Abstract
Diabetes mellitus (DM) is a progressive disorder with severe late complications. Normal wound healing involves a series of complex and well-orchestrated molecular events dictated by multiple factors. In diabetes, wound healing is grossly impaired due to defective, and dysregulated cellular and molecular events at all phases of wound healing resulting in chronic wounds that fail to heal. Carnosine, a dipeptide of alanine and histidine and an endogenous antioxidant is documented to accelerate healing of wounds and ulcers. However, not much is known about its role in wound healing in diabetes. Therefore, we studied the effect of carnosine in wound healing in db/db mice, a mice model of Type 2 DM. Six millimeter circular wounds were made in db/db mice and analyzed for wound healing every other day. Carnosine (100 mg/kg) was injected (I.P.) every day and also applied locally. Treatment with carnosine enhanced wound healing significantly, and wound tissue analysis showed increased expression of growth factors and cytokines genes involved in wound healing. In vitro studies with human dermal fibroblasts and microvascular-endothelial cells showed that carnosine increases cell viability in presence of high glucose. These effects, in addition to its known role as an antioxidant and a precursor for histamine synthesis, provide evidence for a possible therapeutic use of carnosine in diabetic wound healing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impaired wound healing is a major diabetic complication. The pathophysiology of impaired wound healing in diabetes is complex and multifactorial involving neuronal, vascular, biochemical and immunological components (Greenhalgh 2003). Normal wound healing process has four prime overlapping phases: wound hemostasis, inflammation, proliferation and remodeling. These processes are well-co-ordinated and progressive events that restore the mechanical integrity of the skin as a result of dynamic interaction between damaged cells and its extracellular matrix (ECM) (Schultz et al. 2011). In diabetes, the wounds fail to exhibit the normal sequence of molecular interactions and signal transductions inducing dysregulated leukocyte chemotaxis, phagocytosis, extracellular matrix deposition, re-epithelialization, keratinocyte and fibroblast migration and proliferation and cytokine, chemokine and growth factor production in addition to an elevated proteolytic environment that delays wound healing (Galkowska et al. 2006; Guo and DiPietro 2010; Khanna et al. 2010; Lamers et al. 2011; Schultz et al. 2011). Furthermore, impaired angiogenesis, chronic hypoxia, neuropathy and hyperglycemia in diabetes mellitus disrupt wound healing severely (Guo and DiPietro 2010).
In addition to several factors, hyperglycemia-mediated oxidative stress and advanced glycation end products (AGEs) contribute largely to impaired wound healing in diabetes (Peppa et al. 2009). The excess glucose in DM enters the polyol pathway and depletes cytosolic NADPH that is required for the synthesis of the endogenous antioxidant glutathione (GSH). Depletion of GSH renders the cell vulnerable to oxidative injury by free radicals produced during normal cellular functions (Vincent et al. 2004). The situation is aggravated in chronic wounds where free radicals are accumulated due to persistent and uncontrolled production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) during the inflammatory phase leading to a prolonged inflammatory response and stagnation in wound healing (Soneja et al. 2005). Oxidative stress has also been shown to have direct effect on the migratory properties of dermal fibroblasts (Lamers et al. 2011). During hyperglycemia, glucose also undergoes non-enzymatic reactions with primary amino groups of proteins to form the glycated adducts called AGEs. AGEs accumulate in wound tissue by binding to their cell surface receptors, RAGE and modulate the inflammatory phase and proliferative phase of wound healing. AGEs decrease the transendothelial migration of neutrophils leading to delayed inflammatory response, and in the proliferative phase, it causes apoptosis of fibroblasts by glycosylating the extracellular matrix that induces cell cycle arrest in dermal fibroblasts (Peppa et al. 2009).
Oxygenation is also critical to normal wound healing (Schreml et al. 2010). The microenvironment of the early wound is hypoxic and hypoxia plays the role of an early stimulus for tissue repair and angiogenesis (Tandara and Mustoe 2004). The cellular responses to hypoxia are mediated by hypoxia inducible factor 1 alpha (HIF1α) (Semenza 2006). Hyperglycemia disrupts hypoxic signaling by destabilizing HIF1α (Catrina et al. 2004) leading to deleterious effects on wound healing (Botusan et al. 2008). Also, prolonged hypoxia can amplify the early inflammatory response by increasing the levels of oxygen radicals (Sen 2009).
Carnosine, a dipeptide of beta-alanine and l-histidine, is a well-characterized endogenous anti-oxidant and anti-glycating agent (Reddy et al. 2005). In addition, it is an efficient cytosolic buffering agent and a chelator of heavy metals (Quinn et al. 1992). It is also effective in eliminating hypoxia-induced oxidative stress. Studies show that carnosine as a potent antioxidant is protective against hypoxia-ischemic insults in brain injury in rats and in liver injury (Fouad et al. 2007; Fedorova et al. 2006). These results emphasize carnosine to be useful as a prophylactic treatment to protect tissues against hypoxia-induced oxidative stress. Carnosine has been shown to accelerate healing of bleomycin-induced and irradiation-induced pulmonary wounds (Cuzzocrea et al. 2007; Guney et al. 2006). These effects of carnosine have been attributed to its affinity to quench free radicals. In dermal wounds, carnosine promotes granulation, increases the tensile strength and hydroxyproline content in the wound area (Fitzpatrick and Fisher 1982; Nagai et al. 1986). The observed effect has been ascribed to the histamine synthesis from histidine, one of the components of carnosine and the stimulation of collagen synthesis by β-alanine, another component of carnosine. Studies with Sprague–Dawley rats conclude that carnosine as a dietary peptide improves wound healing following surgery when administered as part of a complete enteral formula which might be clinically relevant (Roberts Md et al. 1998).
As mentioned above, the role and efficiency of carnosine in wound healing has been proven, however, it is unclear if carnosine is effective in improving the disturbed wound healing process in diabetes. In our current study, we investigated the effect of carnosine on wound healing in db/db animals, the diabetic mice model and show that carnosine accelerates wound healing in db/db mice and stimulate the expression of growth factors and cytokines involved in wound healing.
Materials and methods
Cell culture
Human dermal fibroblast (HDF) and human dermal microvascular endothelial cells (HDMEC) from Promocell were maintained in a humidified atmosphere with 5 % CO2 at 37 °C. HDFs were grown in DMEM containing 5.5 mM glucose (Gibco) supplemented with 2 mM l-glutamine, 100 IU/ml penicillin and streptomycin (Gibco), and 10 % heat-inactivated FBS. HDMECs were grown in endothelial cell growth medium MV (Promocell). Only cells between passages 4 and 9 were used.
Animals and experimental protocol
C57BL/KsJm/Leptdb (db/db) mice were obtained from Charles River, housed five per cage in a 12-h light/dark cycle at 22 °C and provided ad libitum with standard laboratory food and water. The animals were caged individually before the experiment, handled daily, and then wounded as described in Wound Model. The experimental procedure was approved by the North Stockholm Ethical Committee for Care and Use of Laboratory Animals.
Wound model
The animals were weighed, measured for blood glucose with the OneTouch Ultra 2 blood glucose monitoring system (LifeScan) and then were injected with the first dose of carnosine (Sigma, 100 mg/Kg body weight; IP) or vehicle (water), just before the experiment. Soon after, the animals were anaesthetized with 3 % Isoflurane (Abbott) and their dorsal hair was shaved with an electric clipper followed by complete hair removal with a depilatory cream. The skin was cleaned with alcohol and two full-thickness wounds extending through the panniculus carnosus were made on the dorsum on each side of midline, using a 6-mm biopsy punch. A transparent dressing (Tegaderm; 3 M) was applied to cover the wounds after topical application of 100 μl of carnosine (25 mg/ml in 60 % polyethylene glycol), or vehicle alone. Following the surgical procedure, the animals were individually housed. During the first 2 days, the animals received buprenorphine (SC 0.03 mg/kg) once a day. For histological and gene expression analysis, the wounds were harvested at the end of the study. Carnosine (I.P.) was administered every day and was topically applied through the dressing using a 30-gauge needle every alternate day. The study was evaluated in 5–6 animals per group.
Wound analysis
Digital photographs were recorded at the day of surgery and every other day after wounding. A circular reference was placed alongside the animal to permit correction for the distance between the camera and the animals. The wound area was calculated in pixels with ImageJ 1.32 software (National Institutes of Health), corrected for the area of the reference circle and expressed as percentage of the original area.
Serum analysis
Carnosine concentrations in the serum were assayed by fluorometric determination after derivatization with carbazole-9-carbonyl chloride. Separation was performed by liquid chromatography, according to the method described by Schönherr et al. (Schönherr 2002). The detection limit was 10 nM.
Tissue preparation and histological analysis
After fixation in formalin, the samples were embedded in paraffin and sectioned (5 μm). For histological evaluation, sections were deparaffinized and rehydrated followed by hematoxylin and eosin staining. All slides were evaluated by light microscopy for qualitative evaluation of granulation.
Staining for collagen fibers
The collagen fiber density was visualized by staining the sections with Massons trichrome stain using the standard protocol at the Karolinska Institutet’s Immunohistochemical core facility (Bradbury and Rae 1996).
Cell viability assay
Cell viability assay was performed using the CellTiter 96 AQueous One solution (Promega). Briefly 3 × 104 HDF or HDMEC cells were seeded on a 96 well plate and were grown till confluence. The cells were then starved over-night in DMEM with 0.2 %FBS medium and treated with two different concentrations of Carnosine (50 mM and 50 μM), d-glucose (Sigma, 30 mM), and combinations of glucose and carnosine for 24 h and analyzed for cell viability by measuring the absorbance at 490 nm. The assay involves measurement of the colored formazan product formed as a result of the bioreduction of exogenous chemical compounds into colored products by metabolically active cells. 10 % FBS served as a positive control in this study.
In vitro scratch wound assay/cell migration assay
Cell migration was studied using in vitro scratch wound assay. Both HDF and HDMEC cells were seeded on collagen coated plates and were grown to confluence. Confluent cells were starved over-night in DMEM with 0.2 % FBS medium. The following day, in vitro wound was made by scraping the cell monolayer to create a scratch. The cells were then washed off debris, photographed and treated with the indicated treatments in presence of mitomycin c (10 μM) for 24 h. Mitomycin c was used to suppress cell proliferation and thus distinguish cellular migration. At the end of the study, the cells were photographed again and the cellular migration was quantified using the Image J 1.44p software.
Quantitative RT-PCR
Total RNA was isolated from cells using RNeasy Plus mini Kit (Qiagen) and from skin using an RNeasy Fibrous Tissue Mini Kit (Qiagen). cDNA was synthesized using the first-strand cDNA synthesis kit (Applied Biosystems) according to the manufacturer’s protocol. The primers sequences are listed in Table 1. Real-time PCR was performed in an Applied Biosystems 7300 unit using SYBR Green quantitative PCR Kit (Applied Biosystems). The quality of the quantitative PCR was determined by standard curves and melting curve analysis, and the results were normalized to the house keeping gene GAPDH.
Statistical analysis
Differences between groups for animal experiment were computed using one-way analysis of variance, with the Tukey post hoc test. For other experiments t test was performed. A P value <0.05 was considered statistically significant.
Results
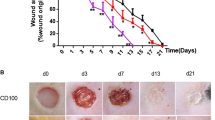
The db/db animals were randomly grouped into either the vehicle (n = 5) or carnosine treated groups (n = 6) after wounding. These animals showed high blood glucose levels (<33.3 mM) and weighed an average of 46.2 ± 3.4 g. The wound area was analyzed as the measure of wound healing after treating the study groups with either the vehicle or carnosine. Carnosine treated animals showed a significant enhancement in wound healing as early as the second day of the treatment. The initial expansion of the wound after surgery was very limited in the treated animals versus the untreated animals (Fig. 1a). The wound healing progressed effectively in treated animals and significant differences in wound area were observed in comparison to the untreated group (Fig. 1a, b). Serum analysis at the end of the treatment showed an increase in serum carnosine level. Serum carnosine concentrations were below detection limit for untreated animals and were clearly detectable (15.6 ± 5 nM) in the carnosine treated animals.
Carnosine treatment improves wound healing in the db/db, type 2 diabetes mice model (a). The rate of wound healing is accelerated in db/db mice after carnosine treatment both locally and intraperitoneally (100 mg/Kg). Values are mean ± SEM and *p < 0.05, ANOVA: p = 0.001. b Macroscopic pictures of representative wounds of each group on day 0, 4, 8 and 12 after wounding
The tissue of the wound area was collected at the end of the study. At the molecular level, growth factors and proteins known to have a role in wound healing were studied (Fig. 2). Growth factors such as insulin-like growth factor 1 (IGF1), transforming growth factor beta (TGFβ), and stromal derived factor 1 (SDF1) were significantly enhanced in wound tissues from treated animals. The mRNA expression of these growth factors was increased by 2.9-fold, 2.5-fold and 1.8-fold, respectively. The remodeling protein collagen 1a which plays a major role in the last phase of wound healing was expressed highly by 3.4-fold in the treated animals. Healing fibroblast express smooth muscle actin 1 (SMA1) more than the normal fibroblast, and the carnosine treated wounds showed an increased expression of SMA1 by 2.4-fold.
Carnosine up-regulates the expression of growth factors and cytokines during wound healing (a). In the carnosine treated wound tissue, the growth factors, insulin growth factor 1 (IGF-1), transforming growth factor beta (TGFβ), fibroblast growth factor (FGF7); the cytokines stromal derived cell factor 1 (SDF1), Interleukin 6 (IL6) and extracellular matrix components collagen 1a (Col-1a) and Smooth muscle actin (SMA) are expressed significantly more in comparison to their control counterparts except for FGF7 and IL6 proteins. Data are normalized to the expression of GAPDH in each sample and presented as the relative expression of the control group. Values are mean ± SEM and *p < 0.05
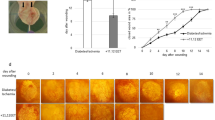
In line with the above results, an intense accumulation of collagen in the treated wound tissue was observed by the green–blue colored staining in the tissue sections (Fig. 3a). The hematoxylin and Eosin staining seen in Fig. 3b, also suggests a better granulation in the treated wound tissue in comparison with the untreated tissue.
Wound tissue granulation and collagen deposition are improved in carnosine treated tissues Image (magnification: 10x) of a Massons trichome staining for collagen fibers and b hematoxylin and eosin staining on sections of wound tissue from vehicle treated and carnosine treated animals. Blue color-stained collagen fibers; Brown color-stained nuclei. Arrows indicate granulation layer (color figure online)
In vitro studies with HDF and HDMEC were performed to analyze the role of carnosine on fibroblast and endothelial cell viability and migration. In cell viability studies, HDFs and HDMECs were starved over night in 0.2 % FBS containing medium and were then treated with two different concentrations of carnosine (Car1, 50 mM; Car2, 50 μM) in presence and absence of glucose (30 mM) or 10 % FBS. FBS served as a positive control and cells treated with FBS showed an increase in cell viability by 30 % in both the cell types. In presence of normal concentration of glucose, treatment with carnosine (50 mM) for 24 h showed a significant increase in endothelial cell viability by 40 % (Fig. 4b) in comparison with the untreated controls (Fig. 4b), while no significant difference was seen with HDFs. Lower concentration of carnosine had no effect on cell viability in both the cell types. Interestingly, in presence of high glucose, the cell viability effect of carnosine (50 mM) was enhanced significantly by 40 % in HDFs and by 270 % in endothelial cells in comparison to untreated cells (Fig. 4a, b, respectively).
Carnosine treatment enhances cell viability in HDF and HDME cells In vitro cell viability of a human dermal fibroblasts (HDFs) and b human dermal endothelial cells (HDMEC) without treatment (c) and after treating them with fetal bovine serum (FBS, 10 %), Glucose (Glu, 30 mM), Carnosine (Car1-50 mM; Car2-50 μM) or a combination of glucose and two concentration of carnosine for 24 h. Values are mean ± SEM and *p < 0.05, **p < 0.01, ***p < 0.001. The results are the mean of four–six different experiments performed in quadruplicates
Migration studies with the scratch assay were performed with two different concentrations of carnosine to determine the role of carnosine in cellular migration of HDFs and HDMECs. Cells were starved over night in 0.2 % FBS containing medium and were then treated with carnosine (Car1, 50 mM; Car2, 50 μM) in presence and absence of glucose (30 mM) for 24 h. In HDMECs, both the carnosine concentrations by themselves had no effect on endothelial cell migration (Fig. 5). However, the lower concentration of carnosine could reverse the high glucose-induced decrease in cellular migration. Carnosine (50 μM) significantly inhibited the anti-migratory effects of high glucose on endothelial cell migration and also increased the cellular migration by 1.4-fold. In HDFs, no significant difference in cellular migration was observed after carnosine treatment with both the concentrations. Interestingly, carnosine (50 μM) both in presence and absence of glucose showed a trend towards enhancing migration in HDFs (Data not shown).
Carnosine affects cell migration more in HDMEs than in HDFs cells In vitro cell migration of human dermal endothelial cells (HDMEC) without treatment (c) and after treating them with Glucose (Glu, 30 mM), Carnosine (Car1-50 mM; Car2–50 μM) and a combination of glucose and two concentration of carnosine for 24 h. Values are mean ± SEM and *p < 0.05. The results are the mean of four different experiments
Discussion
Wound healing in diabetes mellitus is an active area of research because of its high cost, risk of amputation, multifactorial etiology and for a need of better alternative healing agents. In our current study, we demonstrate for the first time that carnosine is significantly effective in diabetic wound healing using the db/db diabetic mouse model.
Earlier investigations on the role of carnosine in wound healing of surgical wounds have shown that carnosine does so by enhancing biosynthesis of glycosaminoglycans and histamine synthesis (Fitzpatrick and Fisher 1982; Vizioli and Almeida 1978; Vizioli et al. 1983). Nagai et al. explain the mechanisms of action of carnosine in wound leading as a combination of histamine synthesis, which causes early effusion of wound that promotes nucleic acid synthesis and β-alanine induced collagen synthesis which together with histamine promotes granulation and faster healing of the wound tissue (Nagai et al. 1986).
In our study, we observed that daily injections and local application of carnosine significantly enhanced the degree of wound healing in our diabetic animal model, db/db mice. Local and intra-peritoneal injections were combined to enhance carnosine availability since microvascular dysfunction and enhanced carnosinase activity is associated with diabetes phenotype as is in our study model (Cho et al. 2006; Riedl et al. 2010; Peters et al. 2011). The difference in the wound size between the control and carnosine treated animals was significant by the 2nd day after wounding and by the 10th day around 70 % of the wounds in treated animals were significantly healed. The mRNA analysis of the wound tissue from the carnosine treated animals shows significant increase in collagen expression. This observation is in agreement with the results of Nagai et al. Tissue sections stained for collagen fibers and granulation also suggest an increase in collagen deposition and a better, uniform granulation in the wound tissue from carnosine treated animals.
In addition, we observe that carnosine treatment significantly enhanced the expression of growth factors. Changes in production of growth factors and cytokines have a negative effect on wound healing in diabetes. IGF-1, known to promote re-epithelialization of keratinocyte, fibroblast proliferation and the induction of endothelial cell chemotaxis has decreased expression in human diabetic skin and diabetic mice (Grant et al. 1987; Tsuboi et al. 1995). Studies in mice show that IGF1 accelerates wound healing in diabetic animal models (Bitar 1997). Carnosine treatment caused a significant increase in IGF1 expression in the wound area suggesting a direct or indirect modulation of IGF1 expression by carnosine enabling improved wound healing. We speculate that an increase in the circulatory levels of IGF1 may also be involved in the carnosine-mediated wound healing in db/db animals. Similarly, TGFβ, well studied for its role in cutaneous wounds in various phases of wound healing (Bhora et al. 1995; Bitar and Labbad 1996), is significantly over-expressed in the wound tissue from carnosine-treated animals. The chemokine SDF1 that plays an essential role in the mobilization and homing of stem/progenitor cells like endothelial progenitor cells (Ceradini et al. 2004; Kucia et al. 2005) is also increased after carnosine treatment in the wound tissue suggesting improved recruitment of stem cells in the wound area. SMA1, the specific marker for myofibroblasts also exhibits increased expression in the carnosine treated wound tissue. These myofibroblasts are specialized fibroblasts that are transiently involved in the wound healing process and during the healing process they repair and remodel the wounded tissue, while the fibroblast from normal tissue simply maintains the matrix around them (Agarwal et al. 2006). Thus, the overexpression of the growth factors and key proteins like SDF1 and SMA in our study may have a role in the accelerated wound healing in the carnosine treated animals than their control counterparts.
Successful wound healing is a co-ordinated effect of several cell types and their complex interaction with cytokines, chemokines and growth factors modulating cellular behavior like cell viability, cell motility or cell differentiation (Barrientos et al. 2008). In vitro studies showed that carnosine treatment could enhance the cellular viability of HDFs and HDMEs. The observed effect was more pronounced in presence of high glucose, projecting carnosine as an attractive compound for use in hyperglycemic conditions. The anti-glycosylation and anti-oxidative effects of carnosine may possibly explain the above observation. With respect to cell migration, carnosine did not exhibit any significant direct effects on HDFs at both the concentrations irrespective of the presence or absence of high glucose in their medium (data not shown). However, with HDMECs carnosine could rescue the anti-migratory effects of high glucose. Lower concentration of carnosine was more effective in inhibiting the negative effects of glucose on endothelial cell migration. These results indicate additional roles of carnosine in proliferative and remodeling phases of wound healing. The effect of carnosine treatment on cell viability of fibroblasts in presence of glucose may have a role in promoting fibroblast-induced collagen deposition and other extracellular matrix components like fibronectin during the proliferative phase and tissue remodeling phase of wound healing. Similarly, enhanced endothelial cell viability and migration in presence of glucose may improve neovascularization in the wound tissue thereby providing more nutrients and oxygen to the wound area enabling faster wound healing. However, the mechanism of action and the molecular players mediating these effects of carnosine in hyperglycemic condition is unknown and will be further studied.
Apart from these effects, the wound healing potential of carnosine may also be due to its ability to alter the generation of free radicals and oxidative stress-induced prolonging of the inflammatory phase of the wound healing process in a diabetic wound. Such a role of carnosine as an anti-oxidant under hyperglycemic conditions is yet to be addressed. Since carnosine also shifts the acid–base balance to higher pH values (Skulachev 1978), it can modify the wound micro-environment and block the activity of acid proteases and thereby enhance wound healing under hyperglycemic conditions.
In conclusion, in this preliminary study, we provide evidence for the role of carnosine in accelerating wound healing in a db/db type 2 diabetes mice model and propose carnosine to be a good candidate for clinical use because of its multifaceted role in wound healing.
References
Agarwal C, Britton Z, Alaseirlis D, Li Y, Wang J (2006) Healing and normal fibroblasts exhibit differential proliferation, collagen production, α-SMA expression, and contraction. Ann Biomed Eng 34(4):653–659. doi:10.1007/s10439-006-9090-z
Bradbury P, Rae K (1996) Connective tissues and stains. In: Bancroft JD, Stevens A (eds) Theory and practice of histological techniques, 4th edition. Churchill Livingstone, New York, pp 113–137
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Perspective article: growth factors and cytokines in wound healing. Wound Repair Regen 16(5):585–601. doi:10.1111/j.1524-475X.2008.00410.x
Bhora F, Dunkin B, Batzri S, Aly H, Bass B, Sidawy A, Harmon J (1995) Effect of growth factors on cell proliferation and epithelialization in human skin. J Surg Res 59:236–244
Bitar M (1997) Insulin-like growth factor-1 reverses diabetes-induced wound healing impairment in rats. Horm Metab Res 29:383–386
Bitar M, Labbad Z (1996) Transforming growth factor-beta and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. J Surg Res 61:113–119
Botusan IR, Sunkari VG, Savu O, Catrina AI, Grünler J, Lindberg S, Pereira T, Ylä-Herttuala S, Poellinger L, Brismar K, Catrina S-B (2008) Stabilization of HIF-1α is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci 105(49):19426–19431
Catrina S, Okamoto K, Pereira T, Brismar K, Poellinger L (2004) Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes 53:3226–3232
Ceradini D, Kulkarni A, Callaghan M, Tepper O, Bastidas N, Kleinman M, Capla J, Galiano R, Levine J, Gurtner G (2004) Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10:858–864
Cho CH, Sung HK, Kim KT, Cheon HG, Oh GT, Hong HJ, Yoo OJ, Koh GY (2006) COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc Natl Acad Sci USA 103(13):4946–4951
Cuzzocrea S, Genovese T, Failla M, Vecchio G, Fruciano M, Mazzon E, Di Paola R, Muià C, La Rosa C, Crimi N, Rizzarelli E, Vancheri C (2007) Protective effect of orally administered carnosine on bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol 292(5):L1095–L1104
Fedorova T, Macletsova M, Kulikov A, Stepanova M, Boldyrev A (2006) Carnosine protects from the oxidative stress induced by prenatal hypoxia. Dokl Biol Sci 408:207–210
Fitzpatrick D, Fisher H (1982) Carnosine, histidine, and wound healing. Surgery 91:56–60
Fouad AA, El-Rehany MA-A, Maghraby HK (2007) The hepatoprotective effect of carnosine against ischemia/reperfusion liver injury in rats. Eur J Pharmacol 572(1):61–68
Galkowska H, Wojewodzka U, Olszewski W (2006) Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen 14:558–565
Grant M, Jerdan J, Merimee T (1987) Insulin-like growth factor-I modulates endothelial cell chemotaxis. J Clin Endocrinol Metab 65:370–371
Greenhalgh DG (2003) Wound healing and diabetes mellitus. Clin Plast Surg 30:37–45
Guney Y, Turkcu U, Hicsonmez A, Andrieu M, Guney H, Bilgihan A, Kurtman C (2006) Carnosine may reduce lung injury caused by radiation therapy. Med Hypotheses 66:957–959
Guo S, DiPietro LA (2010) Factors affecting wound healing. J Dent Res 89(3):219–229
Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo G, Sen C, Roy S (2010) Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One 5(3):e9539
Kucia M, Ratajczak J, Ratajczak M (2005) Bone marrow as a source of circulating CXCR4 + tissue-committed stem cells. Biol Cell 97:133–146
Lamers ML, Almeida MES, Vicente-Manzanares M, Horwitz AF, Santos MF (2011) High glucose-mediated oxidative stress impairs cell migration. PLoS One 6(8):e22865
Nagai K, Suda T, Kawasaki K, Mathuura S (1986) Action of carnosine and beta-alanine on wound healing. Surgery 100:815–821
Peppa M, Stavroulakis P, Raptis SA (2009) Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen 17(4):461–472
Peters V, Schmitt C, Zschocke J, Gross M-L, Brismar K, Forsberg E (2011) Carnosine treatment largely prevents alterations of renal carnosine metabolism in diabetic mice. Amino Acids. doi:10.1007/s00726-011-1046-4
Quinn P, Boldyrev A, Formazuyk V (1992) Carnosine: its properties, functions and potential therapeutic applications. Mol Aspects Med 13:379–444
Reddy VP, Garrett MR, Perry G, Smith MA (2005) Carnosine: a versatile antioxidant and antiglycating agent. Sci Aging Knowl Environ (18):pe12
Riedl E, Koeppel H, Pfister F, Peters V, Sauerhoefer S, Sternik P, Brinkkoetter P, Zentgraf H, Navis G, Henning RH, Van Den Born J, Bakker SJL, Janssen B, van der Woude FJ, Yard BA (2010) N-Glycosylation of carnosinase influences protein secretion and enzyme activity. Diabetes 59(8):1984–1990
Roberts PR, Black KW, Santamauro JT, Zaloga GP (1998) Dietary peptides improve wound healing following surgery. Nutrition 14 (3):266–269. doi:10.1016/s0899-9007(97)00468-1
Schönherr J (2002) Analysis of products of animal origin in feeds by determination of carnosine and related dipeptides by high-performance liquid chromatography. J Agric Food Chem 50:1945–1950
Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P (2010) Oxygen in acute and chronic wound healing. Br J Dermatol 163(2):257–268
Schultz G, Davidson J, Kirsner R, Bornstein P, Herman I (2011) Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 19:134–148
Semenza GL (2006) Regulation of physiological responses to continuous and intermittent hypoxia by hypoxia-inducible factor 1. Exp Physiol 91(5):803–806. doi:10.1113/expphysiol.2006.033498
Sen CK (2009) Wound healing essentials: let there be oxygen. Wound Repair Regen 17(1):1–18
Skulachev V (1978) Membrane-linked energy buffering as the biological function of N +/K + gradient. FEBS Lett 87:171–179
Soneja A, Drews M, Malinski T (2005) Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol Rep 57:108–119
Tandara AA, Mustoe TA (2004) Oxygen in wound healing—more than a nutrient. World J Surg 28(3):294–300
Tsuboi R, Shi C, Sato C, Cox G, Ogawa H (1995) Co-administration of insulin-like growth factor (IGF)-I and IGF-binding protein-1 stimulates wound healing in animal models. J Invest Dermatol 104:199–203
Vincent AM, Russell JW, Low P, Feldman EL (2004) Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 25(4):612–628
Vizioli M, Almeida O (1978) Effects of carnosine on the development of rat sponge-induced granulation. I. General morphology and glycosaminoglycans histophotometr. Cell Mol Biol Incl Cyto Enzymol 23:267–273
Vizioli M, Blumen G, Almeida O, Munhoz C (1983) Effects of carnosine on the development of rat sponge-induced granulation tissue. II. Histoautoradiographic observations on collagen biosynthesis. Cell Mol Biol Incl Cyto Enzymol 29:1–9
Acknowledgments
We thank the Family Erling Persson Foundation, The European Commission project FUNCFOOD (FP7-KBBE-2009-245030), and Swedish Medical Research Council Grant No. 04224 for financial support. A part of this study was supported by the EU-funded specific-target project PREDICTIONS on the identification of risk factors for the development of diabetic nephropathy.
Conflict of interest
There is no commercial or financial interest in the presented work and the authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ansurudeen, I., Sunkari, V.G., Grünler, J. et al. Carnosine enhances diabetic wound healing in the db/db mouse model of type 2 diabetes. Amino Acids 43, 127–134 (2012). https://doi.org/10.1007/s00726-012-1269-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1269-z