Abstract
There are marked decreases in plasma concentrations of cortisol and arginine (an essential amino acid for neonates) as well as intestinal citrulline synthesis in piglets during the first 14 days of life. The objective of this study was to test the hypothesis that increasing plasma cortisol concentrations by cortisol administration may prevent the decline in intestinal citrulline and arginine synthesis from proline, thereby possibly increasing plasma arginine concentration in suckling piglets and their growth. Seven-day-old pigs reared by sows received daily intramuscular injections of hydrocortisone 21-acetate (25 mg/kg) or vehicle solution (saline) (n = 10/group). At 14 days of age, piglets were used to prepare jejunal enterocytes. Cells were incubated at 37 °C for 30 min in oxygenated Krebs buffer containing 5 mM glucose, 2 mM [U-14C]proline, and 2 mM glutamine. Cortisol treatment increased plasma cortisol concentration, mitochondrial proline oxidase and N-acetylglutamate synthase activities, cytosolic argininosuccinate lyase activity, and the intracellular concentrations of N-acetylglutamate and carbamoyl phosphate for citrulline and arginine synthesis. However, cortisol treatment induced the expression of intestinal arginase-II for arginine hydrolysis, resulting in no change in plasma arginine concentration. Administration of cortisol had no effect on milk consumption or the whole-body growth rate of piglets, but increased villus height in the jejunum and ileum. Collectively, these results suggest an important role for proline oxidase and N-acetylglutamate in regulating citrulline and arginine synthesis from proline in pig enterocytes. Because proline catabolism plays an important role in modulating protein synthesis, cell proliferation, and arginine production, our findings may have important implications for understanding the role of proline oxidase in the growth and health of the mammalian small intestine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The milk of most mammals, including humans (Davis et al. 1994) and swine (Wu and Knabe 1994; Wu et al. 2018) is remarkably deficient in arginine despite a particularly high requirement for this amino acid by suckling pigs. Thus, milk-fed neonates must be able to synthesize arginine to grow, develop, and survive. The pig is a useful animal model to elucidate the metabolic pathways for arginine synthesis (Beaumont and Blachier 2020; Wu et al. 2018). The small intestine is the exclusive source of citrulline for the endogenous synthesis of arginine in piglets to compensate for low arginine intake (Wu and Knabe 1994; 1995), and plays an important role in regulating arginine homeostasis in the neonates (Flynn and Wu 1996). Glutamine, glutamate, and proline (abundant amino acids in sow’s milk) are major precursors for the synthesis of citrulline and arginine by enterocytes of postnatal pigs, and the intracellular compartmentalized pathway for the conversion of proline into citrulline and arginine is illustrated in Fig. 1. Of particular interest, the intestinal synthesis of citrulline and arginine from proline decreases markedly in 14-day-old suckling pigs, compared with 0- to 2-day-old pigs (Wu 1997), leading to a deficiency of arginine and suboptimum growth in sow-reared piglets (Wu et al. 2018). Because aberrations of intestinal function and malnutrition remain major factors for high morbidity and mortality in piglets (Hernandez et al. 2020; Kirkden et al. 2013) and because arginine plays a crucial role in intestinal integrity, nutrition, host immunity, and whole-body homeostasis (Zhang et al. 2021), it is imperative to identify means to inhibit the substantial decline in the intestinal synthesis of citrulline and arginine from proline during the suckling period.
Synthesis of citrulline and arginine from proline in the enterocytes of the small intestine of most mammals (including humans and pigs). This metabolic pathway spans both the mitochondria and the cytosol. The conversion of proline into citrulline occurs exclusively in the mitochondria of enterocytes. Arginine is formed from citrulline in the cytoplasm of almost all cell types. In the neonatal small intestine, the near absence of arginase maximizes the output of arginine into the portal circulation. In adults, most of the intestine-derived citrulline is released into the portal circulation, bypasses the liver, and is extracted primarily by the kidneys for arginine synthesis. CP carbamoyl phosphate; CPS-I carbamoylphosphate synthase I; OAT ornithine aminotransferase
Plasma cortisol concentrations in pigs decrease progressively during the suckling period (Hoskinson et al. 1990). We have shown that cortisol enhances the catabolism of glutamine for the synthesis of citrulline and arginine by enterocytes of suckling piglets (Flynn and Wu 1997a, b). On the basis of the previous finding that cortisol increased proline oxidase activity in rat liver (Kowaloff et al. 1977) and cultured cells (Kowaloff et al. 1978), we hypothesized that increasing plasma cortisol concentration by cortisol administration may attenuate the decline in intestinal citrulline and arginine synthesis from proline in suckling pigs. This hypothesis was tested in the present study with the use of 7–14-day-old pigs.
Materials and methods
Chemicals
Unless indicated otherwise, all chemicals used for this study were obtained from Sigma Chemicals (St. Louis, MO).
Animals
This research was approved by Texas A&M University’s Animal Use and Care Committee. Pigs were the offspring of Yorkshire × Landrace sows and Duroc × Hampshire boars, and were housed in the Texas A&M University’s Swine Center. Lactating sows had free access to a diet consisting of the following (%, as-fed basis): corn grain, 57.5; soybean meal (44.5% crude protein), 27.0; cornstarch, 3.07; sugarcane molasses, 2.78; potassium chloride, 0.10; NaCl, 0.35; vitamin-mineral premix, 3.0; vegetable oil, 3.0; dicalcium phosphate, 2.5; and limestone, 0.70 (Rezaei 2015). This diet contained 90% dry matter, 3.32 Mcal of metabolizable energy/kg diet, and 17.5% crude protein [including 1.1% arginine and 1.46% proline as analyzed by high-performance liquid chromatography (HPLC) after acid hydrolysis (Hou et al. 2019; Li and Wu 2021)]. Piglets were allowed to nurse freely during the entire experimental period. Seven-day-old male piglets from five litters (4 piglets/litter) were assigned randomly within litters into two groups (10 piglets/group), and received daily intramuscular injections of either hydrocortisone 21-acetate (25 mg per kg body weight) or vehicle solution (saline, 1 mL) for 7 consecutive days, as described by Wu et al. (2000). This dose of cortisol was chosen because it was found in our previous study to increase the expression of arginase and glutaminase in enterocytes of suckling pigs (Flynn et al. 1997a). Body weights were measured at the beginning and end of the cortisol treatment. At day 0, blood samples (3 mL) were obtained from the umbilical vein of newborn pigs immediately after birth. At days 8 and 14 of age, blood samples (3 mL) were withdrawn from the jugular vein into heparinized tubes at 1 h after suckling. At day 14, after blood sampling, piglets were euthanized for obtaining the small intestine (see below). For comparison with proline oxidase in enterocytes, liver and kidney samples were also obtained. This study was approved by the Texas A&M University Institutional Animal Care and Use Committee.
Preparation of enterocytes
Enterocytes were prepared from the jejunum of piglets, as described previously (Wu et al. 2000). We chose the jejunum but not duodenum and ileum to isolate enterocytes because the metabolic studies involved intensive labor. Briefly, pigs received intramuscular injections of atropine (0.05 mg/kg body weight) followed by ketamine and acetylpromazine (4.76 and 0.24 mg/kg body weight, respectively). After this preanesthetic procedure, 5% halothane was administered via a face mask to achieve a surgical plane of anesthesia. After the abdomen was opened, the duodenum (approximately first 10 cm), jejunum, and ileum were removed and kept in physiological saline until use for cell isolation. In pigs, the jejunum and ileum constitutes approximately 40% and 60%, respectively, of the small intestine length below the duodenum (Wu 2018). Samples (approximately 3 cm) of the duodenum, jejunum, and ileum were obtained and fixed in paraformaldehyde for histological preparation.
Before the isolation of enterocytes, the lumen of the jejunum was washed three times with saline. The jejunum was then filled with oxygenated (95% O2/5% CO2) Ca2+-free Krebs–Henseleit bicarbonate (KHB) buffer supplemented with 5 mM EDTA and 5 mM glucose (Wu et al. 1994), and was placed in a flask containing this buffer. The flasks were incubated in a shaking waterbath (37 °C, 70 oscillations/min) for 20 min. The cells were separated from the jejunal mucosa by gently patting the jejunum with the fingertips for 1 min. Only enterocytes from the villus tip and mid-villus of the jejunum were removed, whereas its crypt cells and the muscularis mucosa were left intact as determined by examining the morphology of intestinal segments before and after cell isolation (Wu and Knabe 1995). Enterocytes were collected by draining the luminal fluid into a polysterene tube (Fisher Scientific, Houston, TX). The cells were washed three times with oxygenated KHB buffer (EDTA-free) containing 2.5 mM CaCl2 and 20 mM Hepes (pH 7.4), by centrifugation at 600 g for 2 min, and then suspended in this buffer.
Determination of activities of arginase, proline oxidase, and the enzymes for converting ∆1-pyrroline-5-carboxylate (P5C) into arginine
Mitochondria and the cytosol were prepared from freshly isolated enterocytes for measuring the activities of arginase, proline oxidase, and the enzymes for converting P5C into arginine (Fig. 1), as described previously (Wu et al. 1996a; Dillon et al. 1999). Enzyme assays were performed at 37 °C at two protein levels (0.2 and 0.5 mg) and at three time points [0, 7.5, and 15 min, as previously described (Wu et al. 1996a, b; Wu et al. 1997; Dillon et al. 1999).
Determining N-acetylglutamate (NAG) synthase activity as well as NAG and carbamoyl phosphate concentrations in enterocyte mitochondria
Freshly isolated enterocytes (~ 100 mg protein) were homogenized at 4 °C in 4 mL buffer (pH 7.4) containing 300 mM mannitol, 1 mM EDTA, 0.2 mM DTT, 5 mM Hepes buffer, and protease inhibitors (5 μg/mL phenylmethylsulfonyl fluoride, 5 μg/mL aprotinin, 5 μg/mL chymostatin, 5 μg/mL pepstatin A). The homogenates were centrifuged at 600 g, 4 °C, for 10 min. The supernatant was centrifuged at 10,000 g, 4 °C, for 10 min. The resultant mitochondrial pellets were suspended in a buffer (pH 7.4) consisting of 20 mM potassium phosphate, 1 mM EDTA, and 0.2 mM DTT. The mitochondria were lysed with 0.2% Triton X-100 (also a stabilizer of NAG synthase), and stored at − 80 °C until use within 3 days for determining NAG synthase activity, as well as NAG and carbamoyl phosphate concentrations.
NAG synthase activity was measured as described by Yamada and Wakabayashi (1991) with modifications. Briefly, the assay mixture (0.2 mL) contained 1 mM L-[U-14C]glutamate (2.5 μCi/μmol; American Radiolabeled Chemicals, St. Louis, MO), 0.5 mM acetyl-CoA, 1 mM arginine, 1 mM EDTA, 50 mM Tris buffer (pH 8.2), and enzyme preparation (0.5–1 mg protein). The mixture was incubated at 25 °C for 0, 15 and 30 min, and the reaction was terminated by the addition of 10 μL of 1 M HCl. To the acidified mixture, unlabeled NAG (100 nmol) was added as an internal standard to correct for the chromatographic recovery of [14C]NAG. The mixture was applied to Dowex 50 W-X8 resin (H+ form, 200–400 mesh (0.6 × 5 cm). The column was washed with 2 mL water, and the 2-mL effluent (containing NAG but no glutamate) was mixed with 38 µL of 1 M KOH and 0.25 mL of 250 mM potassium buffer (pH 7.5). Aminoacylase (0.5 mg) was added to the solution, and the mixture was incubated at 37 °C for 90 min for converting [14C]NAG to [14C]glutamate. The aminoacylase reaction was terminated by the addition of 38 µL of 6 M HCl, and the acidified solution was applied to Dowex 50 W-X8 resin (H+ form, 200–400 mesh) (0.6 × 5 cm). After the column was sequentially washed with 12 mL of water and 4 mL of 1 M HCl, [14C]glutamate was eluted with 8 mL of 1 M HCl into a scintillation vial. An aliquot (0.2 mL) of the collected solution was used for HPLC analysis of the glutamate derived from internal NAG standard for the calculation of NAG recovery, and the remaining solution was measured for 14C-radioactivity using a liquid scintillation counter (Wu et al. 1994).
NAG was determined by HPLC as described by Alonso and Rubio (1985) with modifications. Briefly, the mitochondrial pellet (~ 0.5 mL) were mixed with 1 mL of 1.5 M HClO4, and the acidified solution was neutralized with 0.5 mL of 2 M K2CO3. The extract was adjusted to pH 2 with 1 M HCl, and applied to Dowex 50 W-X8 resin (H+ form, 200–400 mesh) (0.6 × 5 cm). The column was washed with 2 mL H2O, and the effluent was neutralized with 1 M KOH and then mixed with 0.3 mL of 250 mM potassium phosphate buffer (pH 7.5). An aliquot (1 mL) of the solution was incubated with 0.5 mg aminoacylase at 37 °C for 0 or 90 min, and this aminoacylase reaction was terminated by the addition of 0.2 mL of 1.5 M HClO4. The acidified solution was used for glutamate analysis by HPLC.
Carbamoyl phosphate was determined using ornithine and OCT (Sigma Chemicals), as previously described (Wu et al. 1994). Determination of NAG and carbamoyl phosphate included corrections for endogenous amounts of mitochondrial glutamate and citrulline, respectively.
Incubation of enterocytes
For studies of proline catabolism, enterocytes (10 × 106 cells/mL) were incubated at 37 °C for 0 or 30 min in 2 mL of KHB buffer (pH 7.4, saturated with O2/CO2, 19:1) containing 20 mM Hepes, 1% BSA, 5 mM glucose, 2 mM L-[U-14C]proline (2500 dpm/nmol; American Radiolabeled Chemicals, St. Louis, MO) and 2 mM glutamine. Glutamine was used to provide: (1) ammonia for the production of carbamoyl phosphate by the cells (Wu et al. 1994), and (2) glutamate for the conversion of proline-derived P5C into ornithine (Wu 1997). At the end of the incubation, 1 mL of medium plus cells was used for determining intracellular specific activities of [14C]proline, [14C]ornithine and [14C]citrulline, as previously described (Wu 1997). The remaining portion of medium plus cells was acidified with 0.2 mL of 1.5 M HClO4, and used for analyses of amino acids by HPLC involving precolumn derivativization with o-phtaldialdehyde (Wu et al. 1994). Intracellular specific activities of [14C]proline, [14C]ornithine and [14C]citrulline were used to calculate synthesis of ornithine, citrulline and arginine from proline, respectively (Wu 1997). For studies of arginine catabolism, enterocytes (10 × 106 cells/mL) were incubated as described above, except that the medium contained no [14C]proline, unlabeled proline or glutamine, and arginine metabolites were measured by HPLC (Wu et al. 1996a).
Uptake of proline by enterocytes
This was performed as described by Dillon et al. (1999). Briefly, 1 mL of KHB medium (pH 7.4), which contained enterocytes (1 mg protein), 5 mM glucose, 2 mM glutamine, 0.5 and 2 mM proline plus [U-14C]proline (0.05 μCi/mL), and 0.1 mM gabaculine (an inhibitor of ornithine aminotransferase), was incubated at 37 °C for 2 min. Cell suspension was prewarmed to 37 °C before addition to KHB medium (prewarmed to 37 °C). At the end of a 2-min incubation period, 50 μL of [3H]inulin (an extracellular marker; 0.5 μCi/mL) plus 100 μg/mL unlabeled inulin was added to the incubation medium, and 0.25 mL of the mixture was immediately transferred in duplicate to a 1.6-mL microcentrifuge tube which contained 0.7 mL of an oil mixture of bromododecane and dodecane (20:1, vol/vol) overlaid on 0.2 mL of 1.5 M HClO4 (Davis and Wu 1998). Cells were rapidly separated from the medium through the oil layer into the acid layer by centrifugation (12,000×g, 1 min). The upper layer (incubation medium) was removed, and washed three times with KHB buffer. After the oil layer was removed, the acid layer was assayed for 14C and 3H using a dual-channel program in a Packard liquid scintillation counter (Wu et al. 1994). A small amount of 3H radioactivity in the acid layer was used to correct for contamination by the incubation medium, and proline uptake was calculated on the basis of 14C radioactivity in the acid layer and the specific activity of [14C] proline in the incubation medium.
Analysis of plasma cortisol and amino acids
Blood samples withdrawn into heparinized tubes were immediately centrifuged at 2000 g for 5 min at 4 °C to obtain plasma. Cortisol in the plasma was analyzed using a radioimmunoassay kit (Diagnostic Products, Los Angeles, CA), as previously described (Flynn and Wu 1997a). One mL of the remaining plasma was deproteinized with 1 mL of 1.5 M HClO4 and then neutralized with 0.5 mL of 2.0 M K2CO3. Amino acids in neutralized plasma extracts were analyzed by HPLC methods involving precolumn derivatization with o-phtaldialdehyde (Wu et al. 1994).
Histological preparation and examination of intestinal morphology
Preparation and examination of the intestinal morphology was performed as previously described (Wu et al. 1996b). Samples of the duodenum, jejunum, and ileum were split open lengthwise, pinned to cork and floated upside down in paraformaldehyde, allowing the samples to be suspended in solution. The samples were fixed in paraformaldehyde for 24-h after which they were rinsed and kept in 70% ethanol until processed for histology. Ten well-oriented villi per histology slide were measured for villus height, crypt depth, and lamina propria depth (from the base of the villus to the muscularis mucosae) using a microscope (MicromasterTM Model E) with a calibration ruler (×40 magnification; Fisher Scientific, Houston, TX).
Protein determination
Protein in enterocytes, as well as their mitochondria and cytosol was determined by a modified Lowry procedure using BSA as a standard (Wu et al. 1994).
Milk consumption
Milk consumption was estimated by the weigh-suckle-weigh technique using an additional twelve male 7-day-old pigs to avoid an effect of daily handling (i.e., intramuscular administration of saline) on the expression of intestinal enzymes (Wu et al. 2000). There were 6 piglets per treatment group, and they were from 3 litters (4 piglets/litter). Piglets were treated with cortisol or vehicle solution, as described above. At days 1, 4, and 7 of cortisol administration, the body weights of piglets were measured before and after suckling every 1.5 h during a 12-h period from 8:00 AM to 8:00 PM to estimate milk intake.
Statistical analysis
Results are expressed as mean ± SEM. Data for intestinal weight, intestinal morphology, enterocyte metabolism, enterocyte enzyme activities, and plasma amino acid concentrations in the control and cortisol groups were analyzed by the unpaired t test (Steel and Torrie 1980). Data on plasma cortisol concentrations, body weight, and milk intake were analyzed using the two-way analysis of variance for repeated measurement (Lee et al. 2019). Probability values < 0.05 were taken to indicate statistical significance.
Results
Milk consumption
Milk consumption by 8-, 11-, and 14-day-old piglets in the control group was 305 ± 34, 287 ± 32, and 277 ± 30 mL/kg body weight/day (means ± SEM, n = 6 piglets), respectively. Milk consumption by 8-, 11-, and 14-day-old piglets in the cortisol group was 289 ± 40, 272 ± 37, and 262 ± 36 mL/kg body weight/day (means ± SEM, n = 6 piglets), respectively. At days 8, 11, and 14 of age, milk consumption did not differ (P > 0.05) between control and cortisol-treated piglets. However, milk intake by piglets was reduced (P < 0.05) with age.
Plasma cortisol concentrations
There was a marked decrease (P < 0.01) in plasma cortisol concentrations in untreated piglets during the first 14 days of life (Table 1). Daily administration of cortisol prevented the decline in plasma cortisol concentrations. On days 8 and 14 of postnatal life, plasma cortisol concentrations in cortisol-treated piglets were similar to those observed at birth.
Animal and intestinal growth
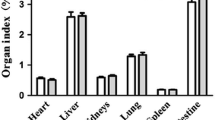
Cortisol treatment had no effect (P > 0.05) on body weight gains (Table 2), but increased (P < 0.05) jejunal and ileal weights by 16 and 18%, respectively (Table 3). Duodenal weights were similar between control and cortisol-treated pigs. The weight of the total small intestine was 17% greater (P < 0.05) in cortisol-treated pigs compared with control pigs. Duodenal, jejunal and ileal lengths did not differ (P > 0.05) between control and cortisol-treated piglets.
Intestinal morphology
Data on intestinal morphology are summarized in Table 4. Cortisol treatment had no effect (P > 0.05) on the villus height, crypt depth, or lamina propria depth of the duodenum, but increased (P < 0.05) villus height in the jejunum and ileum by 13–14%. Cortisol treatment had no effect (P > 0.05) on the crypt depth or lamina propria depth of the jejunum or ileum.
Enzyme activities in enterocytes
Cortisol treatment had no effect (P > 0.05) on the intestinal activities of OAT, OCT or ASS (Table 5). In the presence of optimal concentration of NAG added to the assay solution, CPS-I activity in enterocytes did not differ (P > 0.05) between control and cortisol-treated piglets. Cortisol treatment increased (P < 0.01) proline oxidase, NAG synthase, and ASL activities in enterocytes by 41%, 131%, and 91%, respectively. Arginases I and II were not detected in the enterocytes of control pigs. Interestingly, cortisol administration induced the expression of arginase-II but not arginase-I in pig enterocytes.
Proline oxidase activities in the liver and kidney
Cortisol treatment had no effect (P > 0.05) on proline oxidase activity in the kidney of pigs (1.63 ± 0.11 and 1.71 ± 0.12 nmol/mg protein/min in the control and cortisol groups, respectively; means ± SEM, n = 10). By contrast, cortisol treatment increased (P < 0.05) proline oxidase activity in the liver of pigs by 35% (1.87 ± 0.13 and 2.52 ± 0.16 nmol/mg protein/min in the control and cortisol groups, respectively, means ± SEM, n = 10).
NAG and carbamoyl phosphate concentrations
Cortisol treatment increased (P < 0.05) NAG and carbamoyl phosphate concentrations in enterocyte mitochondria by 106% and 48%, respectively. Because carbamoyl phosphate is utilized for converting ornithine into citrulline, an increase in mitochondrial concentration of carbamoyl phosphate (as a result of elevated NAG concentration) indicated an increase in CPS-I activity in vivo.
Proline and arginine catabolism as well as proline uptake by enterocytes
Large amounts of P5C, ornithine and citrulline were formed from proline in enterocytes (Table 6). Cortisol treatment increased proline utilization, the synthesis of citrulline and arginine from [U-14C]proline (P < 0.01), and the net accumulation of P5C (P < 0.05) in enterocytes of neonatal pigs (Table 6). The rates of ornithine production from 2 mM arginine in jejunal enterocytes were 0.28 ± 0.02 and 1.46 ± 0.11 nmol/mg protein/30 min (mean ± SEM, n =10; P < 0.01) in the control and cortisol-treated 14-day-old piglets, respectively. Cortisol treatment markedly increased (P < 0.01) proline uptake by enterocytes by 66% (4.15 ± 0.38 and 6.87 ± 0.72 nmol/mg protein/min in the control and cortisol groups, respectively; means ± SEM, n = 10).
Plasma concentrations of amino acids, ammonia and urea
Cortisol treatment increased (P < 0.05) plasma concentrations of all branched-chain amino acids (isoleucine, leucine, and valine), lysine, ornithine, and citrulline, and had no effect (P > 0.05) on those of arginine and proline (Table 7) or all other amino acids (Data not shown). Cortisol treatment did not affect (P > 0.05) plasma concentrations of ammonia or urea.
Discussion
Proline is a major substrate for the intestinal synthesis of citrulline and arginine via proline oxidase in neonatal pigs based on in vitro (Wu 1997, 1998) and in vivo (Brunton et al. 1999) studies. This metabolic pathway spans both the mitochondria and the cytosol (Wu and Morris 1998). In most mammals (including humans, pigs, cattle, sheep, and rats) that synthesize arginine de novo, all the necessary reactions for the conversion of glutamine, glutamate and proline into ornithine, citrulline and arginine occur in the same enterocytes (Wu 2021; Wu et al. 2021). This is physiologically and biochemically significant, as extracellular ornithine is a poor substrate for citrulline and arginine formation in these cells due to the complex intracellular compartmentation of amino acid metabolism (Wu and Morris 1998). The postnatal development of pigs is characterized by a progressive decrease in plasma concentrations of cortisol (Hoskinson et al. 1990) and a marked decline in intestinal synthesis of citrulline and arginine during the first 2 weeks of life (Wu 1997). Interestingly, most deaths in neonatal pigs occur during this postnatal period (Zhang et al. 2021), and aberrations of intestinal function and malnutrition remain major factors for high morbidity and mortality in piglets (Kirkden et al. 2013). Because arginine plays a crucial role in intestinal integrity, nutrition and whole body homeostasis in neonates, increasing the intestinal synthesis of citrulline and arginine for the endogenous provision of arginine may provide an attractive strategy for improving neonatal survival and growth (Wu et al. 2018).
As reported for in the rat liver (Kowaloff et al. 1977) and cultured cells (Kowaloff et al. 1978), cortisol administration increased proline oxidase activity in the enterocytes (Table 5) and liver (see the text) of piglets. A novel and important finding of this study is that cortisol treatment increased the mitochondrial concentration of NAG (an allosteric activator of CPS-I; Wu and Morris 1998) due to increased NAG synthase activity in enterocytes. Thus, although CPS-I activity under the in vitro assay conditions in which the enzyme was saturated with substrates and NAG did not differ between the control and cortisol-treated pigs, an elevated concentration of NAG in the mitochondria of enterocytes can allosterically activate CPS-I to generate carbamoyl phosphate from ammonia (a product of glutamine hydrolysis) and bicarbonate. This is consistent with our observation that the mitochondrial concentration of carbamoyl phosphate was 48% greater in enterocytes of cortisol-treated pigs compared with control pigs (Table 5). Thus, cortisol treatment stimulated the conversion of proline-derived ornithine into citrulline in enterocytes despite the lack of change in intestinal activities of OCT and OAT. Note that the activities of both OCT and OAT were particularly high in the mitochondria of pig enterocytes, as reported by Davis and Wu (1998). This result supports the view that proline oxidase and NAG synthase activities, as well as the availabilities (e.g., the proline-derived ornithine and NAG) are important determinants of the intestinal synthesis of citrulline and arginine from proline, and that these two enzymes play critical roles in the regulation of this metabolic pathway (Wu et al. 2004).
As proline is a major substrate for the intestinal synthesis of ornithine, citrulline, and arginine in most mammals including humans and pigs (Wu and Morris 1998), our findings provide a novel means to prevent the striking decline in this metabolic pathway and to augment the endogenous provision of arginine in their bodies. This is of nutritional and physiological importance because there is a particularly high requirement for arginine by neonates and yet the milk of most species studied (including humans and pigs) is remarkably deficient in arginine (Davis et al. 1994; Wu and Knabe 1994). In addition, because the uptake of ornithine, proline and arginine from arterial blood by the small intestine is limited (Wu et al. 1994), increases in the apical uptake of proline by the small-intestinal mucosa and the intestinal synthesis of ornithine, citrulline and arginine in cortisol-treated neonates will provide locally these amino acids for metabolic utilization such as the synthesis of polyamines and NO (Wu et al. 2018). Polyamines play an important role in the growth and remodeling of the neonatal intestine (Wu et al. 2000), whereas NO promotes blood flow in the portal-drained viscera to facilitate nutrient absorption and transport (Durante 2020; Wu 2018).
Intramuscular administration of hydrocortisone 21-acetate (25 mg/kg body weight) resulted in an increase in plasma concentrations of cortisol to the values similar to those in piglets during the perinatal period (Flynn and Wu 1997a, b; Sangild et al. 1995). Such an elevated concentration of cortisol is associated with the rapid somatic growth of the small intestine and maturation of intestinal digestive enzymes (e.g., sucrase and maltase) during neonatal development (Sangild et al. 1995). A single intramuscular administration of hydrocortisone 21-acetate (25 mg/kg body wt) to nursing piglets has been reported to reduce postweaning mortality and improve the growth rate of piglets weaned at 14 days of age (Chapple et al. 1989). In contrast, we found that daily hydrocortisone 21-acetate administration to 7-day-old piglets increased small-intestinal growth in neonatal pigs (Table 3). These studies, along with our previous findings (Wu et al. 2000), suggest a beneficial effect of cortisol on intestinal growth and maturation in neonatal pigs. Elevated concentrations of glucocorticoids are known to induce protein catabolism in tissues, including the skeletal muscle and small intestine (Fry 2016; Urban 2014). For example, administration of a pharmacological dose of dexamethasone (a more potent synthetic glucocorticoid than cortisol) inhibited intestinal growth in neonatal pigs (Burrin et al. 1998). However, as noted by Burrin et al. (1998), whether glucocorticoids promote anabolism or catabolism in the gut likely depends on the concentrations and potency of the circulating glucocorticoid, as well as tissue sensitivity to the hormone. Cortisol acts via both receptor-dependent and transcription-independent mechanisms to generally induce gene expression and nutrient metabolism in cells (Vandewalle et al. 2018), including pig enterocytes (Wu et al. 2000).
Cortisol treatment increased plasma concentrations of BCAA and lysine in piglets, probably due to an increase in whole-body protein degradation and/or a decrease in the tissue-specific catabolism of these amino acids. However, cortisol administration did not blunt overall growth and development as indicated by increased body weight of both cortisol- and saline-treated piglets between 7 and 14 days of age (Table 2). The increases in plasma concentrations of ornithine and citrulline in cortisol-treated pigs are consistent with the increase in the intestinal catabolism of proline (Table 6) and arginine (Flynn and Wu 1997a,b) for ornithine and citrulline production. However, plasma arginine concentration was not enhanced by cortisol treatment, likely because an increase in the endogenous synthesis of citrulline and arginine was offset by the increased catabolism of dietary arginine by the small intestine (the Results section) and of circulatory arginine by extraintestinal tissues (Wu and Morris 1998) via the action of arginase II (Table 5). Thus, cortisol treatment for one week is unlikely to be beneficial for augmenting net arginine provision in sow-reared piglets or improving neonatal pig growth. Consequently, means must be identified to stimulate intestinal citrulline production for endogenous arginine synthesis without a concomitant increase in intestinal or extraintestinal arginine catabolism. One of the effective means is the oral administration of N-carbamoylglutamate (NCG), a structural analog of NAG and a metabolically stable activator of CPS-I (Wu et al. 2004). In addition, it would be important to determine whether treatment with cortisol for a longer period (e.g., 2–4 weeks) will enhance the growth of sow-reared piglets.
The present study may have implications for intestinal health and cancer treatment. Glucocorticoids are known to be an effective agent of chemotherapy for certain cancers and hematopoietic malignancies, including leukemia, lymphomas, and multiple myeloma in neonates (Pufall 2015). Likewise, proline metabolism has been shown to closely link with protein synthesis (Phang 2019), cell proliferation (Phang 1985; Phang et al. 1995), and carcinogenesis in mammals (Phang and Liu 2012) via the elevated production of P5C and reactive oxygen species. There is also a suggestion that proline catabolism plays a role in epigenetic landscape and tumor heterogeneity (D’Aniello et al. 2020). Furthermore, in the small intestine and other tissues, cortisol increases the expression of ASL for the conversion of citrulline into arginine. In the local cells, arginine is used by nitric oxide synthase to generate nitric oxide, which kills tumors (Xu et al. 2002). Of particular note, increased proline catabolism via proline oxidase has been shown to inhibit carcinogenesis in mammalian cells and kill tumors via the actions of reactive oxygen species and possibly other proline metabolites (Phang 2019). Among all tissues studied in pigs (a widely used animal model for studying human nutrition and disease; Burrin and Reeds 1997; Ren et al. 2020), proline oxidase has the highest activity in the small intestine (Dillon et al. 1999; Wu et al. 1997). At present, we are not aware of data on intestinal proline-oxidase activity in humans. It is tempting to speculate that the high expression of proline oxidase in enterocytes may help to explain why malignant tumors of the small intestine are rare in humans (Pan and Morrison 2011) and that increased proline catabolism in the intestinal mucosa may contribute to the therapeutic effect of glucocorticoids to treat various kinds of cancers (Pufall 2015). Future experiments are warranted to test these two novel hypotheses.
In conclusion, the administration of cortisol to neonatal pigs from 7 to 14 days of age stimulated their intestinal growth and the synthesis of citrulline and arginine from proline in the enterocytes of the small intestine. Our results suggest that cortisol is beneficial for attenuating the substantial decline in the intestinal synthesis of citrulline and arginine from proline during the suckling period, and in advancing intestinal maturation for successful early weaning of piglets. Because proline catabolism plays an important role in modulating protein synthesis, cell proliferation and carcinogenesis, our findings of proline catabolism via proline oxidase in pig enterocytes may have important implications for understanding a role of proline metabolism in the growth and health of the mammalian small intestine.
Abbreviations
- ASL:
-
Argininosuccinate lyase
- ASS:
-
Argininosuccinate synthase
- BSA:
-
Bovine serum albumin
- DTT:
-
Dithiothreitol
- HPLC:
-
High-performance liquid chromatography
- KHB:
-
Krebs–Henseleit bicarbonate
- NAG:
-
N-acetylglutamate
- OAT:
-
Ornithine aminotransferase
- OCT:
-
Ornithine carbamoyltransferase
- P5C:
-
∆1-Pyrroline-5-carboxylate
References
Alonso E, Rubio V (1985) Determination of N-acetyl-L-glutamate using high-performance liquid chromatography. Anal Biochem 146:252–259
Beaumont M, Blachier F (2020) Amino acids in intestinal physiology and health. Adv Exp Med Biol 1265:1–20
Brunton JA, Bertolo RF, Pencharz PB, Ball RO (1999) Proline ameliorates arginine deficiency during enteral but not parenteral feeding in neonatal piglets. Am J Physiol 277:E223–E231
Burrin DG, Reeds PJ (1997) Alternative fuels in the gastrointestinal tract. Curr Opin Gastroenterol 13:165–170
Burrin DG, Wester TJ, Davis TA, Florotto ML, Chang X (1998) Dexamethasone inhibits small intestinal growth via increased protein catabolism in neonatal pigs. Am J Physiol 276:E269-277
Chapple RP, Cuaron JA, Easter RA (1989) Temporal changes in carbohydrate digestive capacity and growth rate of piglets in response to glucocorticoid administration and weaning age. J Anim Sci 67:2985–2995
D’Aniello C, Patriarca EJ, Phang JM, Minchiotti G (2020) Proline metabolism in tumor growth and metastatic progression. Front Oncol 10:776
Davis PK, Wu G (1998) Compartimentation and kinetics of urea cycle enzymes in porcine enterocytes. Comp Biochem Physiol 119:527–537
Davis TA, Nguyen HV, Garcia-Bravo R, Fiorotto ML, Jackson EM, Lewis DS, Lee DR, Reeds PJ (1994) Amino acid composition of human milk is not unique. J Nutr 124:1126–1132
Dillon EL, Knabe DL, Wu G (1999) Lactate inhibits citrulline and arginine synthesis from proline in pig enterocytes. Am J Physiol 276:G1079-1086
Durante W (2020) Amino acids in circulatory function and health. Adv Exp Med Biol 1265:39–56
Flynn NE, Wu G (1996) An important role for endogenous synthesis of arginine in maintaining arginine homeostasis in neonatal pigs. Am J Physiol 271:R1149-1155
Flynn NE, Wu G (1997a) Enhanced metabolism of arginine and glutamine in enterocytes of cortisol-treated pigs. Am J Physiol 272:G474-480
Flynn NE, Wu G (1997b) Glucocorticoids play an important role in mediating the enhanced metabolism of arginine and glutamine in enterocytes of postweaning pigs. J Nutr 127:732–737
Fry CS, Nayeem SZ, Dillon EL, Sarkar PS, Tumurbaatar B, Urban RJ, Wright TJ, Sheffield-Moore M, Tilton RG, Choudhary S (2016) Glucocorticoids increase skeletal muscle NF-κB inducing kinase (NIK): links to muscle atrophy. Physiol Rep 4:e13014
Hernandez GV, Smith VA, Melnyk M, Burd MA, Sprayberry KA, Edwards MS, Peterson DG, Bennet DC, Fanter RK, Columbus DA, Steibel JP, Glanz H, Immoos C, Rice MS, Santiago-Rodriguez TM, Blank J, VanderKelen JJ, Kitts CL, Piccolo BD, La Frano MR, Burrin DG, Maj M, Manjarin R (2020) Dysregulated FXR-FGF19 signaling and choline metabolism are associated with gut dysbiosis and hyperplasia in a novel pig model of pediatric NASH. Am J Physiol 318:G582–G609
Hoskinson CD, Chew BP, Wong TS (1990) Age-related changes in mitogen-induced lymphocyte proliferation and polymorphonuclear neutrophil function in the piglet. J Anim Sci 68:2471–2478
Hou YQ, He WL, Hu SD, Wu G (2019) Composition of polyamines and amino acids in plant-source foods for human consumption. Amino Acids 51:1153–1165
Kirkden RD, Broom DM, Andersen IL (2013) Piglet mortality: management solutions. J Anim Sci 91:3361–3389
Kowaloff EM, Granger AS, Phang JM (1977) Glucocorticoid control of hepatic proline oxidase. Metabolism 26:893–901
Kowaloff EM, Phang JM, Granger AS, Downing SJ (1978) Glucocorticoid induction of proline oxidase in LLC-RK1 cells. J Cell Physiol 97:153–160
Lee U, Garcia TP, Carroll RJ, Gilbreath KR, Wu G (2019) Analysis of repeated measures data in nutrition research. Front Biosci 24:1378–1390
Li P, Wu G (2020) Composition of amino acids and related nitrogenous nutrients in feedstuffs for animal diets. Amino Acids 52:523–542
Pan SY, Morrison H (2011) Epidemiology of cancer of the small intestine. World J Gastrointest Oncol 3:33–42
Phang JM (2019) Proline metabolism in cell regulation and cancer biology: recent advances and hypotheses. Antioxid Redox Signal 30:635–649
Phang JM, Liu W (2012) Proline metabolism and cancer. Front Biosci 17:1835–1845
Phang JM, Yeh GC, Scriver CR (1995) Disorders of proline and hydroxyproline metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, vol 1, 7th edn. McGraw-Hill Inc, pp 1125–1146
Pufall MA (2015) Glucocorticoids and cancer. Adv Exp Med Biol 872:315–333
Ren WK, Bin P, Yin YL, Wu G (2020) Impacts of amino acids on the intestinal defensive system. Adv Exp Med Biol 1265:133–151
Rezaei R (2015) Nutritional and regulatory roles for branched-chain amino acids in milk production by lactating sows. PhD Dissertation. Texas A&M University, College Station, Texas, USA
Sangild PT, Sjostrom H, Noren O, Fowden AL, Silver M (1995) The prenatal development and glucocorticoid control of brush-border hydrolases in the pig small intestine. Pediatr Res 37:207–212
Steel RGD, Torrie JH (1980) Principles and procedures of statistics. McGraw-Hill
Urban RJ, Dillon EL, Choudhary S, Zhao Y, Horstman AM, Tilton RG, Sheffield-Moore M (2014) Translational studies in older men using testosterone to treat sarcopenia. Trans Am Clin Climatol Assoc 125:27–42
Vandewalle J, Luypaert A, De Bosscher K, Libert C (2018) Therapeutic mechanisms of glucocorticoids. Trends Endocrinol Metab 29:42–54
Wu G (1997) Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am J Physiol 272:G1382–G1390
Wu G (1998) Intestinal mucosal amino acid catabolism. J Nutr 128:1249–1252
Wu G (2018) Principles of animal nutrition. CRC Press
Wu G (2021) Amino acids: biochemistry and nutrition, 2nd edn. CRC Press, Boca Raton, Florida
Wu G, Knabe DA (1994) Free and protein-bound amino acids in sow’s colostrum and milk. J Nutr 124:415–424
Wu G, Knabe DA (1995) Arginine synthesis in enterocytes of neonatal pigs. Am J Physiol 269:R621-629
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Wu G, Knabe DA, Flynn NE (1994) Synthesis of citrulline from glutamine in pig enterocytes. Biochem J 299:115–121
Wu G, Knabe DA, Flynn NE, Yan W, Flynn SP (1996a) Arginine degradation in developing porcine enterocytes. Am J Physiol 271:G913–G919
Wu G, Meier SA, Knabe DA (1996b) Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr 126:2578–2584
Wu G, Davis PK, Flynn NE, Knabe DA, Davidson JT (1997) Endogenous synthesis of arginine plays an important role in maintaining arginine homeostasis in postweaning growing pigs. J Nutr 127:2342–2349
Wu G, Flynn NE, Knabe DA (2000) Enhanced intestinal synthesis of polyamines from proline in cortisol-treated piglets. Am J Physiol 279:E395-402
Wu G, Knabe DA, Kim SW (2004) Arginine nutrition in neonatal pigs. J Nutr 134:2783S-S2390
Wu G, Bazer FW, Johnson GA, Hou Y (2018) Arginine nutrition and metabolism in growing, gestating, and lactating swine. J Anim Sci 96:5035–5051
Wu G, Meininger CJ, McNeal CJ, Bazer FW, Rhoads JM (2021) Role of L-arginine in nitric oxide synthesis and health in humans. Adv Exp Med Biol 1332:167–188
Xu W, Liu LZ, Loizidou M, Ahmed M, Charles IA (2002) The role of nitric oxide in cancer. Cell Res 12:311–320
Yamada E, Wakabayashi Y (1991) Development of pyrroline-5-carboxylate synthase and N-acetylglutamate synthase and their changes in lactation and aging. Arch Biochem Biophys 291:15–23
Zhang Q, Hou YQ, Bazer FW, He WL, Posey EA, Wu G (2021) Amino acids in swine nutrition and production. Adv Exp Med Biol 1285:81–107
Acknowledgements
This work was supported by Texas A&M AgriLife Research (H-8200). We thank research assistants and students in our laboratory for technical assistance and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
This study was approved by the Institutional Animal Care and Use Committee of Texas A&M University.
Informed consent
No informed consent is required for this study.
Additional information
Communicated by J. M. Phang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dillon, E.L., Wu, G. Cortisol enhances citrulline synthesis from proline in enterocytes of suckling piglets. Amino Acids 53, 1957–1966 (2021). https://doi.org/10.1007/s00726-021-03039-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-021-03039-y