Abstract
Recently, we identified an allelic variant of human carnosinase 1 (CN1) that results in increased enzyme activity and is associated with susceptibility for diabetic nephropathy in humans. Investigations in diabetic (db/db) mice showed that carnosine ameliorates glucose metabolism effectively. We now investigated the renal carnosinase metabolism in db/db mice. Kidney CN1 activity increased with age and was significantly higher in diabetic mice compared to controls. Increased CN1 activity did not affect renal carnosine levels, but anserine concentrations were tenfold lower in db/db mice compared to controls (0.24 ± 0.2 vs. 2.28 ± 0.3 nmol/mg protein in controls; p < 0.001). Homocarnosine concentrations in kidney tissue were low in both control and db/db mice (below 0.1 nmol/mg protein, p = n.s.). Carnosine treatment for 4 weeks substantially decreased renal CN1 activity in diabetic mice (0.32 ± 0.3 in non-treated db/db vs. 0.05 ± 0.05 μmol/mg/h in treated db/db mice; p < 0.01) close to normal activities. Renal anserine concentrations increased significantly (0.24 ± 0.2 in non-treated db/db vs. 5.7 ± 1.2 μmol/mg/h in treated db/db mice; p < 0.01), while carnosine concentrations remained unaltered (53 ± 6.4 in non-treated vs. 61 ± 15 nmol/mg protein in treated db/db mice; p = n.s.). Further, carnosine treatment halved proteinuria and reduced vascular permeability to one-fifth in db/db mice. In renal tissue of diabetic mice carnosinase activity is significantly increased and anserine concentrations are significantly reduced compared to controls. Carnosine treatment largely prevents the alterations of renal carnosine metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
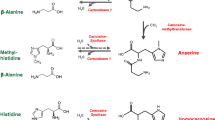
Susceptibility to diabetic nephropathy (DN) is strongly associated with a polymorphism in the CNDP1 gene, encoding the serum carnosinase (CN1) protein. The shortest allelic form (homozygous for the five-leucine allele, the so-called CNDP1 Mannheim) is more common in the absence of nephropathy and associated with lower serum carnosinase activities (Janssen et al. 2005). CN1 is a dipeptidase which catalyses the hydrolysis of the dipeptides carnosine, anserine and homocarnosine (Teufel et al. 2003). CN1 is present in different allosteric conformations (Peters et al. 2010). In mammals, two types of L-carnosine-hydrolyzing enzymes (CN1 and CN2) have been identified. In humans, CN1 is expressed in various tissues and secreted from the liver into the blood, in rats CN1 expression is confined to the kidney (Teufel et al. 2003).
Carnosine, anserine and homocarnosine are the most abundant histidine-containing dipeptides. They are widely distributed in mammals and tissue distributions and concentrations are species specific (Boldyrev and Severin 1990). All three peptides have antioxidant activity (Boldyrev 1993). Carnosine increases ischemia tolerance of neurons and hepatic cells by scavenging of reactive oxygen species (Mozdan et al. 2005) and acts as a natural inhibitor of the angiotensin converting enzyme (Hou et al. 2003). It restores erythrocyte deformability (Aydogan et al. 2008), inhibits protein glycation and cellular senescence (Alhamdani et al. 2005; Hipkiss 2010) and reduces the synthesis of matrix proteins such as fibronectin and collagen type VI of podocytes and mesangial cells (Janssen et al. 2005). In the central nervous system, carnosine meets many criteria for a neurotransmitter (Baslow 2010). Recently, Sauerhöfer et al. (2007) showed that carnosine influences glucose metabolism, however, mesangial expansion, as a sign of nephropathy, was not affected by carnosine treatment in diabetic mice.
In a previous study, we showed that the human serum histidine dipeptide concentrations are not correlated to CN1 activity (Peters et al. 2011). Since the absence of diabetic nephropathy is associated with low CN1 activity, these findings suggest that local effects in the kidney might be of importance. We therefore investigated renal histidine dipeptide metabolism and the impact of exogenous carnosine in db/db mice, a well established model of diabetic nephropathy. Db/db mice are characterized by defective hypothalamic leptin signaling, persistent hyperphagia and obesity, high plasma leptin, glucose and insulin levels and progressive diabetic nephropathy.
Materials and methods
Dipeptide concentrations and CN1 activity
Anserine, carnosine and homocarnosine concentrations were measured fluorometrically by high-performance liquid chromatography as previously described (Schönherr 2002). The samples were diluted with sulfosalicylic acid to precipitate proteins. After derivatization with carbazole-9-carbonyl chloride (CFC), the samples underwent liquid chromatography and quantification by fluorescence. Detection limit was 15 nM. All samples were measured twice, and one sample was spiked with the standards to identify each analyte. Homocarnosine concentrations were comparable with the data obtained by liquid chromatography-tandem mass spectrometry (Jansen et al. 2006).
CN1 activity was determined in serum since EDTA or heparin can inhibit CN1 activity. CN1 activity was assayed according to the method described by Teufel et al. (2003). Briefly, the reaction was initiated by the addition of carnosine to renal tissue homogenate at a pH of 7. The reaction was stopped after defined periods by adding 1% trichloroacetic acid. Liberated histidine was derivatized by adding o-phthaldialdehyde (OPA) and fluorescence was read using a MicroTek plate reader (λ Exc: 360 nm; λ Em: 460 nm). To rule out that the measured carnosinase activity is due to CN2, we measured carnosinase activities with and without addition of bestatin, a specific inhibitor for CN2 (2). Addition of bestatin did not change enzyme activity, the measured carnosinase activities reflect CN1 activity (data not shown). CN1 activity and histidine dipeptide concentrations were related to renal protein concentration (determined by Lowry protein assay).
Db/db mice
Male C57BL/KsJm/Leptdb (db/db) mice (Stock 000662) and their normoglycemic heterozygous littermates were obtained from Charles River (Sulzfeld, Germany). The animals were housed in a 12-h light/dark cycle at 22°C. Standard laboratory food and water was provided ad libitum. The experimental procedure was approved by the North Stockholm Ethical Committee for Care and Use of Laboratory Animals. At the end of each experiment body weight and blood glucose levels were measured. Blood was collected from all animals from the tail tip and glucose was determined using a OneTouch Ultra Blood Glucose meter (LifeScan, Milpitas, CA, USA).
Carnosine and anserine treatment
Treatment of db/db mice started at 8 weeks of age, i.e. before they had developed hyperglycemia and proteinuria. The animals were divided into 4 groups (controls, db/db mice treated and untreated). Each experimental group contained 5 mice. Control and db/db mice received carnosine in high dose (5 g/l of L-carnosine; Sigma, Stockholm, Sweden) in the drinking water. Higher dose was given to assure that the mice were supplied with carnosine. The estimated daily intake was 20 mg carnosine per day and mouse, assuming that the mice drink in average 4 ml per day. After 4 weeks of treatment, carnosinase activity and dipeptide concentrations were measured in kidney tissue. Proteinuria was measured in urine. Spot urines were collected at the end of the treatment period, urinary albumin was measured by an indirect competitive ELISA assay (Excocell, Philadelphia, PA, USA) creatinine was qualified by a chemical analysis based on Jaffe’s reaction of alkaline picrate with creatinine (Exocell, Philadelphia, PA, USA).
Tissue and blood sampling
After 4 weeks of treatment, carnosinase activity and dipeptide concentrations were measured in kidney tissue. The animals were euthanized by carbon-dioxide. Blood samples were collected prior to killing and the kidneys were removed, immediately homogenized in cold buffer containing 20 mM HEPES, 1 mM ethylene glycol-tetraacetic acid (EGTA), 210 mM mannitol and 70 mM sucrose per gram tissue, pH 7.2. The homogenate was centrifuged at 1,500×g for 5 min at 4°C, and the supernatant was kept at −80°C until analysis.
Vascular permeability assay
Vascular permeability was assessed by measurement of Evans Blue leakage from the kidney vessels into the neighboring renal tissue. A 1% solution of Evans blue dye (2 μl/mg BW, Sigma Chemical Co., St. Louis, Missouri, USA) was injected into the tail vein of 12-week old db/db mice 10 min prior to killing. After 10 min, the mice were euthanized by CO2 inhalation, blood samples were collected and the kidneys were removed, blotted dry, and weighed. The Evans blue dye was extracted from the kidney with 1 ml of formamide overnight at 65°C and measured spectrophotometrically at 620 nm (Ibla and Khoury 2006; Matthew et al. 2002).
Morphological and stereological evaluation
Kidneys were quickly removed, and 3–4 mm sections were cut for fixation in formalin. The glomerulosclerosis, tubulointerstitial damage and vascular wall thickness index were assessed on PAS-stained paraffin sections according to a semiquantitative scoring system (scores 0–4) by an observer blinded to the group assignment of the samples.
Statistical analysis
Data are given as mean ± SD. For comparison of three or more groups a one-way analysis of variance was performed, which was followed by post hoc analyses using Tukey’s test. Means were considered different at p < 0.05.
Results
Renal carnosinase activity and histidine-dipeptide concentrations
Carnosinase activity in renal tissue was higher in db/db mice compared to controls (p < 0.01) and increased with age (Table 1) in both groups. Renal carnosine concentrations were similar in db/db and control mice (53 ± 6.4 vs. 42 ± 3.2 nmol/mg protein) whereas renal anserine concentrations were tenfold lower in db/db mice compared to controls (0.24 ± 0.2 nmol/mg protein vs. 2.28 ± 0.31 nmol/mg protein in controls; p < 0.001). Homocarnosine concentrations were low in kidneys from db/db and control mice (0.04 ± 0.03 vs. 0.07 ± 0.06 nmol/mg protein, p = n.s). Whereas CN1 activity increased with age, carnosine, anserine and homocarnosine concentrations were not age-dependent and were in the same range for 12, 21 and 25-week old control mice (data not shown). Blood and liver CN1 activities were below the detection limit in diabetic and control mice.
Treatment with carnosine
Db/db and control mice were treated with carnosine for 4 weeks, treatment started at 8 weeks of age. At the time of killing, the body weight was significantly elevated in db/db animals (38 ± 5 vs. 26 ± 1.8 g in controls, p < 0.001). Carnosine treatment had no effect on body weight in either group.
Treatment with carnosine decreased CN1 activity considerably in db/db mice compared to untreated db/db mice (0.05 ± 0.05 vs. 0.32 ± 0.3 μmol/mg protein/h; p < 0.01) to a level similar as in untreated controls (<0.001 μmol/mg protein/h). In carnosine-treated controls, CN1 activity was increased (Table 2). Renal carnosine concentrations were only slightly increased after carnosine treatment in diabetic, as well as control mice. In contrast, anserine concentrations increased with carnosine treatment about 20-fold in db/db and about sixfold in control mice compared to untreated mice (both p < 0.001; Table 2). Although anserine concentrations were increased by carnosine treatment in diabetic and control mice, anserine levels remained lower in diabetic mice compared to controls (Table 2).
Carnosine treated db/db mice had significantly lower blood glucose levels (17 ± 2 mmol/l) compared to respective untreated db/db mice (23 ± 2 mmol/l, p < 0.05), but had no effect on glycemia in control mice (8 ± 1 mmol/l in both groups).
Proteinuria was significantly higher in db/db mice (99 ± 36 μg albumin/mg creatinine) compared to controls (2.2 ± 1.4 μg albumin/mg creatinine) and could be halved by carnosine treatment (47 ± 7.5 μg albumin/mg creatinine; p < 0.01).
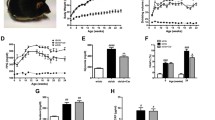
As illustrated in Fig. 1, db/db mice had higher vascular permeability compared to untreated control mice (p < 0.05). Treatment of db/db mice with carnosine significantly reduced vascular permeability to one-fifth compared to untreated db/db mice (p < 0.001) to a level even below the permeability determined in untreated healthy mice.
Vascular permeability was measured in 12-week old mice, carnosine treatment started at 8 weeks. Evans blue was injected intravenously into the tail veins of controls and db/db mice. Extravasation of Evans blue dye at 15 min was much more extensive in db/db mice compared to control. Carnosine treatment significantly decreased vascular permeability in db/db mice (p < 0.001 vs. non treated db/db)
We further compared the morphological indexes of renal damage between treated (n = 7) and non-treated (n = 4) diabetic mice. The glomerulosclerose index (1.5 ± 0.7 in non-treated vs. 1 ± 1.2 in treated diabetic mice; p = 0.39) and tubulointerstitial damage score (0 for both groups) were not affected by carnosine treatment whereas carnosine treatment reduced vascular thickness of small and middle size renal artery sections (vascular damage index: 1.5 ± 0.6 in non-treated vs. 1 ± 0 in treated diabetic mice; p = 0.04).
Discussion
This study for the first time demonstrates marked alterations of renal carnosine metabolism in diabetic mice. Renal CN1 activity is increased whereas tissue anserine concentrations are tenfold reduced. Treatment with carnosine normalizes renal CN1 activity and renal anserine concentrations. Moreover, exogenous carnosine lowered blood glucose levels, proteinuria and renal vascular permeability.
We recently showed that N-glycosylation is essential for appropriate secretion and CN1 activity and that hyperglycemia enhances CN1 secretion and enzyme activity (Riedl et al. 2010). In accordance with these findings, increased CN1 activity in the diabetic mice might be the consequence of hyperglycemia due to the poor glucose control. Carnosine treatment of db/db mice did not affect renal tissue carnosine concentrations, but normalized anserine levels. Anserine can be formed by methylation of carnosine via carnosine-N-methyl transferase (McManus 1962), which explains the increase of renal anserine in carnosine treated mice. Compared to anserine, carnosine is the far better substrate for CN1 (Teufel et al. 2003) and exogenous carnosine is metabolized to anserine or degraded to β-alanine and histidine, while anserine accumulates.
In carnosine-treated diabetic mice, two mechanisms could decrease CN1 activity despite increased substrate concentration. First, lowered blood glucose levels might lower CN1 activity by reduced N-glycosylation (Riedl et al. 2010); second increased anserine levels are known to effectively lower CN1 activity and thus carnosine degradation (Peters et al. 2011). Inhibition of carnosine degradation by CN1 activity by homocarnosine (Peters et al. 2010) can be disregarded in kidney tissue since renal homocarnosine levels are very low.
Whereas carnosine levels remain stable and do not differ between diabetic and control mice, anserine levels are clearly lower in diabetic mice. Although the function of carnosine is better described, previous studies showed that also anserine seems to have several protective functions. Similar to carnosine, anserine was described to affect renal sympathetic nerve activity (Tanida et al. 2010), reduce blood glucose (Kubomura et al. 2010), increase the contribution of the non-bicarbonate buffering action and decrease the bicarbonate buffering action in blood (Suzuki et al. 2006), act as effective transglycating agents in decomposition of aldose-derived Schiff bases (Szwergold 2005), protect neuronal cells against reactive oxygen species (Boldyrev et al. 2004), show dose-dependent angiotensin converting enzyme inhibitory activity (Hou et al. 2003), act as peroxyl radical scavenger to protect the protein modification (Kang et al. 2002) and react as quencher of cytotoxic carbonyls (Aldini et al. 2005).
Beside these similarities, there is some evidence that both dipeptides have also different functions. Whereas carnosine facilitates NO production in endothelial cells, anserine failed to increase NO production (Tanida et al. 2010). Treatment with carnosine, but not anserine, was able to significantly reduce infarct volume and improve neurological function (Min et al. 2008). Further, an increase in the antitumor activity of doxorubicin (Sadzuka and Sonobe 2007) was only described for anserine. Beside these functions, the role of anserine in the kidney is not yet known and remains to be addressed.
In consent with the findings from Sauerhöfer et al. (2007), we found that exogenous carnosine decreased blood glucose concentration in db/db mice. This already may explain some of the beneficial effects such as the reduction in proteinuria. Moreover, treatment with carnosine lowered vascular permeability which cannot be explained by lowered blood glucose. Many proteins are involved in the regulation of vascular permeability, of which the vascular endothelial growth factors (VEGFs) are key regulators (Bates 2010; Lee et al. 2006). A putative role of anserine on vascular permeability needs further evaluation. Interestingly, carnosine treatment of db/db mice improved renal vascular but not glomerular morphology.
Conclusion
In renal tissue of diabetic mice, carnosinase activity is significantly increased and anserine concentrations markedly reduced compared to controls. Carnosine treatment reverses these diabetes-associated alterations of histidine dipeptide metabolism. The concomitant reduction in proteinuria and renal vascular permeability may not only be exerted via the beneficial effects of carnosine on glucose metabolism but also via recovery of renal anserine homeostasis. The specific local mode of action of the different dipeptides and potential of pharmacological interventions deserve further analyses.
References
Aldini G, Facino RM, Beretta G, Carini M (2005) Carnosine and related dipeptides as quenchers of reactive carbonyl species: from structural studies to therapeutic perspectives. Biofactors 24:77–87
Alhamdani M, Al-Azzawie HF, Abbas FK (2005) Decreased formation of advanced glycation end-products in peritoneal fluid by carnosine and related peptides. Perit Dial Int 27:86–89
Aydogan S, Yapislar H, Artis S, Aydogan B (2008) Impaired erythrocytes deformability in H(2)O(2)-induced oxidative stress: protective effect of L-carnosine. Clin Hemorheol Microcirc 39:93–98
Baslow MH (2010) A novel key-lock mechanism for inactivating amino acid neuro-transmitters during transit across extracellular space. Amino Acids 38:51–55
Bates D (2010) Vascular endothelial growth factors and vascular permeability. Cardiovascular Res 87:262–271
Boldyrev AA (1993) Does carnosine possess direct antioxidant activity? Int J Biochem 25:1101–1107
Boldyrev AA, Severin SE (1990) The histidine-containing dipeptides, carnosine and anserine: distribution, properties and biological significance. Adv Enzyme Regul 30:175–194
Boldyrev A, Bulygina E, Leinsoo T, Petrushanko I, Tsubone S, Abe H (2004) Protection of neuronal cells against reactive oxygen species by carnosine and related compounds. Comp Biochem Physiol B Biochem Mol Biol 137:81–88
Hipkiss A (2010) Aging, proteotoxicity, mitochondria, glycation, nad and carnosine: possible inter-relationships and Resolution of the Oxygen Paradox. Front Aging Neurosci 2:10
Hou W, Chen HJ, Lin YH (2003) Antioxidant peptides with angiotensin converting enzyme inhibitory activities and applications for Angiotensin converting enzyme purification. J Agric Food Chem 51:1706–1709
Ibla J, Khoury J (2006) Methods to assess tissue permeability. Methods Mol Biol 341:111–117
Jansen EE, Gibson KM, Shigematsu Y, Jakobs C, Verhoeven NM (2006) A novel, quantitative assay for homocarnosine in cerebrospinal fluid using stable-isotope dilution liquid chromatography–tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 830:196–200
Janssen B, Hohenadel D, Brinkkoetter P et al (2005) Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 54:2320–2327
Kang J, Kim KS, Choi SY, Kwon HY, Won MH, Kang TC (2002) Protective effects of carnosine, homocarnosine and anserine against peroxyl radical-mediated Cu, Zn-superoxide dismutase modification. Biochim Biophys Acta 1570:89–96
Kubomura D, Matahira Y, Nagai K, Jijima A (2010) Effect of anserine ingestion on hyperglycemia and the autonomic nerves in rats and humans. Nutr Neurosci 13:183–188
Lee K, Kim SR, Park SJ et al (2006) Hydrogen peroxide induces vascular permeability via regulation of vascular endothelial growth factor. Am J Respir Cell Mol Biol 35:161–170
Matthew C, Sils IV, Bastille AM (2002) Tissue-specific extravasation of albumin-bound Evans blue in hypothermic and rewarmed rats. Can J Physiol Pharmacol 80:233–243
McManus I (1962) Enzymatic synthesis if anserine in skeletal muscle by N-methylation of carnosine. J Biol Chem 237:1207–1211
Min J, Senut MC, Rajanikant K et al (2008) Differential neuroprotective effects of carnosine, anserine, and N-acetyl carnosine against permanent focal ischemia. J Neurosci Res 86:2984–2991
Mozdan M, Szemraj J, Rysz J, Nowak D (2005) Antioxidant properties of carnosine re-evaluated with oxidizing systems involving iron and copper ions. Basic Clin Pharmacol Toxicol 96:352–360
Peters V, Kebbewar M, Jansen EW et al (2010) Relevance of allosteric conformations and homocarnosine concentration on carnosinase activity. Amino Acids 38:1607–1615
Peters V, Jansen EW, Jakobs C et al (2011) Anserine inhibits carnosine degradation but in human serum carnosinase (CN1) is not correlated with histidine dipeptide concentration. Clin Chim Acta 412:263–267
Riedl E, Koeppel H, Pfister F et al (2010) N-glycosylation of carnosinase influences protein secretion and enzyme activity: implications for hyperglycemia. Diabetes 59:1984–1990
Sadzuka Y, Sonobe T (2007) Anserine induced advantage effects on the antitumor activity of doxorubicin. Food Chem Toxicol 45:985–989
Sauerhöfer S, Yuan G, Braun GS et al (2007) L-carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes 56:2425–2432
Schönherr J (2002) Analysis of products of animal origin in feeds by determination of carnosine and related dipeptides by high-performance liquid chromatography. J Agric Food Chem 50:1945–1950
Suzuki Y, Nakao T, Maemura H et al (2006) Carnosine and anserine ingestion enhances contribution of nonbicarbonate buffering. Med Sci Sports Exerc 38:334–338
Szwergold B (2005) Carnosine and anserine act as effective transglycating agents in decomposition of aldose-derived Schiff bases. Biochem Biophys Res Commun 336:36–41
Tanida M, Shen J, Kubomura D, Nagai K (2010) Effects of anserine on the renal sympathetic nerve activity and blood pressure in urethane-anesthetized rats. Physiol Res 59:177–185
Teufel M, Saudek V, Ledig JP et al (2003) Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J Biol Chem 278:6251–6531
Acknowledgments
We thank Kristina Klingbeil for technical assistance. Part of this study was supported by the EU-funded specific-target project PREDICTIONS on the identification of risk factors for the development of diabetic nephropathy as well as grants by the Deutsche Forschungsgemeinschaft to M. Mack and J Zschocke (Ma2510/3-1 and Zs17/5-1). Family Erling Persson Foundation, The European Commission project FUNCFOOD (FP7-KBBE-2009-245030), Novo Nordic Foundation and Swedish Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peters, V., Schmitt, C.P., Zschocke, J. et al. Carnosine treatment largely prevents alterations of renal carnosine metabolism in diabetic mice. Amino Acids 42, 2411–2416 (2012). https://doi.org/10.1007/s00726-011-1046-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1046-4