Abstract
Several factors (including diets, changes in intestinal fluora, and hormones) regulate postnatal intestinal growth and development. Based on the early studies involving modification of the adrenal gland, pituitary gland or hypothalamus, exogenous glucocorticoids and glucocorticoid receptor antagonists are now used to study glucocorticoid-mediated metabolism of amino acids in the small intestine. Findings from these studies indicate that physiological levels of glucocorticoids stimulate the catabolism of glutamine and proline for the synthesis of citrulline and arginine in enterocytes during weaning. In addition, increases in circulating levels of glucocorticoids enhance expression of arginase, proline oxidase and ornithine decarboxylase, as well as polyamine synthesis from arginine and proline in enterocytes. These actions of the hormones promote intestinal maturation and may have therapeutic effects on intestinal disease (e.g., necrotizing enterocolitis). Molecular aspects, species-specific effects, and developmental responsiveness to glucocorticoids should be taken into consideration in designing both experimental and clinical studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several factors play an important role in the ontogeny of intestinal digestive enzymes in mammals. These factors include diets, changes in intestinal fluora, and hormones. The developmental changes of intestinal digestive enzymes associated with postnatal growth are well documented (Henning 1981). For example, pig intestinal activities of sucrase and maltase increases while lactase activity decreases during the second week of postnatal development (James et al. 1987); these changes in enzyme activity help prepare the piglet for a transition from a milk-based to a grain-based diet. The diet-induced changes in digestive enzyme profile likely results from alterations in actual dietary components (Ferraris 2001). This may help explain the proximal–distal gradient exhibited in many digestive enzymes due to a differential exposure of these intestinal segments to dietary substances (Espinoza et al. 1975, 1976; Greene et al. 1975).
There is also evidence that intestinal fluora may play an important role in regulating the development of intestinal digestive enzymes (Batt et al. 1996). Administration of a probiotic to pigs immediately prior to weaning results in dramatic increases in sucrase activity in the small intestine while the administration of antibiotics prior to weaning results in a delayed decrease in lactase activity in response to weaning (Collington et al. 1990). This suggests that manipulation of bacterial populations in the small intestine will lead to changes in the activities of intestinal digestive enzymes.
Hormonal effects on the development of intestinal digestive enzymes are well established (Chapple et al. 1989). Normal development of the small intestine, for example, is impeded or delayed by adrenalectomy or thyroidectomy (Koldovsky et al. 1964, 1975). Furthermore, administration of hormones to developing animals has been shown to increase the activity of carbohydrate-metabolizing enzymes (Koldovsky et al. 1964) and decrease the activity of milk-utilizing enzymes (James et al. 1987) in the small intestine. Given the recognized importance of amino acids to proper functioning of the small intestine (Rider et al. 2007; Wang et al. 2008a, c; Wu 1998) and whole-body homeostasis (Flynn and Wu 1996), this review will focus on the effects of glucocorticoids on amino acid and polyamine metabolism in the gut.
Early studies of glucocorticoids on intestinal development and metabolism
Initial studies concerning glucocorticoids and the small intestine
Early studies on the effect of glucocorticoids on intestinal development typically involved removal of the respective endocrine gland or modification of the pituitary gland or hypothalamus. Adrenalectomy, for instance, was associated with a delayed increase in intestinal sucrase, maltase and invertase activity and a delayed decrease in intestinal β-galactosidase activity in the weaned rat (Koldovsky et al. 1964, 1975). Additionally, hypophysectomy resulted in a delay of normal elevations of sucrase and maltase activity (Castillo et al. 1991). These studies were important toward gaining better understanding of the effects of glucocorticoids on intestinal development but they did not clearly demonstrate a direct effect of the hormones, because factors such as nonglucocorticoid adrenal secretions or pituitary-dependent factors could not be ruled out.
Effects of glucocorticoid administration on nutrient metabolism
More direct evidence for a role of glucocorticoids in inducing intestinal development came from several studies that reported a stimulatory effect of glucocorticoid administration on carbohydrate metabolism in developing animals. For example, administration of corticosterone to adrenalectomized rats increased the activity of invertase (Koldovsky et al. 1964). In addition, cortisol treatment decreased lactase activity (James et al. 1987) while increasing activities of maltase and aminopeptidase in the small intestine of weanling pigs (Chapple et al. 1989; Sangild 1995). Furthermore, glucocorticoid administration reduced the transcription of cholecystokinin in the small intestine (Ratineau et al. 1996) and enhanced the uptake of glutamine by the canine small intestine (Souba et al. 1985).
Effects of glucocorticoids on intestinal amino acid metabolism
Weaning
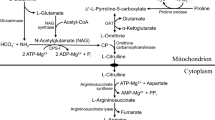
Weaning is characterized by a marked change from a high fat to a high carbohydrate diet (Henning 1981). This change in the diet results in marked alterations in enzyme profiles and selective metabolism in the small intestine. Wu and colleagues discovered an induction of pyrroline-5-carboxylate synthase (P5CS) and arginase in enterocytes of 29-day-old pigs that were weaned at 21 days of age (Wu et al. 1994a; Wu 1995). The enhanced expression of these two enzymes results in the production of large amounts of citrulline from glutamine (Wu et al. 1994a; Wu and Knabe 1995), as well as the synthesis of urea from ammonia (Wu 1995), in enterocytes of post-weaning pigs. Furthermore, the weaning-associated changes in intestinal metabolism of glutamine and arginine are independent of a change in age or diet (Dugan et al. 1995). Because weaning is associated with an increase in plasma concentrations of cortisol in mammals (Henning 1981), it was suggested (Wu et al. 1994a) and subsequently demonstrated (Flynn and Wu 1997a, b; Flynn et al. 1999) that glucocorticoids play an important role in regulating the metabolism of arginine and glutamine in enterocytes. For example, administration of cortisol to 21-day-old suckling pigs (killed at day 29 of age) augmented the activities of argininosuccinate lyase (ASL) and arginase, as well as the production of CO2, ornithine and proline from arginine in the cells (Flynn and Wu 1997a). Intestinal P5CS activity and the formation of citrulline from glutamine were also enhanced in cortisol-treated pigs compared to control pigs (Flynn and Wu 1997b). The P5CS induction is of physiological importance because it promotes the synthesis of citrulline and arginine (an essential amino acid for young pigs; Wu et al. 2004) from glutamine and glutamate in the small intestine. Effects of cortisol on metabolite production and the aforementioned enzyme induction were completely blocked by administration of RU486, a glucocorticoid receptor antagonist (Flynn and Wu 1997a). Interestingly, neither cortisol nor RU486 administration affected ASL activity in enterocytes (Flynn and Wu 1997a, b), indicating that glucocorticoids stimulate the intestinal synthesis of citrulline which can be subsequently released into the circulation. In support of this notion, the release of citrulline by the small intestine is enhanced in post-weaning pigs (Wu et al. 1994b).
Availability of glucocorticoid receptor antagonists (e.g., RU486) has been very helpful in determining whether a developmental increase in enzyme activity is mediated by glucocorticoids. We studied the effect of RU486 on arginine and glutamine metabolism in weanling and age-matched suckling pigs (Flynn and Wu 1997b). We found that RU486 administration to weanling pigs diminished, but did not completely abolish, the increase in arginase activity or the production of ornithine from arginine. RU486 administration had no effect on argininosuccinate synthase (ASS) activity, yet totally blocked the weaning-associated increase in P5CS and ASL activities (Wu et al. 2000c). The enhanced production of CO2, citrulline and ornithine from glutamine in response to weaning was also prevented by RU486 treatment. At the molecular level, RU486 administration had no effect on ASS mRNA levels, but did suppress the weaning-associated rise in arginase and ASL mRNA levels. An additional study (Flynn et al. 1999) demonstrated that the intestinal expression of type-II arginase, but not type-I arginase, was increased in response to weaning or glucocorticoids. This finding has important implications for better understanding of the regulation of arginine metabolism because type-II arginase is a mitochondrial enzyme and type-I arginase is present only in the cytoplasm (Davis and Wu 1998; Wu et al. 2000c). The cortisol induction of type-II arginase in the small intestine of post-weaning pigs may play a major role in tissue remodeling, because proline (a major amino acid for the synthesis of extracellular matrix protein (Krane 2008) and a metabolically versatile nutrient (Phang et al. 2008)) is produced from arginine in enterocytes of weaned pigs via the type-II arginase pathway (Wu 1996; Wu et al. 1997). The induction of P5CS and arginase in enterocytes of post-weaning pigs provides a biochemical basis for the synthesis of proline from glutamine and arginine (Wu 1995; Wu et al. 2000c). This result explains why proline is an essential amino acid for suckling, but not weaned, pigs (Ball et al. 1986; Tan et al. 2008b).
On the basis of current knowledge of glucocorticoid biochemistry (Weigel 1996), a mechanism for cortisol induction of arginine and glutamine metabolism in enterocytes of post-weaning pigs can be proposed. We propose that elevating concentrations of plasma cortisol increases arginase activity and glutamine metabolism in the small intestine through the glucocorticoid receptor (Flynn and Wu 1997a, b; Flynn et al. 1999). Weaning stress increases plasma concentrations of glucocorticoids (Henning 1981). These hormones bind nuclear receptors (Weigel 1996), therefore promoting transcription of the arginase, ASL and P5CS genes. In addition, diet and thyroid hormones likely play a role in mediating the increase in ASS activity and may contribute to approximately 20% of the weaning-associated increase in intestinal arginase activity (Flynn and Wu 1997a, b). The exact mechanism associated with an increase in ASS activity in response to weaning remains to be determined.
Perinatal
Weaning is not the only time when cortisol production is increased in mammals. It has also been established that glucocorticoid production is elevated at the time of birth (Silver and Fowden 1989; Wu et al. 2004). Studies on the effects of prenatal glucocorticoids on postnatal development of carbohydrate metabolism have been conducted (Sangild 1995; Solomon et al. 2001). However, similar studies on amino acid metabolism are lacking. The closest research involving amino acid metabolism in the newborn determined the effects of glucocorticoids on mixed function oxidases in the small intestine of rabbits (Tredger and Chhabra 1980).
Effects of glucocorticoids on intestinal polyamine metabolism
Milk has relatively high concentrations of polyamines (Dorhout et al. 1996), products of arginine catabolism (Wu and Morris 1998). This finding has led scientists to explore the role that polyamines play in intestinal development. Several studies have demonstrated the importance of polyamines in regulating epithelial cell growth and differentiation as well as remodeling in the small intestine (Johnson 1988; Sugita et al. 2007). The role of polyamines in mRNA stability and intestinal growth has been recognized (Wang 2007). To this effect, polyamine administration to weanling rats and piglets increases the maturation of the small intestine and the activities of digestive enzymes (Grèco et al. 2001; Wang et al. 2008a). Expression of ornithine decarboxylase, a key enzyme in polyamine synthesis, is also enhanced in response to weaning in rats and pigs (Lin et al. 1998; Wu et al. 2000c) or glucocorticoid administration (Wang and Johnson 1990; Wu et al. 2000b). Indeed, administration of RU486 to weaned pigs has revealed that cortisol increases the synthesis of polyamines from arginine, proline and ornithine in enterocytes (Wu et al. 2000b). It is noteworthy that the work of Wu and co-workers has shown that arginine is not a major precursor of polyamines in enterocytes of suckling mammals due to the lack of arginase activity (Wu et al. 1996). Importantly, Wu (1997) discovered that enterocytes of both preweaning and post-weaning pigs have a high activity of proline oxidase, which oxidizes proline to P5C. The proline-derived P5C is utilized efficiently for the synthesis of ornithine and polyamines (Wu et al. 2000a, 2008a).
Considerations when using glucocorticoids in intestinal studies
Molecular aspects
Glucocorticoids mediate most of their molecular effects via an interaction between a translocated nuclear receptor and a hormone response element in cellular DNA (Weigel 1996). Binding of glucocorticoids to the receptor is responsible for mediating this interaction, which affects cellular homeostasis. For example, glucocorticoid treatment can cause the preferential expression of certain subunits of an individual protein (Henning et al. 1999). This can change many aspects of protein function, including allosteric regulation, ligand binding, and enzyme kinetics. A thymidine analog study involving glucocorticoids has provided an interesting insight into the role that glucocorticoids play in intestinal maturation (Nanthakumar and Henning 1995). This study examined the effect of bromodeoxyuridine on glucocorticoid induction of brush border enzymes. There were differential effects on individual enzymes; some were affected by bromodeoxyuridine while others were not (Nanthakumar and Henning 1995). The authors suggested that changes in intestinal metabolism brought about by glucocorticoids are due to cellular cooperation between different cell types in the small intestine.
Recently, it has been suggested that glucocorticoid receptor dephosphorylation plays a vital role in regulating nuclear translocation and expression of glucocorticoid receptor in the small intestine (Takabe et al. 2008). Several studies also show that a lack of response to exogenous glucocorticoids during maturation of the small intestine is primarily due to a lack of glucocorticoid receptor expression in the anatomical location or developmental stage being studied. The intestinal NHE3 sodium/proton exchange protein response to glucocorticoids, for example, is both age and anatomically sensitive (Kiela et al. 2000). Additionally, certain inflammatory cytokines have been shown to increase expression of the β form of the glucocorticoid receptor (Orii et al. 2002). This form of the glucocorticoid receptor is known to be an inactive transcription factor and would thus limit the effectiveness of glucocorticoid treatment. Because glucocorticoids do mediate physiological effects independent of the glucocorticoid receptor, it is important to distinguish these effects from glucocorticoid receptor-mediated effects.
Species-specific effects
Any developmental study involving glucocorticoids and the small intestine must take into account the species of animal that is being studied. The mink exhibits a rather late responsiveness to glucocorticoids compared to other species and mink exhibit a decreased metabolic clearance of cortisol that is contrasted with a decreased adrenal responsiveness to ACTH (Elnif and Sangild 1996; Sangild and Elnif 1996). Additionally, the same parameter, when studied across species, can exhibit differential effects. Expression of the Vitamin D receptor in the small intestine, for example, is increased in rats but decreased in mice in response to cortisol administration (Hirst and Feldman 1982). One must also consider the major circulatory glucocorticoid and response to synthetic glucocorticoids in the species being studied.
Developmental responsiveness
As noted above, the lack of responsiveness to glucocorticoids can be developmentally specific. Cross-breeding studies in mice lacking a functional glucocorticoid receptor suggest that responsiveness to glucocorticoids in rodents depends greatly upon age (Gartner et al. 2003). For instance, transcriptional activation of trehalase expression in the small intestine by glucocorticoids is possible in neonatal mice, but fetal administration of glucocorticoids fails to elicit a response (Solomon et al. 2001). In the same study, it was also determined that there was no difference in the observed response to glucocorticoids between 1 and 2 weeks of age. These differential responses are likely due to altered expression of the glucocorticoid receptor as well as the prevalence of a critical mass of responsive cell types in the small intestine. Another important factor to consider is the presence of glucocorticoid-binding proteins in the blood. These proteins could limit the availability of glucocorticoids to targeted cells. Corticosteroid-binding globulin, for instance, exhibits a sharp increase in expression during the first 3 weeks of birth in the rat (Henning 1978).
Physiological and medical implications
Several intestinal diseases can benefit from moderation of glucocorticoid biochemistry. The intestinal pathology of endotoxemia, for example, may be due to the decline in glucocorticoid receptors (Fan et al. 1994). Glucocorticoids have also been implicated in the treatment of such diseases as encapsulating peritoneal sclerosis (Imai et al. 2002) and ulcerative colitis (Kornbluth and Sachar 1997).
Several studies have demonstrated that the increase in plasma glucocorticoids in association with weaning is mirrored in response to the birth process, suggesting that glucocorticoids may play an important role in the development of newborn tissues. Changes in intestinal function are still a major factor for high mortality and morbidity in mammalian neonates, including pigs (Wu et al. 2006, 2008b). This area of study is important to increasing the survival rate of premature infants. Premature infants are prone to an intestinal condition termed necrotizing enterocolitis. This disease is thought to occur as a result of an immature small intestine. Interestingly, the prevention and treatment of necrotizing enterocolitis using glucocorticoids is very much dependent upon the age of the premature infant (Crowley et al. 1990). Glucocorticoids play an important role in maturation of the small intestine in weanling mammals (Wang 2007; Wu et al. 2000b); therefore, these hormones may help in the treatment of diseases (e.g., necrotizing enterocolitis) that are associated with a poorly developed small intestine in premature infants (Bauer et al. 1984; Nanthakumar et al. 2005; Patole 2007).
The exact mechanisms associated with these medical observations are poorly understood but it can be appreciated that the general effect of glucocorticoids on amino acid and polyamine metabolism may play a crucial role (Wu et al. 2000a, b, c). An increase in the production of proline and ornithine from arginine would provide substrates necessary for the production of cellular matrix proteins (collagen) and polyamines (Davis and Wu 1998). These amino acids and their functional metabolites are important for intestinal growth, development, integrity, and immunity (Han et al. 2008; Li et al. 2007), as well as multi-organ functions (Wu et al. 2008c). As mentioned previously, studies of the relationship between glucocorticoids and intestinal amino acid metabolism in the very young neonate or preterm infant are lacking. What is clear, however, is the fact that arginine supplementation does improve intestinal function (Rhoads et al. 2008) and provide protective benefits to early-weaned piglets (Han et al. 2008; Tan et al. 2008a).
Future directions
While glucocorticoids clearly play an important role in regulating amino acid metabolism in the developing intestine, the underlying mechanisms remain largely unknown. Understanding the mode of actions of these hormones will be an important area of biomedical research. One interesting study demonstrated that treatment of neonatal pigs with dexamethasone inhibited intestinal growth (Burrin et al. 1999). In contrast, administration of a physiological dose of cortisol to 21-day-old piglets enhanced the growth of the small intestine (Wu et al. 2000a). Clearly, it is important to determine whether glucocorticoids regulate protein degradation via multiple pathways (e.g., ubiquitin-dependent proteolysis; Kahana 2007) and protein synthesis via mTOR signaling (Deng et al. 2008; Liao et al. 2008) in the small intestine. Additionally, studies are needed to elucidate the complex effects of glucocorticoids on intestinal amino acid metabolism using biochemical techniques, molecular biology, and bioinformatics (He et al. 2008; Hu et al. 2008a, b; Li et al. 2008b; Ou et al. 2007; Wang et al. 2008b, c). Finally, little is known about the role of glucocorticoids in intestinal and whole-body homeostasis of amino acids in mammals, birds, or fish (Aragao et al. 2008; Li et al. 2008a; Wu et al. 2008c), and these studies can be conducted using tracer methodologies (Suryawan et al. 2008). Collectively, this work will greatly advance our knowledge about the physiology and therapeutic uses of glucocorticoids in medicine.
Abbreviations
- ASL:
-
Argininosuccinate lyase
- ASS:
-
Argininosuccinate synthase
- P5C:
-
Pyrroline-5-carboxylate
- P5CS:
-
Pyrroline-5-carboxylate synthase
References
Aragao C, Corte-Real J, Costas B et al (2008) Stress response and changes in amino acid requirements in Senegalese sole (Solea senegalensis Kaup 1858). Amino Acids 34:143–148
Ball RO, Atkinson JL, Bayley HS (1986) Proline as an essential amino acid for the young pig. Br J Nutr 55:659–668
Batt RM, Rutgers HC, Sancak AA (1996) Enteric bacteria: friend or foe? J Small Anim Pract 37:261–267
Bauer CR, Morrison JC, Poole WK et al (1984) A decreased incidence of necrotizing enterocolitis after prenatal glucocorticoid therapy. Pediatrics 73:682–688
Burrin DG, Wester TJ, Davis TA et al (1999) Dexamethasone inhibits small intestinal growth via increased protein catabolism in neonatal pigs. Am J Physiol Endocrinol Metab 276:E269–E277
Castillo RO, Glasscock GF, Noren KM, Reisenauer AM (1991) Pituitary regulation of postnatal small intestinal ontogeny in the rat: differential regulation of digestive hydrolase maturation by thyroxine and growth hormone. Endocrinology 129:1417–1423
Chapple RP, Cuaron JA, Easter RA (1989) Response of digestive carbohydrases and growth to graded doses and administration frequency of hydrocortisone and adrenocorticotropic hormone in nursing piglets. J Anim Sci 67:2974–2984
Collington GK, Parker DS, Armstrong DG (1990) The influence of inclusion of either an antibiotic or a probiotic in the diet on the development of digestive enzyme activity in the pig. Br J Nutr 64:59–70
Crowley P, Chalmers I, Keirse MJ (1990) The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. Br J Obstet Gynaecol 97:11–25
Davis PK, Wu G (1998) Compartmentation and kinetics of urea cycle enzymes in porcine enterocytes. Comp Biochem Physiol B 119:527–537
Deng D, Yin YL, Chu WY et al (2008) Impaired translation initiation activation and reduced protein synthesis in weaned piglets fed a low-protein diet. J Nutr Biochem. doi:10.1016/j.jnutbio.2008.05.014
Dorhout B, van Beusekom CM, Huisman M et al (1996) Estimation of 24-hour polyamine intake from mature human milk. J Pediatr Gastroenterol Nutr 23:298–302
Dugan ME, Knabe DA, Wu G (1995) The induction of citrulline synthesis from glutamine in enterocytes of weaned pigs is not due primarily to age or change in diet. J Nutr 125:2388–2393
Elnif J, Sangild PT (1996) The role of glucocorticoids in the growth of the digestive tract in mink (Mustela vison). Comp Biochem Physiol A 115:37–42
Espinoza J, Hritz A, Kaplan R et al (1975) Regional variation in glycolytic enzyme adaptation to dietary sugars in rat small intestine. Am J Clin Nutr 28:453–458
Espinoza J, Clark SB, Hritz A, Rosensweig NS (1976) Regulation of rat proximal intestinal glycolytic enzyme activity by ileal perfusion with glucose. Gastroenterology 71:295–298
Fan J, Gong XQ, Wu J et al (1994) Effect of glucocorticoid receptor (GR) blockade on endotoxemia in rats. Circ Shock 42:76–82
Ferraris RP (2001) Dietary and developmental regulation of intestinal sugar transport. Biochem J 360:265–276
Flynn NE, Wu G (1996) An important role for endogenous synthesis of arginine in maintaining arginine homeostasis in neonatal pigs. Am J Physiol Regul Integr Comp Physiol 271:R1149–R1155
Flynn NE, Wu G (1997a) Glucocorticoids play an important role in mediating the enhanced metabolism of arginine and glutamine in enterocytes of postweaning pigs. J Nutr 127:732–737
Flynn NE, Wu G (1997b) Enhanced metabolism of arginine and glutamine in enterocytes of cortisol-treated pigs. Am J Physiol Gastrointest Liver Physiol 272:G474–G480
Flynn NE, Meininger CJ, Kelly K et al (1999) Glucocorticoids mediate the enhanced expression of intestinal type II arginase and argininosuccinate lyase in postweaning pigs. J Nutr 129:799–803
Gartner H, Graul MC, Oesterreicher TJ et al (2003) Development of the fetal intestine in mice lacking the glucocorticoid receptor (GR). J Cell Physiol 194:80–87
Grèco S, Niepceron E, Hugueny I et al (2001) Dietary spermidine and spermine participate in the maturation of galactosyltransferase activity and glycoprotein galactosylation in rat small intestine. J Nutr 131:1890–1897
Greene HL, Stifel FB, Hagler L, Herman RH (1975) Comparison of the adaptive changes in disaccharidase, glycolytic enzyme and fructose diphosphatase activities after intravenous and oral glucose in normal men. Am J Clin Nutr 28:1122–1125
Han J, Liu YL, Fan W et al (2008) Dietary l-arginine supplementation alleviates immunosuppression induced by cyclophosphamide in weaned pigs. Amino Acids. doi:10.1007/s00726-008-0184-9
He QH, Kong XF, Wu G et al (2008) Metabolomic analysis of the response of growing pigs to dietary l-arginine supplementation. Amino Acids. doi:10.1007/s00726-008-0192-9
Henning SJ (1978) Plasma concentrations of total and free corticosterone during development in the rat. Am J Physiol Gastrointest Liver Physiol 235:G451–G456
Henning SJ (1981) Postnatal development: coordination of feeding, digestion, and metabolism. Am J Physiol 241:G199–G214
Henning SJ, Oesterreicher TJ, Osterholm DE et al (1999) Meprin mRNA in rat intestine during normal and glucocorticoid-induced maturation: divergent patterns of expression of alpha and beta subunits. FEBS Lett 462:368–372
Hirst M, Feldman D (1982) Glucocorticoid regulation of 1, 25(OH)2vitamin D3 receptors: divergent effects on mouse and rat intestine. Endocrinology 111:1400–1402
Hu CA, Khalil S, Zhaorigetu S et al (2008a) Human Δ1-pyrroline-5-carboxylate synthase: function and regulation. Amino Acids 35:665–672
Hu CA, Williams DB, Zhaorigetu S et al (2008b) Functional genomics and SNP analysis of human genes encoding proline metabolic enzymes. Amino Acids 35:655–664
Imai H, Nakamoto H, Fucshima R et al (2002) Glucocorticoid protects against the development of encapsulating peritoneal sclerosis on peritoneal dialysis. Adv Perit Dial Conf 18:124–130
James PS, Smith MW, Tivey DR, Wilson TJ (1987) Epidermal growth factor selectively increases maltase and sucrase activities in neonatal piglet intestine. J Physiol 393:583–594
Johnson LR (1988) Regulation of gastrointestinal mucosal growth. Physiol Rev 68:456–502
Kahana C (2007) Ubiquitin dependent and independent protein degradation in the regulation of cellular polyamines. Amino Acids 33:225–230
Kiela PR, Guner YS, Xu H et al (2000) Age- and tissue-specific induction of NHE3 by glucocorticoids in the rat small intestine. Am J Physiol Cell Physiol 278:C629–C637
Koldovsky O, Chytil F, Muzcenkova H (1964) Effect of adrenalectomy and diet on the activity of beta-galactosidase in the small intestine during the postnatal development of the rat. Experientia 20:87–89
Koldovsky O, Jumawan J, Palmieri M (1975) Effect of thyroidectomy on the activity of alpha-glucosidases and acid hydrolases in the small intestine of rats during weaning. J Endocrinol 66:31–36
Kornbluth A, Sachar DB (1997) Ulcerative colitis practice guidelines in adults. American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 92:204–211
Krane SM (2008) The importance of proline residues in the structure, stability and susceptibility to proteolytic degradation of collagens. Amino Acids 35:703–710
Li P, Yin YL, Li DF et al (2007) Amino acids and immune function. Br J Nutr 98:237–252
Li P, Mai KS, Trushenski J, Wu G (2008a) New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids. doi:10.1007/s00726-008-0171-1
Li P, Kim SW, Li XL et al (2008b) Dietary supplementation with cholesterol and docosahexaenoic acid affects concentrations of amino acids in tissues of young pigs. Amino Acids. doi:10.1007/s00726-008-0196-5
Liao XH, Majithia A, Huang XL, Kimmel AR (2008) Growth control via TOR kinase signaling, an intracellular sensor of amino acids and energy availability, with crosstalk potential to proline metabolism. Amino Acids 35:761–770
Lin CH, Correia L, Tolia K et al (1998) Early weaning induces jejunal ornithine decarboxylase and cell proliferation in neonatal rats. J Nutr 128:1636–1642
Nanthakumar NN, Henning SJ (1995) Distinguishing normal and glucocorticoid-induced maturation of intestine using bromodeoxyuridine. Am J Physiol Gastrointest Liver Physiol 268:G139–G145
Nanthakumar NN, Young C, Ko JS et al (2005) Glucocorticoid responsiveness in developing human intestine: possible role in prevention of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 288:G85–G92
Orii F, Ashida T, Nomura M et al (2002) Quantitative analysis for human glucocorticoid receptor alpha/beta mRNA in IBD. Biochem Biophys Res Commun 296:1286–1294
Ou DY, Li DF, Cao YH et al (2007) Dietary supplementation with zinc oxide decreases expression of the stem cell factor in the small intestine of weanling pigs. J Nutr Biochem 18:820–826
Patole S (2007) Prevention and treatment of necrotising enterocolitis in preterm neonates. Early Hum Dev 83:635–642
Phang JM, Donald SP, Pandhare J, Liu YM (2008) The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids 35:681–690
Ratineau C, Roche C, Chuzel F et al (1996) Regulation of intestinal cholecystokinin gene expression by glucocorticoids. J Endocrinol 151:137–145
Rhoads JM, Niu XM, Surendran S et al (2008) Arginine stimulates intestinal epithelial cell migration via a mechanism requiring both nitric oxide and p70s6k signaling. J Nutr 138:1652–1657
Rider JE, Hacker A, Mackintosh CA et al (2007) Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids 33:231–240
Sangild PT (1995) Stimulation of gastric proteases in the neonatal pig by a rise in adrenocortical secretion at parturition. Reprod Fertil Dev 7:1293–1298
Sangild PT, Elnif J (1996) Intestinal hydrolytic activity in young mink (Mustela vison) develops slowly postnatally and exhibits late sensitivity to glucocorticoids. J Nutr 126:2061–2068
Silver M, Fowden AL (1989) Pituitary-adrenocortical activity in the fetal pig in the last third of gestation. Q J Exp Physiol 74:197–206
Solomon NS, Gartner H, Oesterreicher TJ, Henning SJ (2001) Development of glucocorticoid-responsiveness in mouse intestine. Pediatr Res 49:782–788
Souba WW, Smith RJ, Wilmore DW (1985) Effects of glucocorticoids on glutamine metabolism in visceral organs. Metabolism 34:450–456
Sugita Y, Takao K, Toyama Y, Shirahata A (2007) Enhancement of intestinal absorption of macromolecules by spermine in rats. Amino Acids 33:253–260
Suryawan A, O’Connor PMJ, Bush JA et al (2008) Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids. doi:10.1007/s00726-008-0149-z
Takabe S, Mochizuki K, Goda T (2008) De-phosphorylation of GR at Ser203 in nuclei associates with GR nuclear translocation and GLUT5 gene expression in Caco-2 cells. Arch Biochem Biophys 475:1–6
Tan B, Li X, Kong X et al (2008a) Dietary l-arginine supplementation enhances the immune status in early-weaned piglets. Amino Acids. doi:10.1007/s00726-008-0155-1
Tan BE, Yin YL, Liu ZQ et al (2008b) Dietary l-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids. doi:10.1007/s00726-008-0148-0
Tredger JM, Chhabra RS (1980) Factors affecting the properties of mixed-function oxidases in the liver and small intestine of neonatal rabbits. Drug Metabol Dispos 8:16–22
Wang JY (2007) Polyamines and mRNA stability in regulation of intestinal mucosal growth. Amino Acids 33:241–252
Wang JY, Johnson LR (1990) Gastric and duodenal mucosal ornithine decarboxylase and damage after corticosterone. Am J Physiol 258:G942–G950
Wang W, Qiao S, Li D (2008a) Amino acids and gut function. Amino Acids. doi:10.1007/s00726-008-0152-4
Wang JJ, Wu G, Zhou HJ, Wang FL (2008b) Emerging technologies for amino acid nutrition research in the post-genome era. Amino Acids. doi:10.1007/s00726-008-0193-8
Wang JJ, Chen LX, Li P et al (2008c) Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr 138:1025–1032
Weigel NL (1996) Steroid hormone receptors and their regulation by phosphorylation. Biochem J 319:657–667
Wu G (1995) Urea synthesis in enterocytes of developing pigs. Biochem J 312:717–723
Wu G (1996) An important role for pentose cycle in the synthesis of citrulline and proline from glutamine in porcine enterocytes. Arch Biochem Biophys 336:224–230
Wu G (1997) Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am J Physiol Gastrointest Liver Physiol 272:G1382–G1390
Wu G (1998) Intestinal mucosal amino acid catabolism. J Nutr 128:1249–1252
Wu G, Knabe DA (1995) Arginine synthesis in enterocytes of neonatal pigs. Am J Physiol Regul Integr Comp Physiol 269:R621–R629
Wu G, Morris SM (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Wu G, Knabe DA, Flynn NE (1994a) Synthesis of citrulline from glutamine in pig enterocytes. Biochem J 299:115–121
Wu G, Borbolla AG, Knabe DA (1994b) The uptake of glutamine and release of arginine, citrulline and proline by the small intestine of developing pigs. J Nutr 124:2437–2444
Wu G, Knabe DA, Flynn NE et al (1996) Arginine degradation in developing porcine enterocytes. Am J Physiol Gastrointest Liver Physiol 271:G913–G919
Wu G, Davis PK, Flynn NE et al (1997) Endogenous synthesis of arginine plays an important role in maintaining arginine homeostasis in postweaning growing pigs. J Nutr 127:2342–2349
Wu G, Flynn NE, Knabe DA (2000a) Enhanced intestinal synthesis of polyamines from proline in cortisol-treated piglets. Am J Physiol Endocrinol Metab 279:E395–E402
Wu G, Flynn NE, Knabe DA, Jaeger LA (2000b) A cortisol surge mediates the enhanced polyamine synthesis in porcine enterocytes during weaning. Am J Physiol Regul Integr Comp Physiol 279:R554–R559
Wu G, Meininger CJ, Kelly K et al (2000c) A cortisol surge mediates the enhanced expression of pig intestinal pyrroline-5-carboxylate synthase during weaning. J Nutr 130:1914–1919
Wu G, Jaeger LA, Bazer FW, Rhoads JM (2004) Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. J Nutr Biochem 15:442–451
Wu G, Bazer FW, Wallace JM, Spencer TE (2006) Intrauterine growth retardation: implications for the animal sciences. J Anim Sci 84:2316–2337
Wu G, Bazer FW, Datta S et al (2008a) Proline metabolism in the conceptus: implications for fetal growth and development. Amino Acids 35:691–702
Wu G, Bazer FW, Datta S et al (2008b) Intrauterine growth retardation in livestock: implications, mechanisms and solutions. Arch Anim Breed 51(special issue 1):4–10
Wu G, Bazer FW, Davis TA et al (2008c) Arginine metabolism and nutrition in growth, health and disease. Amino Acids. doi:10.1007/s00726-008-0210-y
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flynn, N.E., Bird, J.G. & Guthrie, A.S. Glucocorticoid regulation of amino acid and polyamine metabolism in the small intestine. Amino Acids 37, 123–129 (2009). https://doi.org/10.1007/s00726-008-0206-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-008-0206-7