Abstract
A green, convenient, and efficient one-pot synthesis of a new class of spiro[indolinepyranopyrimidine] derivatives was achieved in good yields by the multi-component reaction of N-alkyl-1-(methylthio)-2-nitroethenamine derived from the addition of various amines to nitroketene dithioacetal with isatin and barbituric acid derivatives in water at reflux conditions. Notably, the present method offers desirable advantages including good yields, use of water as green solvent, absence of catalyst, simple workup procedure, and easy purification process with no chromatographic technique.
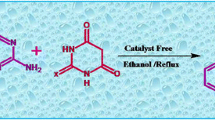
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Indole derivatives have been a topic of substantial research interest and continue to be one of the most active areas of heterocyclic chemistry. They exhibit a wide range of biological activities [1,2,3,4] such as antibacterial, antimicrobial, antiviral, antifungal, antihypertensive, anti-inflammatory [5], antitumor [6], anticancer, anti-HIV, antioxidant [7], antimalarial, anticonvulsant [8], and anti-alzheimer [9] properties. In addition, it was reported that sharing of the indole 3-carbon atom in the formation of spiroindole derivatives (Fig. 1) significantly improves biological properties [10]. Spiroindoles have generated considerable synthetic interest due to their occurrence in diverse natural products and notable biological activities [11,12,13,14,15,16,17,18,19,20,21].
Pyranopyrimidine derivatives are very important and valuable compounds, due to their potential importance in the medicine and biological fields [22]. They have diverse pharmacological properties such as antimalarial, antibacterial [23], antifungal, antiviral, antitumor [23, 24], antibronchitic [25, 26], anti-AIDS [27], antipyretic [28], anti-inflammatory [29], and antihypertensive [30] evaluation activities. Considering the above reports, the development of new and simple synthetic methods for the efficient preparation of the spiroindoles containing pyranopyrimidine fragment could potentially lead to a series of structurally and biologically interesting heterocycles.
During the past decades, specific strategies have been reported for the synthesis of spiroindole-annulated heterocycles. In 2010, Bazgir et al. reported an efficient, one-pot synthesis of spiro[chromenopyrimidineindoline] from cyclohexane-1,3-diones, isatins, and barbituric acids in refluxing water in the presence of p-TSA for 10 h (Scheme 1, entry a) [10, 13]. In 2015, Esmaeili et al. developed a rapid and convenient protocol for the synthesis of novel spiro-oxindole derivatives in excellent yields by the three-component reaction of malononitrile, isatin, and 2,3-dihydro-5H-[1,3]thiazolo[3,2-a]pyrimidine-5,7(6H)-dione in the presence of diisopropylethylamine (Scheme 1, entry b) [14]. In 2018, Deka et al. described a simple and cost-effective, micelle-catalyzed one-pot strategy for the synthesis of spiro[indolinepyranopyrazoles] by reacting isatins, malononitrile, and 3-methyl-1H-pyrazol-5(4H)-ones in water at room temperature (Scheme 1, entry c) [11]. Herein we report an environmentally benign synthesis of a new class of spiro[indolinepyranopyrimidine] derivatives via a catalyst free, one-pot, multi-component condensation reaction of various amines, nitroketene dithioacetal, isatin derivatives, and barbituric acids in refluxing water (Scheme 1, entry d).

Results and discussion
In this paper, we would like to report an easy and efficient procedure for synthesizing novel spiro[indolinepyranopyrimidine] derivatives. The products were obtained from the addition of various amines 1 to nitroketene dithioacetal 2 with isatin 3 and barbituric acid derivatives 4 in water as a green solvent at reflux conditions (Scheme 2).

Several solvents in the presence and absence of catalyst were examined to develop standard reaction conditions and the results are summarized in Table 1. Experimental results showed that the reaction proceeded very cleanly with good yield when the EtOH and water were used as solvent at reflux conditions without any catalyst (Table 1, entries 2 and 6). The yield of product was low, when the water was used as solvent at 60 °C and room temperature (Table 1, entries 7 and 8). Also the yield of product was low, when the reaction was performed in the presence of piperidine or Et3N as catalyst in EtOH (Table 1, entries 3 and 4). The reaction did not proceed well, when the CH3CN and DMF were used as solvent (Table 1, entries 9 and 11). Also the reaction did not work in CHCl3, so a lot of spots were observed on TLC (Table 1, entry 10).
As shown in Table 2, various primary amines, isatin derivatives, and barbituric acids were tolerated. The reaction proceeds cleanly under the same reaction conditions to afford a series of spiro[indolinepyranopyrimidine] derivatives 5a–5i in 64–81% yields.
The structures of compounds 5a–5i were elucidated from their mass, IR, and 1H and 13C NMR spectra. The IR spectrum of 5a showed absorption bonds due to the NH groups at 3267 and 3194 cm−1, and C=O groups at 1721 and 1615 cm−1. Stretching frequencies related to the Ar and NO2 groups appeared at 1528, 1463, and 1384 cm−1, respectively. The 1H NMR spectrum of 5a exhibited a doublet recognized as arising from the CH3 group (δ = 3.14 ppm, 3JHH = 5.1 Hz), one AB quartet due to CH2 group (4.85 ppm), one multiplet for NHCH3 group (10.55 ppm) and two singlets for NH groups (11.19 and 12.58 ppm), together with characteristic signals for the aromatic moiety (6.55–7.51 ppm). 1H-decoupled 13C NMR spectrum showed 20 distinct signals in agreement with the proposed structure. Resonances due to CH3, CH2, spiro carbon and three C=O groups appeared at δ = 29.3, 44.6, 48.2, 156.9, 161.3, and 175.7 ppm, respectively.
A plausible mechanistic pathway for the formation of 5 is outlined in Scheme 3. Initially, the Knoevenagel condensation between isatin 3 and barbituric acid 4 derivatives affords 7 which undergoes Michael addition with N-alkyl-1-(methylthio)-2-nitroethenamine 6 (derived from the addition of various amines 1 to nitroketene dithioacetal 2) to give 8. Thus the intermediate 8 undergoes imine-enamine tautomerisation to form 9 followed by O-cyclization to form 5 via the elimination of MeSH (Scheme 3).

Conclusion
In conclusion, we have developed a simple, green and novel one-pot, multi-component synthesis of spiro[indolinepyranopyrimidine] derivatives, through sequential Knoevenagel condensation, Michael addition, and O-cyclization sequences in refluxing water, without any catalyst. This procedure offers several advantages, such as use of water as a green solvent, good yields of products, easy accessibility of reactants, easy workup procedure, and high atom economy.
Experimental
The various amines, nitroketene dithioacetal, isatin, barbituric acid, and other chemicals and solvents were obtained from Merck and Aldrich and were used without further purification. NMR spectra were recorded with a Bruker DRX-300 Avance instrument (300 MHz for 1H and 75.4 MHz for 13C) with DMSO-d6 and CDCl3 as solvent. Chemical shifts are given in ppm (δ), and coupling constant (J) are reported in hertz (Hz). Melting points were measured with an electrothermal 9100 apparatus. Mass spectra were recorded with an Agilent 5975C VL MSD with Triple-Axis Detector operating at an ionization potential of 70 eV. IR spectra were measured with Bruker Tensor 27 spectrometer. Elemental analyses for C, H, and N were performed using a PerkinElmer 2004 series [II] CHN elemental analyzer.
General procedure for the synthesis of product 5
A mixture of various amines (1 mmol), 0.165 gnitroketene dithioacetal (1 mmol) and 10 cm3 H2O in a 50 cm3 flask was refluxed for 6 h. After completion of the reaction (monitored by TLC, ethyl acetate/n-hexane, 6:4), isatin derivatives (1 mmol) and barbituric acids (1 mmol) were added to the reaction mixture, and it was stirred under reflux for 7–10 h. Then, the reaction mixture was cooled to room temperature and filtered to give the crude product. The solid was washed with water to give pure product 5 in good yield.
1-Benzyl-7′-(methylamino)-6′-nitrospiro[indoline-3,5′-pyrano[2,3- d ]pyrimidine]-2,2′,4′(1′ H ,3′ H )-trione (5a, C 22 H 17 N 5 O 6 )
White solid; m.p.: 308–310 °C (dec.); yield: 0.304 g (68%); IR (KBr): \(\bar{\nu }\) = 3267 and 3194 (NH), 1721 (C=O), 1615 (C=O), 1528 (Ar), 1463 and 1384 (NO2) cm−1; MS (EI, 70 eV): m/z (%) = 447 (M+, 11), 390 (9), 347 (5), 299 (30), 247 (10), 169 (8), 91 (100), 51 (2); 1H NMR (300 MHz, DMSO-d6): δ = 3.14 (d, 3JHH = 5.1 Hz, 3H), 4.85 (AB q, 2H), 6.55 (d, 3JHH = 7.5 Hz, 1H), 6.86 (t, 1H), 7.07 (t, 1H), 7.21–7.30 (m, 4H), 7.51 (d, 3JHH = 7.2 Hz, 2H), 10.55 (m, 2H), 11.19 (s, 1H), 12.58 (br s, 1H) ppm; 13C NMR (75.4 MHz, DMSO-d6): δ = 29.3 (NHCH3), 44.6 (CH2), 48.2 (C-spiro), 89.3, 107.5, 108.4, 122.2, 123.3, 127.4, 127.6, 128.6, 128.7, 130.3, 137.1, 145.6, 149.3, 151.9, 156.9 (C=O), 161.3 (C=O), 175.7 (C=O) ppm.
1-Methyl-7′-(methylamino)-6′-nitrospiro[indoline-3,5′-pyrano[2,3- d ]pyrimidine]-2,2′,4′(1′ H ,3′ H )-trione (5b, C 16 H 13 N 5 O 6 )
White solid; m.p.: 320–322 °C (dec.); yield: 0.237 g (64%); IR (KBr): \(\bar{\nu }\) = 3246 (NH), 1711 (C=O), 1656 (C=O), 1533 (Ar), 1467 and 1388 (NO2) cm−1; MS (EI, 70 eV): m/z (%) = 371 (M+, 60), 310 (44), 284 (25), 240 (11), 211 (100), 171 (83), 143 (18), 114 (24), 91 (30), 57 (17); 1H NMR (300 MHz, DMSO-d6): δ = 3.11 (s, 6H), 6.87–7.19 (m, 4H), 10.48 (m, 1H), 11.11 (s, 1H), 12.55 (br s, 1H) ppm; 13C NMR (75.4 MHz, DMSO-d6): δ = 26.9 (CH3), 29.2 (CH3), 48.0 (C-spiro), 89.3, 107.4, 107.7, 122.0, 123.1, 128.7, 130.3, 146.2, 149.3, 151.8, 156.9 (C=O), 161.0 (C=O), 175.4 (C=O) ppm.
7′-(Methylamino)-6′-nitrospiro[indoline-3,5′-pyrano[2,3- d ]pyrimidine]-2,2′,4′(1′ H ,3′ H )-trione (5c, C 15 H 11 N 5 O 6 )
White solid; m.p.: 354–356 °C (dec.); yield: 0.257 g (72%); IR (KBr): \(\bar{\nu }\) = 3382 and 3235 (NH), 1725 (C=O), 1693 (C=O), 1531 (Ar), 1472 and 1325 (NO2) cm−1; MS (EI, 70 eV): m/z (%) = 357 (M+, 39), 313 (9), 283 (63), 240 (100), 197 (94), 168 (85), 140 (55), 103 (35), 57 (69); 1H NMR (300 MHz, DMSO-d6): δ = 3.09 (s, 3H), 6.67–7.12 (m, 4H), 10.45 (s, 2H), 11.10 (s, 1H), 12.45 (br s, 1H) ppm; 13C NMR (75.4 MHz, DMSO-d6): δ = 29.2 (NHCH3), 48.4 (C-spiro), 89.4, 107.8, 108.9, 121.3, 123.3, 128,5, 131.0, 144.8, 149.4, 151.8, 156.9 (C=O), 161.1 (C=O), 176.7 (C=O) ppm.
7′-(Isopropylamino)-6′-nitrospiro[indoline-3,5′-pyrano[2,3- d ]pyrimidine]-2,2′,4′(1′ H ,3′ H )-trione (5d, C 17 H 15 N 5 O 6 )
White solid; m.p.: 255–260 °C (dec.); yield: 0.288 g (75%); 1H NMR (300 MHz, DMSO-d6): δ = 1.13 (d, 3H), 1.30 (d, 3H), 4.15–4.30 (m, 1H), 6.60–7.05 (m, 4H), 7.60 (br s, 1H), 9.30 (s, 1H), 10.15 (s, 1H), 10.65 (d, 1H) ppm; 13C NMR (75.4 MHz, DMSO-d6): δ = 20.8 (CH3), 22.8 (CH3), 44.6 (CH), 49.5 (C-spiro), 85.0, 108.4, 108.6, 120.7, 122.4, 127.5, 132.8, 144.9, 157.8, 158.4, 161.3 (C=O), 163.8 (C=O), 178.1 (C=O) ppm.
7′-(Ethylamino)-6′-nitrospiro[indoline-3,5′-pyrano[2,3- d ]pyrimidine]-2,2′,4′(1′ H ,3′ H )-trione (5e, C 16 H 13 N 5 O 6 )
White solid; m.p.: 306–312 °C (dec.); yield: 0.259 g (70%); 1H NMR (300 MHz, DMSO-d6): δ = 1.24 (t, 3JHH = 6.9 Hz, 3H), 3.51–3.59 (m, 2H), 6.68–7.12 (m, 4H), 10.47 (s, 1H), 10.59 (t, 3JHH = 5.7 Hz, 1H), 11.12 (s, 1H) ppm; 13C NMR (75.4 MHz, DMSO-d6): δ = 15.6 (CH3), 37.5 (CH2), 48.4 (C-spiro), 89.4, 107.6, 108.9, 121.3, 123.3, 128.5, 131.0, 144.8, 149.3, 151.7, 156.4 (C=O), 161.1 (C=O), 176.7 (C=O) ppm.
1′,3′-Dimethyl-7′-(methylamino)-6′-nitrospiro[indoline-3,5′-pyrano[2,3- d ]pyrimidine]-2,2′,4′(1′ H ,3′ H )-trione (5f, C 17 H 15 N 5 O 6 )
White solid; m.p.: 287–289 °C (dec.); yield: 0.312 g (81%); IR (KBr): \(\bar{\nu }\) = 3431 and 3192 (NH), 1726 (C=O), 1687 (C=O), 1455 and 1355 (NO2) cm−1; MS (EI, 70 eV): m/z (%) = 385 (M+, 53), 339 (55), 324 (100), 280 (37), 228 (24), 197 (41), 157 (31), 114 (22), 58 (35); 1H NMR (300 MHz, DMSO-d6): δ = 3.00 (s, 3H), 3.16 (d, 3JHH = 4.8 Hz, 3H), 3.45 (s, 3H), 6.69–7.11 (m, 4H), 10.48 (s, 1H) ppm; 13C NMR (75.4 MHz, DMSO-d6): δ = 28.2 (NHCH3), 29.4 (NCH3), 30.0 (NCH3), 48.9 (C-spiro), 90.0, 107.8, 108.9, 121.2, 123.4, 128.6, 130.9, 144.9, 149.7, 150.7, 156.6 (C=O), 159.0 (C=O), 176.6 (C=O) ppm.
7′-(Benzylamino)-6′-nitrospiro[indoline-3,5′-pyrano[2,3- d ]pyrimidine]-2,2′,4′(1′ H ,3′ H )-trione (5 g, C 21 H 15 N 5 O 6 )
Orange solid; m.p.: 240–242 °C (dec.); yield: 0.294 g (68%); IR (KBr): \(\bar{\nu }\) = 3422 and 3217 (NH), 1702 (C=O), 1687 (C=O), 1641 (C=O), 1605 (Ar), 1515 and 1326 (NO2), 1461 and 1388 (NO2) cm−1; MS (EI, 70 eV): m/z (%) = 433 (M+, 2), 417 (6), 283 (9), 240 (20), 197 (19), 168 (25), 133 (45), 91 (100), 51 (19); 1H NMR (300 MHz, DMSO-d6): δ = 4.63 (ABX, JAB = 66 Hz, JAX = JBX = 5.7 Hz, δA = 4.52 and δB = 4.74 ppm, 2H), 7.18–7.72 (m, 9H), 8.20 (t, 1H), 9.37 (t, 3JHH = 5.7 Hz, 1H) ppm; 13C NMR (75.4 MHz, DMSO-d6): δ = 43.2 (CH2), 44.4 (C-spiro), 118.5, 124.3, 126.6, 127.1, 127.6, 127.9, 128.1, 128.6, 128.8, 129.8, 133.6, 138.8, 140.1, 141.7, 147.9, 149.0, 163.5 (C=O) ppm.
7′-(Benzylamino)-1′,3′-dimethyl-6′-nitrospiro[indoline-3,5′-pyrano[2,3- d ]pyrimidine]-2,2′,4′(1′ H ,3′ H )-trione (5 h, C 19 H 19 N 5 O 6 )
White solid; m.p.: 306–312 °C (dec.); yield: 0.309 (67%); 1H NMR (300 MHz, DMSO-d6): δ = 2.96 (s, 3H), 3.23 (s, 3H), 4.82 (A2X, d, JAX = 6.0 Hz, δA = 4.82, 2H), 6.70–7.43 (m, 9H), 10.53 (s, 1H), 10.98 (t, 3JHH = 6.0 Hz, 1H) ppm; 13C NMR (75.4 MHz, DMSO-d6): δ = 28.2 (NCH3), 29.9 (NCH3), 45.9 (CH2), 48.9 (C-spiro), 90.0, 108.2, 109.0, 121.3, 123.4, 127.2, 128.0, 128.7129.2, 130.8, 137.6, 144.9, 149.6, 150.6, 156.2 (C=O), 159.0 (C=O), 176.6 (C=O) ppm.
7′-(Benzylamino)-6′-nitro-2′-thioxo-2′,3′-dihydrospiro[indoline-3,5′-pyrano[2,3- d ]pyrimidine]-2,4′(1′ H )-dione (5i, C 21 H 15 N 5 O 5 S 5 )
Orange solid; m.p.: 240–242 °C (dec.); yield: 0.314 g (70%); 1H NMR (300 MHz, DMSO-d6): δ = 4.63 (ABX, JAB = 65 Hz, JAX = JBX = 5.4 Hz, δA = 4.52 and δB = 4.74, 2H), 7.18–7.67 (m, 11H), 8.17 (br s, 1H), 9.35 (br s, 1H) ppm; 13C NMR (75.4 MHz, DMSO-d6): δ = 43.2 (CH2), 44.5 (C-spiro), 118.5, 124.3, 126.6, 127.1, 127.6, 127.9, 128.1, 128.6, 128.8, 129.8, 133.6, 138.8, 140.1, 141.7, 147.9, 149.0, 163.5 (C=S) ppm.
References
Humphrey GR, Kuethe JT (2006) Chem Rev 106:2875
Akhlaghi MF, Amidi S, Esfahanizadeh M, Daeihamed M, Kobarfard F (2014) Iran J Pharm Res 13:35
Inman M, Moody CJ (2013) Chem Sci 4:29
Liu H, Domling A (2009) J Org Chem 74:6895
Sachdeva H, Saroj R, Dwivedi D (2014) Sci World J 2014:1
Hassaneen HME, Pagni RM (2010) Z Naturforsch 65b:1491
Darehkordi A, Rahmani F, Hashemi V (2013) Tetrahedron Lett 54:4689
Singh N, Kumar K (2017) Am Inst Phys 1860:020058
Ismail MM, Kamel MM, Mohamed LW, Faggal SI (2012) Molecules 17:4811
Jadidi K, Ghahremanzadeh R, Bazgir A (2009) J Comb Chem 11:341
Devi J, Kalita SJ, Deka DC (2018) ChemistrySelect 3:1512
Abdel-Rahman AH, Keshk EM, Hanna MA, El-Bady SM (2004) Bioorg Med Chem 12:2483
Khanna P, Panda SS, Khanna L, Jain SC (2014) Mini Rev Org Chem 11:73
Esmaeili AA, Amini-Ghalandarabad S, Mesbah F, Tasmimi M, Izadyar M, Fakhari AR, Salimi AR (2015) Tetrahedron 71:2458
Ghahremanzadeh R, Sayyafi M, Ahadi S, Bazgir A (2009) J Comb Chem 11:393
Ghahremanzadeh R, Amanpour T, Bazgir A (2010) J Heterocycl Chem 47:46
Kalita SJ, Das B, Deka DC (2017) ChemistrySelect 2:5701
Chen C, Lv C, Liang J, Jin J, Wang L, Wu C, Shen R (2017) Molecules 22:1295
Niknam K, Piran A, Karimi Z (2016) J Iran Chem Soc 13:859
Sun J, Gong H, Sun Y, Yan CG (2013) Mol Divers 17:627
Wang PF, Chen C, Chen H, Han LS, Liu L, Sun H, Wen X, Xu QL (2017) Adv Synth Catal 359:2339
Kazemi M, Shiri L, Kohzadi H (2017) J Mater Environ Sci 8:3410
Ziarani GM, Faramarzi S, Lashgari N, Badiei A (2014) J Iran Chem Soc 11:701
Bhat AR, Shalla AH, Dongr RS (2017) J Saudi Chem Soc 21:S305
Yu J, Wang H (2005) Synth Commun 35:3133
Ziarani GM, Faramarzi S, Asadi S, Badiei A, Bazl R, Amanlou M (2013) Daru J Pharm Sci 21:3
Maleki N, Shakarami Z, Jamshidian S, Nazari M (2016) Acta Chem Iasi 24:20
Abdel-Razik HH (2003) J Chin Chem Soc 50:887
Reheim MAMA, Hafiz ISA, Elian MA (2016) Heterocycl Commun 22:311
Bhat AR, Shalla AH, Dongre RS (2016) J Taibah Univ Sci 10:9
Acknowledgements
We gratefully acknowledge the financial support of this research from Imam Khomeini International University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghadiri, S., Bayat, M. & Hosseini, F.S. A simple and environmentally benign synthesis of novel spiro[indoline-3,5′-pyrano[2,3-d]pyrimidine] derivatives in water. Monatsh Chem 150, 1079–1084 (2019). https://doi.org/10.1007/s00706-019-2356-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-019-2356-6