Abstract
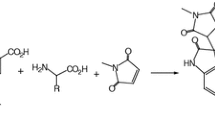
Piperidinium spirooxindoline-pyridineolate has been prepared via one-pot multicomponent reaction of isatin, malononitrile, cyanoacetohydrazide, and piperidine in water or ethanol medium at room temperature. In addition, the synthesis of two indole-substituted 2-pyridones from indole-3-carbaldehyde, malononitrile, and cyanoacetohydrazide in the presence of piperidine is described.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Performing reactions in water as a green medium instead of toxic organic solvents in organic synthesis is raised as one of the main principles of green chemistry due to decreasing release of toxic solvents in the environment and consequently decreasing pollution, especially in the drug development process [1]. Performing a synthetic reaction in an aqueous medium provides many benefits, including enhancement of the reactivity and selectivity of synthetic reactions, high product yield, convenient purification methods, enhanced rate of reaction, shorter reaction times, mild reaction conditions, and no need to protect groups in drug synthesis [2]. The selectivity and increasing rate of organic reactions in water are due to hydrophobic interactions of water with organic precursors. Therefore, it is recognized that water is a noteworthy solvent for various organic synthetic procedures and many MCRs have been introduced in the aqueous medium [3, 4]. There are various articles related to the formation of spirooxindole scaffolds in water, but most of them need the use of different kinds of catalysts [5, 6].
Spirooxindoles are valuable therapeutic cores with unique structure that have attracted wonderful attention from academic and industry specialists due to their rigidity, unique three-dimensional stereochemistry which leads to a good affinity of them to three-dimensional proteins [7,8,9], and a broad range of biological properties including anticancer [7, 8], antiviral activities [9], anticonvulsant, antimicrobial, potential antileukemic, antidiabetic, and local anesthetic [10,11,12], which can also be applied as a synthetic intermediate for the synthesis of several kinds of drugs or drug precursors. Some selected medicinal compounds comprising a spirooxindole scaffold are shown in Fig. 1; for instance, cipargamin displays an antimalarial agent [13], polycyclic alkaloid mitraphylline has been found as an anti-inflammatory agent [14], spirotryprostatin A and spirotryprostatin B exhibit microtubule assembly inhibition [15], and pteropodine and isopteropodine reduce the action of muscarinic serotonin receptors [16]. Due to the significant medicinal value and structural uniqueness of these attractive synthetic targets, numerous synthetic methods were reported on the synthesis of spirooxindoles using a variety of substances [12, 17,18,19,20,21], but most of the previous methods used toxic solvents, stoichiometric catalysts, and transition metals. In the past 2 years, significant progress has been acquired in the reported synthetic procedures for these valuable libraries of N-heterocycles [22].
On the other hand, in medicinal science, half of all medicines are supplied as salts. Therefore, finding the appropriate salt for the medicinal purpose is the key step in the preclinical process of drug production. Since the salt formation of pharmaceutically active substances improves the physical properties, the synthesis of drugs in the form of salt has become significantly more important in the pharmaceutical industry [23,24,25,26]. One of the scaffolds most commonly used for the preparation of salt of drugs is piperidine.
According to our studies, the synthesis of organic piperidinium salts containing spirooxindole cores has not been explored unless piperidinium salt has been used as a catalyst in its synthesis [27,28,29]. Therefore, in the continuation of our attempts to construct important heterocyclic products [30,31,32], we describe here the synthesis of novel organic salts, namely piperidin-1-ium 1',2'-diamino-3',5'-dicyano-2-oxo-1'H-spiro[indoline-3,4'-pyridin]-6'-olate, that is, the presence of spirooxindole and pyridine as heterocyclic structures in a single compound often increases the biological properties of each other significantly. The reaction proceeds through a one-pot, multicomponent condensation between isatin, cyanoacetohydrazide, malononitrile, and piperidine in green synthetic conditions. Cyanoacetohydrazide as valuable synthetic unit recently has attracted the most consideration, providing versatile and suitable precursors for the formation of a broad spectrum of polyfunctional organic products. Since N and C centers have nucleophilic properties, this precursor can be used as an ambident nucleophile [33].
Results and discussion
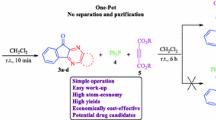
Regarding the biological importance of spirooxindoline scaffolds in medicinal science, we describe a rapid and efficient procedure for the construction of an ionic compound containing a piperidinium cation and a spirooxindoline pyridine olate anion. This piperidinium salt is obtained through a one-pot, four-component condensation of malononitrile, isatin, cyanoacetohydrazide, and piperidine as one of the reactants in stoichiometric ratio which can also act as a promoter in H2O/EtOH at ambient temperature in 5–120 min, with 82–99% yield (Scheme 1). As regards that the reaction between cyanoacetohydrazide and isatin results in imine formation, it in continuation could be cyclized via involving C=N group and enolic OH to produce the oxadiazolo-2-spiroindoline compound [34] and prevents the formation of the expected product 5; therefore, at first, the reaction of isatin and malononitrile was performed, and then cyanoacetohydrazide and piperidine were added. Since the product’s structure is a combination of spirooxindole and pyridine, it probably inherits the biological properties of both pharmacophore moieties. The good to excellent yield and the regioselectivity of this procedure distinguished the reaction as a novel protocol for the formation of spirooxindole pyridine in the anionic form with various functional groups via the use of isatin derivatives.
In Scheme 2, a rational mechanism is proposed for this one-step four-component reaction. It is possible that the first step is the formation of Michael acceptor I that occurs through the Knoevenagel condensation between isatin 1 and malononitrile 2, removing the water molecule as confirmed by the formation of a precipitate. Then, the nucleophilic attack of anion form of cyanoacetohydrazide 3' (generated using piperidine) as an activated C–H compound to the Knoevenagel intermediate I in a Michael addition type reaction produces intermediate II, which after consecutive enol–keto tautomerization in the presence of piperidine as a promoter generates a piperidinium intermediate III. In path a, the resulting salt intermediate III undergoes intramolecular N-cyclization via attack of the amido-NH nitrogen atom to nitrile group generates intermediate IV, that continues by imine–enamine tautomerization leading to final compound 5, that pyridine remains in the form of pyridinium cation near to anionic heterocyclic nucleus via ionic interaction. The spectral data analyses showed that product 6 of path b is formed by nucleophilic addition of hydrazine-NH2 to nitrile group which is expected to be stronger nucleophile compared to the amido-NH nitrogen atom and led to the formation of the seven-membered ring instead of six-membered ring (one singlet broad peak is observed for NH2 attached to N in δ = 4.64 ppm and D2O exchangeable) [35, 36]. So the described four-component reaction shows high regioselectivity for the formation of product 5 (Scheme 2). Generally, the mechanistic studies indicate Knoevenagel condensation/Michael addition/enol–keto tautomerism/N-cyclization/imine–enamine tautomerism sequential processes, in which Michael acceptor is produced in a separate step as the key intermediate.
In the following, the reaction of isatin 1, malononitrile 2, and cyanoacetohydrazide 3 was selected as a pattern for survey-optimized reaction conditions (Table 1). The study started by assessing the reaction without using piperidine as component 4 or in the presence of piperidine 4 in the amount of 0.5 mmol in ethanol at ambient temperature; no conversion happened during 24 h (entries 1 and 2, respectively). But, when the reaction was done using piperidine 4 in the amount of 1.0 mmol in ethanol at room temperature, the desired product precipitated from the solvent and was obtained as an ionic salt in 90% yield and in the time of 60 min (entry 3). In another attempt, another type of basic catalyst (Et3N) was added to the original mixture, but the target compound did not form, and a number of spots appeared on TLC (entry 4). The effect of temperature in reaction has been surveyed using piperidine (1.0 mmol) at reflux for 24 h, and the target compound did not form (entry 5). The choice of solvent was found to be crucial for this condensation. When polar solvents such as ethanol, acetonitrile, and water were applied as the reaction solvent (entries 3, 6, and 7), the reaction gave the desired product in acceptable efficiency due to good solubility of precursors. Among these polar solvents, water could significantly improve both the reaction rate and the product yield (entry 6). It is worth noting that in the case of derivatives 5d–i, the ethanol solvent is more suitable and obtained desired products with greater efficiency.
After the reaction optimization, we investigated the reaction scope using N- and 5-substituted isatins (Fig. 2). The reactions perform cleanly and completely using various precursors to produce a library of spiro compounds 5a–i in 82–99% yields. The existence of chlorine or brome substituents on the aromatic ring of the isatin affects the process and obtained expected compounds for a long time with less efficiency. We also used ethyl cyanoacetate, methyl cyanoacetate, and cyanoacetamide as active methylene structures other than malononitrile to enhance the variety of the products structurally, but the reaction did not proceed.
The structure of the final products was confirmed by spectroscopic data, including 1H NMR, 13C NMR, FT-IR, and mass spectra. The mass spectrum of product 5a has shown a molecular ion peak with the removal of piperidine at m/z 294 value, which was in accordance with the offered structure. The 1H NMR spectrum of 5a displayed two multiplets for the CH2 groups of piperidine (δ 1.53–1.62 ppm for 6H of CH2 and δ 2.95–2.99 ppm for 4H of NCH2), one multiplet for 2H of the +NH2 group of piperidinium (δ 3.40–4.09 ppm, D2O exchangeable) that is located under the peak of water, one singlet broad peak for NH2 attached to N (δ 4.64 ppm, D2O exchangeable), one singlet broad for the NH2 group (δ 6.00 ppm, D2O exchangeable), the aromatic region of the spectrum for the aromatic moieties (δ 6.70–7.11 ppm), and one singlet broad for the NH group of isatin (δ 9.89 ppm, D2O exchangeable). In the 1H-decoupled 13C NMR spectrum of 5a, the appearance of one peak at δ 163.0 ppm was specified as one amide carbonyl group, two nitrile-attached carbons show two different chemical shifts (δ 50.8, 52.3 ppm), and the special peaks of Cspiro,=CNH2, and=C–O−, specified at δ 56.5, 156.3, and 181.6 ppm, verified the selective synthesis of 5a. In 1H-decoupled 13C NMR spectrum of some derivatives, special peaks of Cspiro and one of the CN groups did not appear due to the quaternary C-atom.
In the following, a new one-pot three-component reaction developed for the formation of 1,6-diamino-4-(1H-indol-3-yl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile 8 via reaction of indole-3-carbaldehyde 7, malononitrile 2, and cyanoacetohydrazide 3 using piperidine as a Brønsted–Lowry basic catalyst in H2O or EtOH as reaction medium at room temperature condition (Scheme 3). When indole-3-carbaldehyde 7 was applied as the carbonyl-containing precursor instead of isatin, the reaction could result in the oxidized indole oxopyridine derivatives, but if we use isatin, the dehydration operation at the end does not occur and the enol–keto tautomerization directs to the respective piperidinium spirooxindoline pyridine olate anion.
A rational mechanism for the construction of indole oxopyridine 8 is proposed in Scheme 4. Initially, the reaction of activated malononitrile 2 with indole-3-carbaldehyde 7 using piperidine as a catalyst results in the Knoevenagel intermediate VI. The methylene carbon of cyanoacetohydrazide 3 is activated with the piperidine and undergoes Michael type reaction with Knoevenagel intermediate VI, producing intermediate VII which undergoes intramolecular cyclization via nucleophilic addition of –NH to nitrile group that affords the corresponding intermediate VIII. Consecutive imine–enamine tautomerization that continues with dehydrogenation produces the expected product 8. In the case of product 8, due to the stabilization of oxopyridine core via the resonance with indole, this structure tends to oxidize and generate a double bond using air oxidation. The spectral data analyses showed that product 9 of path b due to nucleophilic addition of hydrazine-NH2 group to the nitrile, which is expected to be stronger nucleophile compared to the amido-NH nitrogen atom for the formation of a seven-membered ring, was not formed (Scheme 4) [35, 36]. Also in this work, indole-3-carboxaldehyde was used instead of isatin, which led to products 8a–b with moderate efficiency (Fig. 3).
Conclusion
In this paper, we have described a new and highly efficient method for the synthesis of piperidin-1-ium 1',2'-diamino-3',5'-dicyano-2-oxo-1'H-spiro[indoline-3,4'-pyridin]-6'-olate as an organic salt by reacting isatin, malononitrile, cyanoacetohydrazide, and piperidine in H2O or EtOH at room temperature under catalyst-free conditions. Furthermore, indole-3-carboxaldehyde was used instead of isatin, which led to indole-substituted oxopyridine with moderate efficiency. The use of various isatin derivatives in an eco-friendly method produced a range of skeletally distinct and biologically important organic salt with spirooxindole-based heterocyclic structure.
Experimental
General
The different isatin, malononitrile, cyanoacetohydrazide, piperidine, indole-3-carbaldehyde, triethylamine, and solvents were purchased from Sigma-Aldrich and Fluka Co. consumed without further purification. IR spectra: Bruker Tensor 27 spectrometer. NMR spectra: Bruker DRX-300 Avance instrument (300 MHz for 1H and 75.4 MHz for 13C) with DMSO-d6 as solvents. Chemical shifts are represented in parts per million (ppm), and coupling constants (J) are stated in hertz (Hz). Mass spectra: Agilent 5975C VL MSD with Triple-Axis detector operating at an ionization potential of 70 eV. Elemental analyses were carried out using a PerkinElmer 2004 series [II] CHN elemental analyzer.
General method for the synthesis of 5a–i
Isatin derivatives (1.0 mmol), malononitrile (0.066 g 1.0 mmol), and 10 mL solvent (as reported in Table 2) were added in a 50-mL round-bottomed flask, and mixture was magnetically stirred at room temperature. After the end of the first stage (5 min) which is confirmed by the appearance of precipitate, cyanoacetohydrazide (0.148 g, 1.0 mmol) and piperidine (108 µL, 1.0 mmol) were added. The solution was stirred at ambient temperature for a time as indicated in Fig. 2. The completion time of reaction was defined by TLC (ethyl acetate/n-hexane, 5:5). In purification step, the acquired solid was filtered and washed with H2O or EtOH and eventually dried in the oven in 100 °C to obtain compounds 5a–i.
General method for the synthesis of 8a–b
A mixture of indole-3carbaldehyde derivatives (1.0 mmol), malononitrile (0.066 g 1.0 mmol), cyanoacetohydrazide (0.148 g, 1.0 mmol), piperidine (20% mol), and 10 mL solvent (as reported in Table 3) in a 50-mL round-bottomed flask was stirred for a time indicated in Fig. 3 and controlled by TLC (ethyl acetate/n-hexane, 5:5). In the following, the precipitate was filtered to obtain the raw compound. The raw product was purified by washing with H2O or EtOH and dried in an oven in 150 °C to give compound 6 and verified by 1H NMR and 13C NMR.
Piperidin-1-ium 1',2'-diamino-3',5'-dicyano-2-oxo-1'H-spiro[indoline-3,4'-pyridin]-6'-olate (5a): White solid, m.p.: 249–250 °C, yield 0.375 g (99%); IR (KBr) (νmax/cm−1): 3460, 3357, 3320 (NH2 and NH), 2825–3031 (NH2+), 2166 (CN), 1707 (C=O), 1612, 1569 (C=C), 1352 (C–N), 1161 (C–O), 734 (Ar). 1H NMR (300 MHz, DMSO-d6): δ 1.53–1.62 (6H, m, CH2), 2.95–2.99 (4H, m, NCH2), 3.40–4.09 (2H, m, +NH2, D2O exchangeable), 4.64 (2H, br s, N–NH2, D2O exchangeable), 6.00 (2H, br s, NH2, D2O exchangeable), 6.70 (1H, d, 3JHH = 7.5 Hz, ArH), 6.92 (1H, d, 3JHH = 7.2 Hz, ArH), 7.06–7.11 (2H, m, ArH), 9.89 (1H, br s, NH, D2O exchangeable). 13C NMR (75.4 MHz, DMSO-d6): δ 22.3 (CH2), 22.9 (2CH2), 44.4 (2NCH2), 50.8, 52.3 (2C–CN), 56.5 (Cspiro), 109.4, 109.5 (2CN), 122.0, 122.1, 125.0, 128.0. 136.9, 141.5 (Ar), 156.3 (=CNH2), 163.0 (C=O), 181.6 (=C–O−); MS (EI, 70 eV): m/z (%) = 294 (15) [M—piperidine]+, 195 (36), 170 (17), 133 (28), 104 (40), 84 (100), 56 (82), 44 (85). Anal. Calcd for C19H21N7O2 (379.18): C, 60.15; H, 5.58; N, 25.84 Found C, 59.95; H, 5.51; N, 26.10.
Piperidin-1-ium 1',2'-diamino-5-chloro-3',5'-dicyano-2-oxo-1'H-spiro[indoline-3,4'-pyridin]-6'-olate (5b): White solid, m.p.: 255–257 °C, yield 0.392 g (95%); IR (KBr) (νmax/cm−1): 3462, 3357, 3327 (NH2 and NH), 2846–3040 (NH2+), 2159 (CN), 1708 (C=O), 1617, 1568 (C=C), 1347 (C–N), 1159 (C–O), 734 (Ar), 690 (C–Cl). 1H NMR (300 MHz, DMSO-d6): δ 1.52–1.61 (6H, m, CH2), 2.95–3.58 (4H, m, NCH2), 3.15–3.76 (2H, m, +NH2), 4.58 (2H, br s, N–NH2), 6.00 (2H, br s, NH2), 6.70 (1H, d, 3JHH = 8.1 Hz, ArH), 7.03 (1H, s, ArH), 7.10 (1H, d, 3JHH = 8.1 Hz, ArH), 9.99 (1H, br s, NH). 13C NMR (75.4 MHz, DMSO-d6): δ 22.2 (CH2), 22.8 (2CH2), 44.3 (2NCH2), 51.4, 51.5 (2C–CN), 110.8, 110.9 (2CN), 122.1, 124.9, 126.1, 127.9, 134.1, 140.4 (Ar), 156.4 (=CNH2), 162.9 (C=O), 181.8 (=C–O−); MS (EI, 70 eV): m/z (%) = 329 (0.09) [M + 1]+, 328 (0.2) [M—piperidine]+, 140 (7), 84 (30), 66 (100), 41 (33). Anal. Calcd for C19H21ClN7O2 (413.14): C, 55.14; H, 4.87; N, 23.69 Found C, 54.95; H, 5.21; N, 24.06.
Piperidin-1-ium 1',2'-diamino-5-bromo-3',5'-dicyano-2-oxo-1'H-spiro[indoline-3,4'-pyridin]-6'-olate (5c): White solid, m.p.: 261–263 °C, yield 0.411 g (90%); IR (KBr) (νmax/cm−1): 3461, 3355, 3324 (NH2 and NH), 2733–3077 (NH2+), 2155 (CN), 1707 (C=O), 1615, 1568 (C=C), 1343 (C–N), 1156 (C–O) 734 (Ar), 628 (C–Br). 1H NMR (300 MHz, DMSO-d6): δ 1.52–1.61 (6H, m, CH2), 2.84–2.99 (4H, m, NCH2), 3.25–3.82 (2H, m, +NH2), 4.57 (2H, br s, N–NH2), 5.99 (2H, br s, NH2), 6.66 (1H, d, 3JHH = 8.4 Hz, ArH), 7.13 (1H, s, ArH), 7.24 (1H, d, 3JHH = 7.5 Hz, ArH), 9.99 (1H, br s, NH). 13C NMR (75.4 MHz, DMSO-d6): δ 22.2 (CH2), 22.8 (2CH2), 44.3 (2NCH2), 51.4, 51.5 (2C-CN), 111.4, 113.8 (2CN), 119.0, 127.5, 127.6, 130.6, 139.5, 140.8 (Ar), 156.3 (=CNH2), 162.8 (C=O), 181.2 (=C–O−); MS (EI, 70 eV): m/z (%) = 374 (0.02) [M + 2]+, 372 (0.03) [M—piperidine]+, 152 (4), 84 (100), 70 (9), 56 (39), 42 (23).
Piperidin-1-ium 1',2'-diamino-1-benzyl-3',5'-dicyano-2-oxo-1'H-spiro[indoline-3,4'-pyridin]-6'-olate (5d): Yellow solid, m.p.: 208 °C, yield 0.445 g (95%); IR (KBr) (νmax/cm−1): 3461, 3358, 3326 (NH2), 2825–3024 (NH2+), 2166 (CN), 1707 (C=O), 1613, 1568 (C=C), 1353 (C–N), 1162 (C–O), 742 (Ar). 1H NMR (300 MHz, DMSO-d6): δ 1.42–1.69 (6H, m, CH2), 2.81–2.97 (4H, m, NCH2), 3.58–3.81 (2H, m, +NH2), 4.62 (2H, br s, N–NH2), 4.83 (2H, s, CH2), 5.99 (2H, br s, NH2), 6.59 (1H, d, 3JHH = 7.2 Hz, ArH), 6.96–7.05, 7.10–7.32 (8H, m, ArH). 13C NMR (75.4 MHz, DMSO-d6): δ 22.2 (CH2), 22.7 (2CH2), 43.1 (CH2), 44.3 (2NCH2), 50.7, 52.51 (2C–CN), 56.5 (Cspiro), 109.0, 109.1 (2CN), 122.0, 123.0, 124.9, 127.4, 127.5, 128.1, 128.8, 228.9, 136.6, 142.0 (Ar), 156.5 (=CNH2), 163.0 (C=O), 180.0 (=C–O−); MS (EI, 70 eV): m/z (%) = 384 (24) [M—piperidine]+, 293 (15), 223 (15), 91 (100), 65 (13). Anal. Calcd for C26H27N7O2 (469.22): C, 66.51; H, 5.80; N, 20.88. Found C, 67.12; H, 6.02; N, 21.23.
Piperidin-1-ium 1',2'-diamino-3',5'-dicyano-1-ethyl-2-oxo-1'H-spiro[indoline-3,4'-pyridin]-6'-olate (5e): Brown solid, m.p.: 220–221 °C, yield 0.366 g (90%); IR (KBr) (νmax/cm−1): 3337, 3104 (NH2), 2813–3031 (NH2+), 2217 (CN), 1720 (C=O), 1653, 1586 (C=C), 1354 (C–N), 1172 (C–O) 735 (Ar). 1H NMR (300 MHz, DMSO-d6): δ 1.17 (3H, t 3JHH = 7.2 Hz, CH3), 1.36–1.88 (6H, m, CH2), 2.80–3.15 (4H, m, NCH2), 3.51–3.92 (2H, s, CH2), 4.33 (2H, br s, N–NH2), 5.08–5.38 (2H, m, +NH2), 5.65 (2H, br s, NH2), 6.79 (1H, d, 3JHH = 7.5 Hz, ArH), 7.03 (1H, d, 3JHH = 7.2 Hz, ArH). 7.17–7.26 (2H, m, ArH). 13C NMR (75.4 MHz, DMSO-d6): δ 19.0 (CH3), 22.2 (CH2), 22.9 (2CH2), 44.4 (2NCH2), 50.7 (CH2), 52.3, 52.4 (2C–CN), 56.5 (Cspiro), 109.4, 109.5 (2CN), 122.0, 122.1, 125.0, 128.0, 137.0, 141.5 (Ar), 156.3 (=CNH2), 163.0 (C=O), 181.6 (=C–O−); MS (EI, 70 eV): m/z (%) = 322 (0.03) [M—piperidine]+, 195 (13), 84 (100), 56 (47), 44 (19).
Piperidin-1-ium 1',2'-diamino-3',5'-dicyano-1-methyl-2-oxo-1'H-spiro[indoline-3,4'-pyridin]-6'-olate (5f): Brown solid, m.p.: 238–239 °C, yield 0.350 g (89%); 1H NMR (300 MHz, DMSO-d6): δ 1.42–1.61 (6H, m, CH2), 2.90–2.99 (4H, m, NCH2), 3.05 (3H, s, CH3), 3.48–3.80 (2H, m, +NH2), 4.63 (2H, br s, N–NH2), 6.00 (2H, br s, NH2), 6.88 (1H, d, 3JHH = 7.2 Hz, ArH), 7.00 (1H, d, 3JHH = 7.2 Hz, ArH). 7.14–7.21 (2H, m, ArH). 13C NMR (75.4 MHz, DMSO-d6): δ 22.2 (CH2), 22.7 (2CH2), 26.4 (CH3), 44.2 (2NCH2), 50.3, 52.2 (2C–CN), 62.1 (Cspiro), 108.5, 108.6 (2CN), 122.9, 124.5, 124.7, 128.8, 143.2 (Ar), 156.5 (=CNH2), 162.7 (C=O), 182.4 (=C–O−).
Piperidin-1-ium 1',2'-diamino-1-benzyl-5-chloro-3',5'-dicyano-2-oxo-1'H-spiro[indoline-3,4'-pyridin]-6'-olate (5 g): Brown solid, m.p.: 110–113 °C, yield 0.452 g (90%); 1H NMR (300 MHz, DMSO-d6): δ 1.40–1.68 (6H, m, CH2), 2.88–3.05 (4H, m, NCH2), 3.58–3.81 (2H, m, +NH2), 4.59 (2H, br s, N–NH2), 4.83 (2H, s, CH2), 6.04 (2H, br s, NH2), 6.63 (1H, d, 3JHH = 8.2 Hz, ArH), 7.06–7.42 (7H, m, ArH). 13C NMR (75.4 MHz, DMSO-d6): δ 22.3 (CH2), 22.8 (2CH2), 43.2 (CH2), 44.3 (2NCH2), 51.3, 51.4 (2C–CN), 56.5 (Cspiro), 110.5, 110.6 (2CN), 124.8, 127.0, 127.3, 127.4, 127.6, 127.8, 128.8, 128.9, 136.3, 140.8 (Ar), 156.6 (=CNH2), 162.9 (C=O), 181.2 (=C–O−).
Piperidin-1-ium 1',2'-diamino-5-chloro-3',5'-dicyano-1-methyl-2-oxo-1'H-spiro[indoline-3,4'-pyridin]-6'-olate (5 h): Cream solid, m.p.: 116–118 °C, yield 0.375 g (88%); IR (KBr) (νmax/cm−1): 3465, 3323 (NH2), 2856–2941 (NH2+), 2160 (CN), 1700 (C=O), 1607 (C=C), 1341 (C–N), 1135 (C–O) 731 (Ar), 695 (C–Cl). 1H NMR (300 MHz, DMSO-d6): δ 1.36–1.75 (6H, m, CH2), 2.86–3.04 (4H, m, NCH2), 3.03 (3H, s, CH3), 3.50–3.80 (2H, m, +NH2), 4.56 (2H, br s, N–NH2), 5.99 (2H, br s, NH2), 6.92 (1H, d, 3JHH = 7.6 Hz, ArH), 7.06 (1H, s, ArH), 7.21 (1H, d, 3JHH = 7.6 Hz, ArH). 13C NMR (75.4 MHz, DMSO-d6): δ 22.2 (CH2), 22.7 (2CH2), 26.6 (CH3), 44.2 (2NCH2), 51.2, 51.2 (2C–CN), 109.9, 110.0 (2CN), 122.2, 124.5, 124.6, 126.8, 128.2, 142.0 (Ar), 156.5 (=CNH2), 162.7 (C=O); MS (EI, 70 eV): m/z (%) = 343 (2) [M + 1]+, 342 (5) [M—piperidine]+, 181 (56), 152 (67), 117 (41), 84 (100), 56 (28).
Piperidin-1-ium 1',2'-diamino-5-bromo-3',5'-dicyano-1-methyl-2-oxo-1'H-spiro[indoline-3,4'-pyridin]-6'-olate (5i): Brown solid, m.p.: 107–110 °C, yield 0.386 g (82%); 1H NMR (300 MHz, DMSO-d6): δ 1.42–1.69 (6H, m, CH2), 2.84–2.93 (4H, m, NCH2), 3.03 (3H, s, CH3), 3.51–3.80 (2H, m, +NH2), 4.56 (2H, br s, N–NH2), 6.00 (2H, br s, NH2), 6.87 (1H, d, 3JHH = 8.4 Hz, ArH), 7.17 (1H, s, ArH), 7.35 (1H, d, 3JHH = 8.4 Hz, ArH). 13C NMR (75.4 MHz, DMSO-d6): δ 22.2 (CH2), 22.7 (2CH2), 26.5 (CH3), 44.3 (2NCH2), 51.1, 51.3 (2C–CN), 56.5 (Cspiro), 110.4, 114.7 (2CN), 121.8, 127.3, 127.4, 130.9, 142.3 (Ar), 156.5 (=CNH2), 162.7 (C=O).
1,6-Diamino-4-(1H-indol-3-yl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile (8a): Yellow solid, m.p.: 267–270 °C, yield 0.235 g (81%); IR (KBr) (νmax/cm−1): 3430, 3370, 3322 (NH2), 2206 (CN), 1625 (C=O), 1582 (C=C), 1339 (C–N), 1109 (C–O) 756 (Ar). 1H NMR (300 MHz, DMSO-d6): δ 5.62 (2H, s, N–NH2), 7.09–7.21 (2H, m, ArH), 7.44–7.50 (2H, m, ArH), 7.81 (1H, s, C=CH), 8.30 (2H, br s, NH2), 11.84 (1H, s, NH). 13C NMR (75.4 MHz, DMSO-d6): δ 74.8 (C–CN), 86.6 (C–CN), 109.3 (NH–CH=C), 116.8 (CN), 117.7 (CN), 112.7, 120.4, 120.5, 122.5, 125.0, 136.5 (Ar), 128.2 (NH–CH=C), 157.3 (CN–C=C), 157.4 (C–NH2), 160.2 (C=O); MS (EI, 70 eV): m/z (%) = 290 (5) [M]+, 130 (20), 77 (28), 57 (54), 43 (100).
1,6-Diamino-4-(1-methyl-1H-indol-3-yl)-2-oxo-1,2-dihydropyridine-3,5-dicarbonitrile (8b): White solid, m.p.: > 300 °C, yield 0.240 g (79%). 1H NMR (300 MHz, DMSO-d6): δ 3.89 (3H, s, CH3), 5.62 (2H, s, N–NH2), 7.11–7.56 (4H, m, ArH), 7.81 (1H, s, C=CH), 8.31 (2H, br s, NH2). 13C NMR (75.4 MHz, DMSO-d6): δ 33.4 (CH3), 74.7 (=C–CN), 86.5 (C–CN), 109.3 (NH–CH=C), 116.8 (CN), 117.7 (CN), 111.1, 120.5, 120.5, 122.5, 125.4, 137.0 (Ar), 131.8 (NH–CH=C), 154.1 (CN–C=C), 157.4 (C–NH2), 160.2 (C=O).
Abbreviations
- TLC:
-
Thin-layer chromatography
- RT:
-
Room temperature
- MeCN:
-
Acetonitrile
- NR:
-
No reaction
- FT-IR:
-
Fourier-transform infrared
- MCR:
-
Multicomponent reaction
References
Tauro SJ, Gawad JB (2013) Green chemistry: A boon to pharmaceutical synthesis. Int J Sci Res 2:67–69. https://doi.org/10.15373/22778179/JULY2013/22
Simon MO, Li CJ (2012) Green chemistry oriented organic synthesis in water. Chem Soc Rev 41:1415–1427. https://doi.org/10.1039/C1CS15222J
Bigi F, Carloni S, Ferrari L, Maggi R, Mazzacani A, Sartori G (2001) Clean synthesis in water. Part 2: Uncatalysed condensation reaction of Meldrum’s acid and aldehydes. Tetrahedron Lett 42:5203–5205. https://doi.org/10.1016/S0040-4039(01)00978-9
Clark JH, Macquarrie DJ (2008) (Eds.). Handbook of Green Chemistry and Technology. John Wiley & Sons. https://doi.org/10.1002/9780470988305
Li Y, Chen H, Shi C, Shi D, Ji S (2010) Efficient one-pot synthesis of spirooxindole derivatives catalyzed by l-proline in aqueous medium. J Comb Chem 12:231–237. https://doi.org/10.1021/cc9001185
Yan LJ, Wang YC (2016) Recent advances in green synthesis of 3, 3′-spirooxindoles via isatin–based one–pot multicomponent cascade reactions in aqueous medium. ChemistrySelect 1:6948–6960. https://doi.org/10.1002/slct.201601534
Yu B, Yu DQ, Liu HM (2015) Spirooxindoles: Promising scaffolds for anticancer agents. Eur J Med Chem 97:673–698. https://doi.org/10.1016/j.ejmech.2014.06.056
Yu B, Yu Z, Qi PP, Yu DQ, Liu HM (2015) Discovery of orally active anticancer candidate CFI-400945 derived from biologically promising spirooxindoles: Success and challenges. Eur J Med Chem 95:35–40. https://doi.org/10.1016/j.ejmech.2015.03.020
Ye N, Chen H, Wold EA, Shi PY, Zhou J (2016) Therapeutic potential of spirooxindoles as antiviral agents. ACS Infect Dis 2:382–392. https://doi.org/10.1021/acsinfecdis.6b00041
Zhou LM, Qu RY, Yang GF (2020) An overview of spirooxindole as a promising scaffold for novel drug discovery. Expert Opin Drug Discovery 15:603–625. https://doi.org/10.1080/17460441.2020.1733526
Panda SS, Jones RA, Bachawala P, Mohapatra PP (2017) Spirooxindoles as potential pharmacophores. Mini-Rev Med Chem 17:1515–1536. https://doi.org/10.2174/1389557516666160624125108
Pavlovska TL, Redkin RG, Lipson VV, Atamanuk DV (2016) Molecular diversity of spirooxindoles. Synth Biol Activity Mol Divers 20:299–344. https://doi.org/10.1007/s11030-015-9629-8
Hien TT, White NJ, Thuy-Nhien NT, Hoa NT, Thuan PD, Tarning J, Hamed K (2017) Estimation of the in vivo MIC of cipargamin in uncomplicated Plasmodium falciparum malaria. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.01940-16
Rojas-Duran R, González-Aspajo G, Ruiz-Martel C, Bourdy G, Doroteo-Ortega VH, Alban-Castillo J, Deharo E (2012) Anti-inflammatory activity of Mitraphylline isolated from uncaria tomentosa bark. J Ethnopharmacol 143:801–804. https://doi.org/10.1016/j.jep.2012.07.015
Sebahar PR, Osada H, Usui T, Williams RM (2002) Asymmetric, stereocontrolled total synthesis of (+) and (−)-spirotryprostatin B via a diastereoselective azomethine ylide [1, 3]-dipolar cycloaddition reaction. Tetrahedron 58:6311–6322. https://doi.org/10.1016/S0040-4020(02)00630-0
Kang TH, Matsumoto K, Tohda M, Murakami Y, Takayama H, Kitajima M, Watanabe H (2002) Pteropodine and isopteropodine positively modulate the function of rat muscarinic M1 and 5-HT2 receptors expressed in Xenopus oocyte. Eur J Pharmacol 444:39–45. https://doi.org/10.1016/s0014-2999(02)01608-4
Mei GJ, Shi F (2018) Catalytic asymmetric synthesis of spirooxindoles: recent developments. Chem Commun 54:6607–6621. https://doi.org/10.1039/C8CC02364F
Youseftabar-Miri L, Hosseinjani-Pirdehi H, Akrami A, Hallajian S (2020) Recent investigations in the synthesis of spirooxindole derivatives by Iranian researchers. J Iran Chem Soc 17:2179–2231. https://doi.org/10.1007/s13738-020-01921-2
Xia M, Ma RZ (2014) Recent progress on routes to spirooxindole systems derived from isatin. J Heterocycl Chem 51:539–554. https://doi.org/10.1002/jhet.1114
Ball-Jones NR, Badillo JJ, Franz AK (2012) Strategies for the enantioselective synthesis of spirooxindoles. Org Biomol Chem 10:5165–5181. https://doi.org/10.1039/C2OB25184A
Bariwal J, Voskressensky LG, Van der Eycken EV (2018) Recent advances in spirocyclization of indole derivatives. Chem Soc Rev 47:3831–3848. https://doi.org/10.1039/C7CS00508C
Nasri S, Bayat M, Mirzaei F (2021) Recent strategies in the synthesis of spiroindole and spirooxindole scaffolds. Top Curr Chem 379:1–37. https://doi.org/10.1007/s41061-021-00337-7
Stahl PH, Wermuth CG (2003) Handbook of pharmaceutical salts: properties, selection and use. J Med Chem 46:1277. https://doi.org/10.1021/jm030019n
Berry DJ, Steed JW (2017) Pharmaceutical cocrystals, salts and multicomponent systems; intermolecular interactions and property based design. Drug Delivery Rev 117:3–24. https://doi.org/10.1016/j.addr.2017.03.003
Głowacki ED, Irimia-Vladu M, Bauer S, Sariciftci NS (2013) Hydrogen-bonds in molecular solids–from biological systems to organic electronics. J Mater Chem B 1:3742–3753. https://doi.org/10.1039/C3TB20193G
Fatahpour M, Hazeri N, Maghsoodlou MT, Sadeh FN, Lshkari M (2018) One-pot multicomponent synthesis of piperidinium 3,3’-(arylmethylene) bis (2-hydroxynaphthalene-1,4-diones): NMR spectroscopic and X-ray structure characterization. Turk J Chem 42:908–917. https://doi.org/10.3906/kim-1712-52
Baharfar R, Asghari S, Zaheri F, Shariati N (2017) Three-component synthesis of novel spirooxindole–furan derivatives using pyridinium salts. C R Chim 20:359–364. https://doi.org/10.1016/j.crci.2016.07.001
Sun J, Shen GL, Huang Y, Yan CG (2017) Formation of diverse polycyclic spirooxindoles via three-component reaction of isoquinolinium salts, isatins and malononitrile. Sci Rep 7:41024. https://doi.org/10.1038/srep41024
Bashkar M, Bavadi M, Ghaderi E, Niknam K (2020) Synthesis of mono-and bis-spirooxindole derivatives on water using double salt of aluminum sulfate–sulfuric acid as a reusable catalyst. Mol Diversity. https://doi.org/10.1007/s11030-020-10091-5
Bayat M, Nasri S, Notash B (2017) Synthesis of new 3-cyanoacetamide pyrrole and 3-acetonitrile pyrrole derivatives. Tetrahedron 73:1522–1527. https://doi.org/10.1016/j.tet.2017.02.005
Bayat M, Nasri S (2017) A catalyst-free approach to regioselective synthesis of multi-functional 1H-pyrrolo[1,2-a]fused[1,3]diazaheterocycle using ketene dithioacetals in water–ethanol media. Tetrahedron Lett 58:3107–3111. https://doi.org/10.1016/j.tetlet.2017.06.076
Mohammadi A, Bayat M, Nasri S (2019) Catalyst-free four-component domino synthetic approach toward versatile multicyclic spirooxindole pyran scaffolds. RSC Adv 9:16525–16533. https://doi.org/10.1039/C9RA03214B
Hosseini H, Bayat M (2018) Cyanoacetohydrazides in synthesis of heterocyclic compounds. Top Curr Chem 376:1–67. https://doi.org/10.1007/s41061-018-0218-z
Allam YA, Nawwar GA (2002) Facile synthesis of 3-spiroindolines. Heteroat Chem 13:207–210. https://doi.org/10.1002/hc.10020
Hosseini H, Bayat M (2018) An efficient and ecofriendly synthesis of highly functionalized pyridones via a one-pot three-component reaction. RSC Adv 8:27131–27143. https://doi.org/10.1039/C8RA05690K
Elmoghayar MRH, El-Agamey AGA, Nasr MYAS, Sallam MMM (1984) Activated nitriles in heterocyclic synthesis. Part III. Synthesis of N-amino-2-pyridone, pyranopyrazole and thiazolopyridine derivatives. J Heterocycl Chem 21:1885–1887. https://doi.org/10.1002/jhet.5570210660
Acknowledgements
We thank the Iran National Science Foundation (Grant No. 98004758) for financial support. We acknowledge the support of this research from Imam Khomeini International University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mirzaei, F., Bayat, M. & Nasri, S. A one-pot synthesis of piperidinium spirooxindoline-pyridineolates and indole-substituted pyridones in aqueous or ethanol medium. Mol Divers 26, 2039–2048 (2022). https://doi.org/10.1007/s11030-021-10313-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-021-10313-4