. S-nitrosylation was lower for diabetic mice compared to controls (Biotin Switch Assay)
Discussion
CN1 degrades histidine-dipeptides such as carnosine and anserine which have significant cytoprotective properties. In diabetic mice, the activity of CN1 is increased approximately twofold and histidine-containing dipeptide levels are reduced (Peters et al. 2012). The mechanisms leading to the up-regulation of CN1 activity in diabetes have been unknown. In the present study, we confirm increased CN1 activity in diabetic mice. This increase was not due to changes in the rate of transcription, protein synthesis or N-glycosylation required for protein secretion and enzyme activity (Riedl et al. 2010). Neither glucose, leptin nor insulin influenced CN1 activity in vitro; incubation of renal tissue with glucose did not modify CN1 activity of db/db and WT mice. In contrast, post-translational CN1 modifications substantially altered CN1 activity.
Carbonylation is a common form of oxidative protein modification leading to the semialdehyde derivatives of lysine, arginine, proline, and threonine (Curtis et al. 2012; Dalle-Donne et al. 2006; Ruskovska and Bernlohr 2013). Carbonyl derivatives of lysine, cysteine, and histidine can be formed by secondary reactions, but the quantitatively most important product of carbonylation is glutamic semialdehyde from arginine (Nystrom 2005). In silico analysis (available online at http://www.lcb.cnrs-mrs.fr/CSPD/; Maisonneuve et al. 2009) predicted two sites in the CN1 amino acid sequence prone to carbonylation located at position 95–99 and at position 333–336. The putative carbonylation site at position 95–99 is located in the active site, a highly conserved metal binding site of CN1 and seems to be responsible for the effects described. In view of the highly reactive nature of hydroxyl radicals, further reactions beyond carbonylation may occur, resulting in heterogeneous oxidative modification products such as methionine sulphoxide, dityrosine, as well cysteine oxidation products. These may also affect the conformation of the active site. As shown by the present experiments, the cysteine residues undergo S-nitrosylation which reduces CN1 activity, and carbonylation which increases CN1 activity. Modifications of cysteine at position 102 regulate CN1 activity while cysteine at position 229 does not appear to be relevant for enzyme function. The active site of CN1 contains no cysteine residues but Cys102 is located in the same β-strand as His106. It is possible that S-nitrosylation of Cys102 causes derangement of the local environment, impairs the coordination of zinc ions, and inhibits catalysis. Further analysis of the modified proteins should include high-resolution mass spectroscopy. Of note, the in vitro setting differs substantially from in vivo conditions. MG concentration used in the cell-free experiments to demonstrate post-translational modifications within 30 min were about 100-fold higher than that reported for intracellular MG concentrations (Dobler et al. 2006). On the other hand, higher MG concentrations have been observed in diabetic patients on dialysis (Lapolla et al. 2005), and our in vivo findings in mice support the impact of carbonylation on CN1 activity.
The regulation of CN1 activity by post-translational modification was not only shown in vitro, but also in the renal tissue of db/db mice. Under conditions of increased oxidative stress and reduced GSH concentrations, as observed under diabetic conditions, we demonstrated increased carbonylation of CN1 and increased CN1 activity. Surprisingly, we found no effect of high glucose levels on CN1 N-glycosylation and activity. Under conditions of lower NO2/NO3, and consequently decreased S-nitrosylation, as also seen under diabetic conditions, CN1 activity was higher. Reduced S-nitrosylation of CN1 under diabetic conditions could moreover result from increased generation of peroxynitrite.
Increased activity of CN1 would be an explanation for the increased turnover of histidine-dipeptides in diabetes. The shortest allelic form of the CN1 is associated with lower CN1 activity and protects from diabetic nephropathy in patients with type 2 diabetes, a correlation of serum CN1 activity with serum HDP concentrations, however, has not been established (Peters et al. 2011). In diabetic rodents a decrease in carnosine content was reported in retina, kidney and liver (Mong et al. 2011; Pfister et al. 2011; Riedl et al. 2011). In contrast, Stegen et al. (2015) demonstrated an increase in muscle carnosine concentrations with obesity and progressive glucose intolerance in rodents and in male humans with type 2 diabetes. We now demonstrate a decrease in renal carnosine concentration in the kidney of diabetic rodents in correlation with the tissue CN1 activity. CN1 activity is not the only mechanism by which renal turnover of histidine dipeptides is regulated. Two enzymes, carnosine synthase (Drozak et al. 2010) which regulates the ATP-dependent conjugation of histidine and alanine, and carnosine methyltransferase (Drozak et al. 2013) which methylates carnosine to anserine, have recently been identified as regulatory enzymes of carnosine metabolism. Likewise, the impact of histidine resulting from carnosine degradation needs further investigations. Histidine is known as a carbonyl scavenger (Orioli et al. 2007), but high concentrations may have toxic effects (Holliday and McFarland 1996; Letzien et al. 2014). Further studies are required to determine whether these enzymes are altered in diabetes and whether post-translational modifications play a role in their regulation.
Conclusions
We demonstrate that increased CN1 activity of diabetes is due to post-translational modifications of CN1 by reactive metabolites. Thus, increased HDP cleavage by CN1 under diabetic conditions, reducing the scavenging capacity for reactive metabolites by HDP to render them harmless, further increases CN1 activity in a positive feedback loop. Interference with this vicious circle, e.g. by inhibition of post-translational modification of CN1 or by suppression of CN1 activity represent intriguing therapeutic strategies for mitigation of diabetic complications such as DN.
Abbreviations
- CN1:
-
Carnosinase 1
- ROS:
-
Reactive oxygen species
- RCS:
-
Reactive carbonyl species
- NO:
-
Nitric oxide
- HDP:
-
Histidine-dipeptides
- H2O2 :
-
Hydrogen peroxide
- MG:
-
Methylglyoxal
- DN:
-
Diabetic nephropathy
References
Aldini G, Facino RM, Beretta G, Carini M (2005) Carnosine and related dipeptides as quenchers of reactive carbonyl species: from structural studies to therapeutic perspectives. BioFactors (Oxford, England) 24:77–87
Alhamdani M, Al-Azzawie HF, Abbas FK (2007) Decreased formation of advanced glycation end-products in peritoneal fluid by carnosine and related peptides. Perit Dial Int 27:86–89
Ansurudeen I et al (2012) Carnosine enhances diabetic wound healing in the db/db mouse model of type 2 diabetes. Amino Acids 43:127–134. doi:10.1007/s00726-012-1269-z
Babizhayev MA, Lankin VZ, Savel’Yeva EL, Deyev AI, Yegorov YE (2013) Diabetes mellitus: novel insights, analysis and interpretation of pathophysiology and complications management with imidazole-containing peptidomimetic antioxidants. Recent Pat Drug Deliv Formul 7:216–256
Barski OA et al (2013) Dietary carnosine prevents early atherosclerotic lesion formation in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol 33:1162–1170. doi:10.1161/atvbaha.112.300572
Baylis C (2012) Nitric oxide synthase derangements and hypertension in kidney disease. Curr Opin Nephrol Hypertens 21:1–6. doi:10.1097/MNH.0b013e32834d54ca
Boldyrev AA, Aldini G, Derave W (2013) Physiology and pathophysiology of carnosine. Physiol Rev 93:1803–1845. doi:10.1152/physrev.00039.2012
Budzen S, Rymaszewska J (2013) The biological role of carnosine and its possible applications in medicine. Adv Clin Exp Med Off Organ Wroc Med Univ 22:739–744
Chang CY et al (2010) Crystal structure and mutational analysis of aminoacylhistidine dipeptidase from Vibrio alginolyticus reveal a new architecture of M20 metallopeptidases. J Biol Chem 285:39500–39510. doi:10.1074/jbc.M110.139683
Cheang WS et al (2011) Endothelial nitric oxide synthase enhancer reduces oxidative stress and restores endothelial function in db/db mice. Cardiovasc Res 92:267–275. doi:10.1093/cvr/cvr233
Curtis JM, Hahn WS, Long EK, Burrill JS, Arriaga EA, Bernlohr DA (2012) Protein carbonylation and metabolic control systems. Trends Endocrinol Metab 23:399–406. doi:10.1016/j.tem.2012.05.008
Dalle-Donne I, Aldini G, Carini M, Colombo R, Rossi R, Milzani A (2006) Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med 10:389–406 (pii:010.002.11)
Decker E, Livisay SA, Zhou S (2000) A re-evaluation of the antioxidant activity of purified carnosine. Biochemistry (Mosc) 65:766–770
Dobler D, Ahmed N, Song L, Eboigbodin KE, Thornalley PJ (2006) Increased dicarbonyl metabolism in endothelial cells in hyperglycemia induces anoikis and impairs angiogenesis by RGD and GFOGER motif modification. Diabetes 55:1961–1969. doi:10.2337/db05-1634
Drozak J, Veiga-da-Cunha M, Vertommen D, Stoobant V, Van Schaftingen E (2010) Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1). J Biol Chem 2685:9346–9356
Drozak J, Chrobok L, Poleszak O, Jagielski AK, Derlacz R (2013) Molecular identification of carnosine N-methyltransferase as chicken histamine N-methyltransferase-like protein (hnmt-like). PLoS One 8:e64805. doi:10.1371/journal.pone.0064805
Forrester M, Foster MW, Benhar M, Stamler JS (2009) Detection of protein S-nitrosylation with the biotin switch technique. Free Radic Biol Med 46:119–126
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(829–837):837a–837d. doi:10.1093/eurheartj/ehr304
Foster MW, Hess DT, Stamler JS (2009) Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med 15:391–404. doi:10.1016/j.molmed.2009.06.007
Freedman BI, Bostrom M, Daeihagh P, Bowden DW (2007) Genetic factors in diabetic nephropathy. Clin J Am Soc Nephrol 2:1306–1316. doi:10.2215/CJN.02560607
Gould N, Doulias PT, Tenopoulou M, Raju K, Ischiropoulos H (2013) Regulation of protein function and signaling by reversible cysteine S-nitrosylation. J Biol Chem. doi:10.1074/jbc.R113.460261
Hartmanova T, Tambor V, Lenco J, Staab-Weijnitz CA, Maser E, Wsol V (2013) S-Nitrosoglutathione covalently modifies cysteine residues of human carbonyl reductase 1 and affects its activity. Chem Biol Interact. doi:10.1016/j.cbi.2012.12.011
Hipkiss AR (2011) Energy metabolism, proteotoxic stress and age-related dysfunction—protection by carnosine. Mol Aspect Med 32:267–278
Holliday R, McFarland GA (1996) Inhibition of the growth of transformed and neoplastic cells by the dipeptide carnosine. Br J Cancer 73:966–971
Hou W, Chen HJ, Lin YH (2003) Antioxidant peptides with Angiotensin converting enzyme inhibitory activities and applications for Angiotensin converting enzyme purification. J Agric Food Chem 51:1706–17093
Janssen B et al (2005) Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 54:2320–2327 (pii: 54/8/2320)
Kohen R, Yamamoto Y, Cundy KC, Ames BN (1988) Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc Natl Acad Sci USA 85:3175–3179
Lapolla A, Reitano R, Seraglia R, Sartore G, Ragazzi E, Traldi P (2005) Evaluation of advanced glycation end products and carbonyl compounds in patients with different conditions of oxidative stress. Mol Nutr Food Res 49:685–690. doi:10.1002/mnfr.200400093
Letzien U, Oppermann H, Meixensberger J, Gaunitz F (2014) The antineoplastic effect of carnosine is accompanied by induction of PDK4 and can be mimicked by l-histidine. Amino Acids 46:1009–1019. doi:10.1007/s00726-014-1664-8
Maisonneuve E, Ducret A, Khoueiry P, Lignon S, Longhi S, Talla E, Dukan S (2009) Rules governing selective protein carbonylation. PLoS One 4:e7269. doi:10.1371/journal.pone.0007269
Masha A, Dinatale S, Allasia S, Martina V (2011) Role of the decreased nitric oxide bioavailability in the vascular complications of diabetes mellitus. Curr Pharm Biotechnol 12:1354–1363 (pii:BSP/CPB/E-Pub/-00061-12-5)
Min J et al (2008) Differential neuroprotective effects of carnosine, anserine, and N-acetyl carnosine against permanent focal ischemia. J Neurosci Res 86:2984–2991. doi:10.1002/jnr.21744
Mong MC, Chao CY, Yin MC (2011) Histidine and carnosine alleviated hepatic steatosis in mice consumed high saturated fat diet. Eur J Pharmacol 653:82–88. doi:10.1016/j.ejphar.2010.12.001
Mooyaart AL et al (2010) Association between CNDP1 genotype and diabetic nephropathy is sex specific. Diabetes 59:1555–1559. doi:10.2337/db09-1377
Mozdan M, Szemraj J, Rysz J, Nowak D (2005) Antioxidant properties of carnosine re-evaluated with oxidizing systems involving iron and copper ions. Basic Clin Pharmacol Toxicol 96:352–360
Nakagawa K, Ueno A, Nishikawa Y (2006) Interactions between carnosine and captopril on free radical scavenging activity and angiotensin-converting enzyme activity in vitro Yakugaku zasshi. J Pharm Soc Jpn 126:37–42
Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R (2008) Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol 153:6–20. doi:10.1038/sj.bjp.0707395
Nystrom T (2005) Role of oxidative carbonylation in protein quality control and senescence. EMBO J 24:1311–1317. doi:10.1038/sj.emboj.7600599
Orioli M, Aldini G, Benfatto MC, Facino RM, Carini M (2007) HNE Michael adducts to histidine and histidine-containing peptides as biomarkers of lipid-derived carbonyl stress in urines: LC-MS/MS profiling in Zucker obese rats. Anal Chem 79:9174–9184. doi:10.1021/ac7016184
Peters V et al (2010) Relevance of allosteric conformations and homocarnosine concentration on carnosinase activity. Amino Acids 38:1607–1615. doi:10.1007/s00726-009-0367-z
Peters V et al (2011) Anserine inhibits carnosine degradation but in human serum carnosinase (CN1) is not correlated with histidine dipeptide concentration. Clin Chim Acta 412:263–267. doi:10.1016/j.cca.2010.10.016
Peters V, Schmitt CP, Zschocke J, Gross ML, Brismar K, Forsberg E (2012) Carnosine treatment largely prevents alterations of renal carnosine metabolism in diabetic mice. Amino Acids 42:2411–2416. doi:10.1007/s00726-011-1046-4
Peters V et al (2014) Carnosine treatment in combination with ACE inhibition in diabetic rats. Regul Pept. doi:10.1016/j.regpep.2014.09.005
Pfister F et al (2011) Oral carnosine supplementation prevents vascular damage in experimental diabetic retinopathy. Cell Physiol Biochem 28:125–136. doi:10.1159/000331721
Pollock JS, Pollock DM (2011) Endothelin, nitric oxide, and reactive oxygen species in diabetic kidney disease. Contrib Nephrol 172:149–159. doi:10.1159/000329054
Riedl E et al (2010) N-glycosylation of carnosinase influences protein secretion and enzyme activity: implications for hyperglycemia. Diabetes 59:1984–1990. doi:10.2337/db09-0868
Riedl E et al (2011) Carnosine prevents apoptosis of glomerular cells and podocyte loss in STZ diabetic rats. Cell Physiol Biochem 28:279–288. doi:10.1159/000331740
Ruskovska T, Bernlohr DA (2013) Oxidative stress and protein carbonylation in adipose tissue—implications for insulin resistance and diabetes mellitus. J Proteomics. doi:10.1016/j.jprot.2013.04.002
Sena CM, Pereira AM, Seica R (2013) Endothelial dysfunction—a major mediator of diabetic vascular disease. Biochim Biophys Acta 1832:2216–2231. doi:10.1016/j.bbadis.2013.08.006
Somineni HK, Boivin GP, Elased KM (2014) Daily exercise training protects against albuminuria and angiotensin converting enzyme 2 shedding in db/db diabetic mice. J Endocrinol 221:235–251. doi:10.1530/joe-13-0532
Stegen S et al (2015) Muscle histidine-containing dipeptides are elevated by glucose intolerance in both rodents and men. PLoS One 10:e0121062. doi:10.1371/journal.pone.0121062
Tanida M, Shen J, Kubomura D, Nagai K (2010) Effects of anserine on the renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Physiol Res 59:177–185
Teufel M, Saudek V, Ledig JP, Bernhardt A, Boularand S, Carreau A, Cairns NJ, Carter C, Cowley DJ, Duverger D, Ganzhorn AJ, Guenet C, Heintzelmann B, Laucher V, Sauvage C, Smirnova T (2003) Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J Biol Chem 278:6251–6531
Velez S, Nair NG, Reddy VP (2008) Transition metal ion binding studies of carnosine and histidine: biologically relevant antioxidants Colloids and surfaces B. Biointerfaces 66:291–294. doi:10.1016/j.colsurfb.2008.06.012
Vistoli G et al (2009) Design, synthesis, and evaluation of carnosine derivatives as selective and efficient sequestering agents of cytotoxic reactive carbonyl species. ChemMedChem 4:967–975. doi:10.1002/cmdc.200800433
Voziyan P, Brown KL, Chetyrkin S, Hudson B (2014) Site-specific AGE modifications in the extracellular matrix: a role for glyoxal in protein damage in diabetes. Clin Chem Lab Med CCLM FESCC 52:39–45. doi:10.1515/cclm-2012-0818
Wagner AH et al (2011) Tyrosine nitration limits stretch-induced CD40 expression and disconnects CD40 signaling in human endothelial cells. Blood 118:3734–3742. doi:10.1182/blood-2010-11-320259
Acknowledgments
We thank Kristina Klingbeil and Nadine Scholz for excellent technical assistance. Part of this study was supported by grants of the Deutsche Forschungsgemeinschaft (SFB 1118 and Ma2510/3-1 and Zs17/5-1), by the Dietmar Hopp Foundation, St. Leon-Roth, and the Doktor Pfleger Foundation, Bamberg, Germany.
Conflict of interest
The authors declare that they have no conflict of interest.
Statement on the welfare of animals
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: C. Sale.
Rights and permissions
About this article
Cite this article
Peters, V., Lanthaler, B., Amberger, A. et al. Carnosine metabolism in diabetes is altered by reactive metabolites. Amino Acids 47, 2367–2376 (2015). https://doi.org/10.1007/s00726-015-2024-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2024-z