Abstract
A glassy carbon electrode (GCE) was consecutively modified with amino groups and phosphate groups, and then loaded with Zr(IV) ions. Fourier transform infrared spectrophotometry, field-emission scanning electron microscopy, energy dispersive X-ray spectroscopy and cyclic voltammetry were used to characterize the morphologies and electrochemical properties. The sensor was used to detect p-nitrophenyl-substituted organophosphorus pesticides, with methyl-parathion (MP) as the model analyte. Under optimized conditions, the oxidation current of square wave voltammetry (typically measured at around −0.28 V vs. saturated calomel electrode) increases linearly in the 1.0 to 100 ng mL−1 MP concentration range, and the detection limit is 0.25 ng mL−1 (at a signal to noise ratio of 3). Average recoveries from (spiked) real water samples are 99.9–102.2%, with relative standard deviations of 0.3–2.6% (n = 3) at three levels. The reliability and accuracy of the method was validated by HPLC.

Zr(IV) modified GCE is prepared via three steps. The electrode shows high specificity and selectivity towards methyl‐parathion. And the linear range is 1.0 ‐ 100.0 ng mL‐1 with the detection limit as low as 0.25 ng mL‐1 with SWV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorus pesticides (OPPs) have been used widely for plants protecting [1]. Unfortunately, studies discover that they are dangerous, and some of them even are ‘extremely hazardous’ to human [2]. Thereafter, many countries and organizations regulate the maximum residue limits (MRLs) in food for OPPs [3]. For example, as regulated by the European Union, MRLs should be less than 0.1 μg/L and 0.5 μg/L for a single and the total OPPs, respectively [4]. As known, most of OPPs are easily volatile, and the residue amount of them is very small in samples [5]. For them, the mostly used method is gas or liquid chromatography coupled with mass spectrometry or other detectors, which requires complex sample pretreatment prior to analysis to diminish high matrix effect [6].
Electrochemical methods are much more robust and relativerly lower cost. However, the sensitivity and specificity of the method using conventional electrodes is far lower than those of regulations. To overcome above defects, a set of chemically modified electrodes (CMEs) were developed by physically or chemically immobilized acetylcholinesterase with/null choline oxidase enzyme or organophosphorus hydrolase enzyme or both on the surface of glass carbon, Au, or carbon paste electrode [7,8,9]. Although the above enzyme-based CMEs show high sensitivity towards OPPs, low stability, short life time and excessive false positive rate limit them applied in practice [10].
As one main principle group of OPPs, p-Nitrophenyl-substituted OPPs (PNP-OPPs) exhibits good electrochemical activity [11]. Such a characteristic makes it possible to directly determine PNP-OPPs based on the electrochemical signal from their selves instead from enzyme catalytic products of them. Therefore, a series of CMEs were reported for PNP-OPPs detecting, including ordered mesoporous carbon, Au nanoparticles (NPs) -coated multiwall carbon nanotube and Pd NPs, etc. [12,13,14]. To those, the key issue is how to guarantee the amount of OPPs on the surface of the electrode big enough and improve the specificity of the electrode.
Utilizing the special characteristics of zirconia, a series of ZrO2 NPs CMEs are prepared by immobilized ZrO2 NPs on single-layer graphene oxide sheet, gold electrode/film via electrodeposition or physic coating [15]. They show excellent selectivity and specificity towards OPPs, as well as good electrochemical activity and chemical inertness. In contrast to ZrO2 NPs, we prepared several Zr(IV) cations modified silica, magnetic nanocomposites or polymeric particles through self-assembled monolayer (SAM) technique, and successfully used for solid-phase extraction (SPE) of phosphopeptides and OPPs [16]. Besides the high specificity, selectivity and stability, Zr(IV) SAM also shows one well-ordered structure and strong adhesion [17, 18]. In this work, Zr(IV) cations was modified on the surface of glass carbon electrode (GCE) via SAM, and used as the Zr-GCE for PNP-OPPs detection with methyl parathion (MP) as the model. The electrochemical characteristic of the Zr- GCE was studied and the feasibility for OPPs detection was investigated.
Experimental
Materials
Sodium nitrite, p-phenylenediamine, hydrochloric acid and 2,4,6-collidine were bought from Sigma-Aldrich (https://www.sigmaaldrich.com/china-mainland.html); Citric acid was from Tianjin Komeo Chemical Co., Ltd. (http://public.company.lookchem.cn/); Phosphorus oxychloride (POCl3), Zirconium oxychloride octahydrate (ZrOCl2·8H2O) was from Tianjin Chemical Reagent Co., Ltd. (http://www.chemreagent.com/); Anhydrous acetonitrile, potassium ferrocyanide trihydrate (K4[Fe(CN)6]·H2O), potassium ferricyanide (K3[Fe(CN)6]), Nitrobenzene and glycine were from Aladdin Ltd. (http://www.aladdin-e.com/); Methyl-parathion (MP) in acetone (100 μg mL−1) was from the National standard material center (http://www.gbw-china.com/). High purified water was obtained by FDY2002-SUV system (http://qdflom.chinajnhb.com/). All other chemicals were analytical grade and used without further purification.
MP standard solutions were prepared by directly diluted the standard solution with highly purified water to multi-levels, and stored at 4 °C in the dark before use.
Apparatus and electrodes
The size and morphology of the Zr(IV) modified GCE was recorded by a SU8010 field-emission scanning electron microscope (http://www.hitachi.com/) with an operating voltage of 5.0 kV. Local elemental composition was determined using an ISIS-2000 energy-dispersive spectroscopy (http://www.questar.com.cn/productsst_ygoxfordnjnpyeds.html). Fourier transform infrared (FT-IR) spectra were obtained with a Vertex 70 FT-IR spectrometer (https://www.bruker.com/cn.html). Electrochemical tests were done by a CHI 660D electrochemical workstation (http://www.chinstr.com/) consisted of a conventional three-electrode system: Zr(IV) modified GCE as working, SCE as reference and platinum as counter electrode, respectively.
Preparation of the Zr(IV)-modified glassy carbon electrode (GCE)
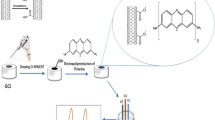
The schematic diagram for the preparation of the Zr(IV) chemically modified electrode (CME) is shown in Fig. 1. It consisted of three steps. In brief, after cleaned as Wang’s work [19], GCE was immersed in the 0.5 M HCl solution containing 6.0 mM p-phenylenediamine and 10.0 mg NaNO2 overnight to advise amino group modified GCE (NH2-GCE) [20]. Subsequently, NH2-GCE was immersed in anhydrous acetonitrile solution containing 40 mM POCl3 and 40 mM 2, 4, 6-collidine overnight to prepare phosphate group derived electrode (P-GCE) [21]. Finally, P-GCE was immersed of in 50 mM ZrOCl2 solution overnight under gentle stirring to fabricate Zr(IV) SAM layer on the electrode through chelating interaction between Zr(IV) and phosphate group [22]. The resulted electrode was donated as Zr-GCE.
Determination of MP
Firstly, Zr-GCE was used just as one typical SPE material for MP extraction at first, but only included incubation and washing steps. In short, Zr-GCE was immersed in pH 7.0 0.1 M KCl solution containing one exact concentration of MP for 3 min to capture MP; then it was removed out and washed with 100 μL pH 7.0 0.1 M KCl solution for three times to reduce or diminish non-specific absorption. And then, Zr-GCE captured MP was used as the working electrode and SWV test was performed with potential sweeping from +1 V to −1.2 V, a step potential of 4 mV, amplitude of 20 mV and frequency of 25 Hz. The amount of MP was determined based on the cathodic peak current at −0.28 V. Supporting electrolyte solution was pH 7.0, 0.1 M KCl solution. All tests were done triplicates at room temperature.
Results and discussion
Characterization of the bare glassy carbon electrode (GCE) and Zr-GCE
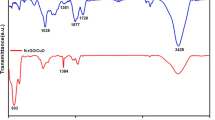
The topologies of GCE before/after modification were determined by FESEM. As can be seen from Fig. 2a, it changes from smooth (the insert) to a microravine-like. Such a phenomenon is facilitate to increase the specific surface area, enhance the adsorption capacity, and improve the electrochemical response of the the electrode. Figure 2b, EDS spectrum, confirms Zr(IV) assembled on the electrode successfully. The same conclusion also can be drawn from Fig. 2c. Compared with curve I obtained from the bare GCE, there are many absorption peaks in curve II derived from NH2-GCE. In which, absorption bands at 1500–1600 and 3000–3100 cm−1 represent the C=C skeletal vibration and the C–H stretching vibration of the benzene ring, respectively [20]. It demonstrates that NH2 groups are modified on the surface of GCE successfully. And in curve III obtained from Zr-GCE, one more broad absorption appears at 860–1200 cm−1, which is the characteristic absorption of phosphate group. Compared with the previous report [23], it shifts to lower wavenumber a little, which is ascribed to the effect of the immobilized Zr(IV) [16].
Electrochemical behavior the Zr-GCE
The microscopic area of Zr-GCE
Scan rate was investigated for the study of electrochemical behavior as Hosseini’s work [24]. The CV test was conducted in pH 7.0 0.1 M KCl containing 5 mM Fe(CN)6 4− redox pair. With the scan rate increasing from 20 to 100 mV s−1, the cathodic peak currents (Ipc) increase and the anodic peak currents (Ipa) decrease, as shown in Fig. 3a. The linear regression equation are Ipc = −0.7550 + 2.5635v1/2 with R2 = 0.9998 and Ipa = −1.6872 - 3.8808v1/2 with R2 = 0.9995, respectively. The results indicate that the electron transfer process occurring at Zr-NH2 composite film was a surface-confined process [25].
And then the microscopic area of the Zr-GCE is calculated with Randles–Sevcik equation:
where Ipa is the the anodic peak current, F is Faraday’s constant (96,485 C mol−1), R is the universal gas constant (8.314 J mol−1 K−1), T is the absolute temperature (298 K), A (cm2) is the microscopic areas of the electrode, n is the number of electron transfer, D0 (cm2 s−1) is diffusion coefficient, C0 (mol cm−3) is the concentration of probe in the supporting electrolyte solution, and v (mV s−1) is the scan rate. For 5 mM Fe(CN)6 4− in the pH 7.0 0.1 M KCl, n = 1 and D0 = 0.65 × 10−5 cm2 s−1 at 20 °C [26]. Based on the slope of Ip vs v1/2, the microscopic area of Zr-GCE is 0.7476 cm2, which is about ten times larger than that of the bare GCE (0.0707 cm2) [27]. It means that the electrochemical performance of the bare GCE will be significantly increased after the modification.
Electrochemical impedance spectra of modified electrode
EIS and CV tests were performed for further characterization of electrode surface modifications. The cyclic behaviors of the bare GCE and Zr-GCE were done at a scan rat of 50 mV s−1 in pH 7.0 0.1 M KCl containing 5 mM [Fe(CN)6]3−/4- as probe. As shown in Fig. 4a, the peak current of the probe decreases and the peak potential separation increases after the modification. It indicates that Zr(IV) SAM slightly prevents the electron transfer of the redox couple to the surface of the electrode [28]. EIS tests were conducted in the range of 0.1 to 10,000 Hz in pH 7.0 0.1 M KCl with 5 mM Fe(CN)63−/4- pair as the probe. As shown in Fig. 4b, the semicircle diameter of Nyquist plots obtained from Zr-GCE is larger than that of the bare GCE. And the calculated electron transfer resistances (Rets) are 3500 Ω and 1065 Ω at high frequencies to Zr-GCE and the bare GCE, respectively. Just as that of CV tests, the same conclusion can be drawn based on the results of EIS tests.
Optimization of the method
The following parameters were optimized: (a) Sample pH value; (b) accumulation time; (c) supporting electrolyte. Respective data and Figures are given in the Electronic Supporting Material. The following experimental conditions were found to give best results under pH value of 7.0, the accumulation time of 3 min and the supporting electrolyte of KCl.
Electrochemical detection of MP
Under the optimized conditions, SWV tests were done to detect 60 ng mL−1 MP in pH 7.0 0.1 M KCl solution with the bare GCE and Zr-GCE, separately. As seen from Fig. 5, a well-defined and strong reduction peak appears with Zr-GCE as the working electrode, while it is hard to find the respond of MP from the bare GCE. The reduction peak may be attributed to the reduction of the nitro group to the hydroxylamine group [29]:

At first, MP in solution accumulated on the surface of the electrode based on the strong and specific chelating interaction between Zr(IV) and phosphate group in MP. It takes only 3 min to reach adsorption equilibrium. When SWV test performed, the nitro group was transferred to the hydroxylamine group in the case of protons and electrons involved. Here Zr(IV) also may be participated in the electro-reduction of MP, but it needs to do further experiments to testify the assumption.
Moreover, standard curve method was conducted. As shown in Fig. 6a, well-defined SWV responses are observed. Ipc increases linearly with the MP concentration increasing in the range of 1.0–100.0 ng mL−1 with R2 of 0.9939 (Fig. 6b). The limit of detection (based on signal/noise (S/N) = 3) and the limit of quantification (LOQ) to MP (based on S/N = 10) are 0.25 and 0.83 ng mL−1, respectively. To the best of our knowledge, it is the minimum LOD for MP detection among the published data with SWV (Table 1). Such a low LOD can be ascribed to the high specificity and the strong interaction between Zr(IV) and phosphate group in MP. Above results indicates that Zr-GCE is a good electrochemical sensor for the detection of MP with low RSD, high sensitivity and good recovery.
The enrichment process of MP was also monitored by SWV with a Zr-GCE as the substrate (Fig. 6c). Based on the above data and equation below [34], the maximum surface coverage of MP is 2.6190 × 10−10 moL cm−2 (equal to 4.87 ng) in 150 ng mL−1 MP solution.
where Ip is the cathodic peak current (amperes), n is the number of electrons transferred (4 in this case), v is the scan rate (mV s−1), A is the geometric area of the electrode (cm2), the gas constant R is 8.314 J K−1 mol−1, the temperature is 301 K, the temperature is 293 K, and Faraday’s constant is F is 96,485 C mol−1.
Selectivity and specificity
To evaluate the selectivity of Zr-GCE to MP, a series of experiments were performed in parallel with other electroactive substance possibly coexisted in solution. 1) Zr-GCE was immersed pH 7.0 0.1 M KCl solution containing 20 ng mL−1 MP. Three minutes later, the electrode was removed out and washed with pH 7.0 0.1 M KCl solution for three times, and then immersed in the electrolyte solution (pH 7.0 0.1 M KCl solution) containing 30 ng mL−1 nitrobenzene. As shown in Fig. 7a, there are two peaks appeared separately at different potentials, one is from MP and the other from nitrobenzene. 2) Zr-GCE was immersed in pH 7.0 0.1 M KCl solution containing 20 ng mL−1 MP and 30 ng mL−1 nitrobenzene or 30 ng mL−1 fenpropathrin (one analog of pyrethrin pesticide), respectively. After incubation and washing steps as described above, Zr-GCE was put into the electrolyte solution. Only one peak, corresponding to MP, appears, as can be seen from Fig. 7b, c. It demonstrates the high selectivity and selectivity of the electrode towards MP.
Square wave voltammograms of Zr-GCE incubated in (a) 20 ng mL−1 MP solution and conducted in the electrolyte solution containing 30 ng mL−1 nitrobenzene, (b) a mixed solution containing 20 ng mL−1 MP and 30 ng mL−1 nitrobenzene, (c) a mixed solution containing 20 ng mL−1 MP and 30 ng mL−1 fenpropathrin, The electrolyte solution: pH 7.0 0.1 M KCl
Stability and reproducibility
After being stored at 4 °C for five days, Zr-GCE was used to detect 20 ng mL−1 MP under optimized conditions with SWV. It can still keep 94.60% of its initial response. Moreover, the relative standard deviation (RSD) of the current responses to 20 ng mL−1 MP is below 3.1% with three independently prepared Zr-GCEs. The results demonstrate the good stability and high reproducibility Zr-GCE having.
Interference studies
The interference for MP detecting may come from coexisting substances, such as cations, ions and its analogs. Here, the selectivity of Zr-GCE was investigated using the chronoamperometric experiment as our previous report [27]. As can be seen that the i-t curve obtained from 60 ng mL−1 MP shows few change after successively adding different ions of K+, Mg2+, Na+, Cl−, SO42− and PO43−, and organic molecules of Vitamin C and glycine (with/null redox ability), and compounds of hydroquinone and p-nitrophenol (containing nitro-groups). It should be pointed that each concentration of above ions and molecules is 0.1 M in the solution which is about 2 × 106 times higher than 60 ng mL−1 (2 × 10−7 M) MP.
Analysis of real water samples
Real water sample was collected from Jing Lake (Chengdu, China). After collected, it was immediately filtered through a 0.45 μm PTFE membrane. And then an aliquot of 10 mL was removed and diluted to 50 mL with pH 7.0 0.1 M KCl solution, in which MP was spiked to final concentrations of 0, 10, 20 or 40 ng mL−1. Subsequently, SWV test was tested. To validate the reliability and accuracy of the method, the same set of samples were also analyzed by HPLC [35]. The chromatographic conditions were listed in the Electronic Supplementary Material. And typical HPLC chromatograms are including in Fig. S3. Table 2 summarized the results obtained from SWV and HPLC. There is no significant difference between the results of two methods.
Conclusion
A glassy carbon electrode (GCE) was modified by immobilizing Zr(IV) on the GCE via phosphonate groups as the bridge. The electrode was successfully used for the fast determination of methyl-parathion (MP) in water samples. The linear range was as wide as three orders of magnitude with the LOD of 0.25 ng mL−1. Moreover, it also shows high reproducibility and stability. The results indicated that Zr-GCE can be a promising alternate for the fast electroanalysis of OPPs in real samples as low as in ppb level. However, the preparation consists of three steps. Hence it need to develop more readily method for the preparation of such kind of Zr(IV) phosphonate based CMEs.
References
Jiang B, Dong P, Zheng J (2018) A novel amperometric biosensor based on covalently attached multilayer assemblies of gold nanoparticles, diazo-resins and acetylcholinesterase for the detection of organophosphorus pesticides. Talanta 183:114–121
WHO (2003) WHO recommended classification of pesticides by hazard and guidelines to classification 2000-01. Angel Losada Vázquez 32(2):99–115
Zhao E, Han L, Jiang S, Wang Q, Zhou ZH (2006) Application of a single-drop microextraction for the analysis of organophosphorus pesticides in juice. J Chromatogr A 1114(2):269–273
Manuela S, Barbara H, Peter P (2004) Determination of organophosphorus pesticides using membrane-assisted solvent extraction combined with large volume injection-gas chromatography-mass spectrometric detection. J Chromatogr A 1040(2):251–258
Sharma D, Nagpal A, Pakade YB, Katnoria JK (2010) Analytical methods for estimation of organophosphorus pesticide residues in fruits and vegens: a review. Talanta 82(4):1077–1089
Lacorte S, Barceló D (1996) Determination of parts per trillion levels of organophosphorus pesticides in groundwater by automated on-line liquid-solid extraction followed by liquid chromatography/atmospheric pressure chemical ionization mass spectrometry using positive and negative i. Anal Chem 68(15):2464–2470
Min W, Feng S (2017) Amperometric determination of organophosphate pesticides using a acetylcholinesterase based biosensor made from nitrogen-doped porous carbon deposited on a boron-doped diamond electrode. Microchim Acta 184(9):3461–3468
Zhao H, Ji X, Wang B, Wang N, Li X, Ni R, Ren J (2015) An ultra-sensitive acetylcholinesterase biosensor based on reduced graphene oxide-au nanoparticles-β-cyclodextrin/Prussian blue-chitosan nanocomposites for organophosphorus pesticides detection. Biosens Bioelectron 65:23–30
Zhai C, Sun X, Zhao W, Gong Z, Wang X (2013) Acetylcholinesterase biosensor based on chitosan/prussian blue/multiwall carbon nanotubes/hollow gold nanospheres nanocomposite film by one-step electrodeposition. Biosens Bioelectron 42:124–130
Zeng Y, Yu D, Yu Y, Zhou T, Shi G (2012) Differential pulse voltammetric determination of methyl parathion based on multiwalled carbon nanotubes-poly(acrylamide) nanocomposite film modified electrode. J Hazard Mater 217-218:315–322
Liu G, Lin Y (2005) Electrochemical stripping analysis of organophosphate pesticides and nerve agents. Electrochem Commun 7(4):339–343
Pan D, Ma S, Bo X, Guo L (2011) Electrochemical behavior of methyl parathion and its sensitive determination at a glassy carbon electrode modified with ordered mesoporous carbon. Microchim Acta 173(1–2):215–221
Ma JC, Zhang WD (2011) Gold nanoparticle-coated multiwall carbon nanotube-modified electrode for electrochemical determination of methyl parathion. Microchim Acta 175(3–4):309–314
Huang B, Zhang WD, Chen CH, Yu YX (2010) Electrochemical determination of methyl parathion at a Pd/MWCNTs-modified electrode. Microchim Acta 171(1–2):57–62
Wang M, Li Z (2008) Nano-composite ZrO2 /au film electrode for voltammetric detection of parathion. Sensors Actuators B Chem 133(2):607–612
Jiang L, Huang T, Feng S, Wang J (2016) Zirconium(IV) functionalized magnetic nanocomposites for extraction of organophosphorus pesticides from environmental water samples. J Chromatogr A 1456:49–57
Kohli P, Blanchard GJ (2006) Probing interfaces and surface reactions of zirconium phosphate/phosphonate multilayers using 31P NMR spectrometry. Langmuir 16(2):695–701
Takahiro T, Yoji K, Hideo H, Yoshinori Y, Nobuhiro K, Nobukazu KA, Mallouk TE (2006) Anodic electrodeposition of highly oriented zirconium phosphate and polyaniline-intercalated zirconium phosphate films. J Am Chem Soc 128(51):16634–16640
Wang L, Dong J, Wang Y, Cheng Q, Yang M, Cai J, Liu F (2016) Novel signal-amplified Fenitrothion electrochemical assay, based on glassy carbon electrode modified with dispersed graphene oxide. Sci Report 6:23409
Zhu D, Li Q, Pang X, Liu Y, Wang X, Chen G (2016) A sensitive electrochemical impedance immunosensor for determination of malachite green and leucomalachite green in the aqueous environment. Anal Bioanal Chem 408(20):5593–5600
Feng S, Ye M, Zhou H, Jiang X, Jiang X, Zou H, Gong B (2007) Immobilized zirconium ion affinity chromatography for specific enrichment of phosphopeptides in phosphoproteome analysis. Mol Cell Proteomics 6(9):1656–1665
Ou J, Li X, Feng S, Dong J, Dong X, Kong L, Ye M, Zou H (2007) Preparation and evaluation of a molecularly imprinted polymer derivatized silica monolithic column for capillary electrochromatography and capillary liquid chromatography. Anal Chem 79(2):639–646
Forato F, Liu H, Benoit R, Fayon F, Charlier C, Fateh A, Defontaine A, Tellier C, Talham DR, Queffã©Lec C (2016) Comparison of zirconium phosphonate-modified surfaces for immobilizing Phosphopeptides and phosphate-tagged proteins. Langmuir 32 (22):5480–5490
Hosseini H, Ahmar H, Dehghani A, Bagheri A, Fakhari AR, Amini MM (2013) Au-SH-SiO2 nanoparticles supported on metal-organic framework (au-SH-SiO2@cu-MOF) as a sensor for electrocatalytic oxidation and determination of hydrazine. Electrochim Acta 88:301–309
Devadas B, Rajkumar M, Chen SM, Yeh PC (2014) A novel voltammetric p-nitrophenol sensor based on ZrO2 nanoparticles incorporated into a multiwalled carbon nanotube modified glassy carbon electrode. Anal Methods 6(13):4686–4691
Ding SJ, Chang BW, Wu CC, Lai MF, Chang HC (2005) Impedance spectral studies of self-assembly of alkanethiols with different chain lengths using different immobilization strategies on au electrodes. Anal Chim Acta 554(1–2):43–51
Li Z, Yue Y, Hao Y, Feng S, Zhou X (2018) A glassy carbon electrode modified with cerium phosphate nanotubes for the simultaneous determination of hydroquinone, catechol and resorcinol. Microchim Acta 185(4):215
Narang J, Chauhan N, Pundir CS (2011) A non-enzymatic sensor for hydrogen peroxide based on polyaniline, multiwalled carbon nanotubes and gold nanoparticles modified au electrode. Analyst 136(21):4460–4466
Du D, Liu J, Zhang XY, Cui XL, Lin Y (2011) One-step electrochemical deposition of a graphene-ZrO2 nanocomposite: preparation, characterization and application for detection of organophosphorus agents. J Mater Chem 21(22):8032–8037
Chen S, Huang J, Du D, Li J, Tu H, Liu D, Zhang A (2011) Methyl parathion hydrolase based nanocomposite biosensors for highly sensitive and selective determination of methyl parathion. Biosens Bioelectron 26(11):4320–4325
Govindasamy M, Chen SM, Mani V, Akilarasan M, Kogularasu S, Subramani B (2017) Nanocomposites composed of layered molybdenum disulfide and graphene for highly sensitive amperometric determination of methyl parathion. Microchim Acta:1–9
Zhang T, Zeng L, Han L, Li T, Zheng C, Wei M, Liu A (2014) Ultrasensitive electrochemical sensor for p-nitrophenyl organophosphates based on ordered mesoporous carbons at low potential without deoxygenization. Anal Chim Acta 822:23–29
Tang X, Zhang T, Liang B, Han D, Zeng L, Zheng C, Li T, Wei M, Liu A (2014) Sensitive electrochemical microbial biosensor for p-nitrophenylorganophosphates based on electrode modified with cell surface-displayed organophosphorus hydrolase and ordered mesopore carbons. Biosens Bioelectron 60:137–142
Shaoqin L, Bjoern Bredenkötter A, Volkmer D (2002) The structure of self-assembled multilayers with Polyoxometalate nanoclusters. J Am Chem Soc 124(41):12279–12287
Dhull V (2018) Fabrication of AChE/SnO2-cMWCNTs/cu nanocomposite-based sensor electrode for detection of methyl parathion in water. Int J Anal Chem 2018:2874059
Acknowledgements
This work was supported by the National Natural Science Foundation of China [NSFC, Grant Nos. 21775126 and 21565023].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Y. Yue and L. Jiang was considered as co-first authors
Electronic supplementary material
ESM 1
(DOCX 245 kb)
Rights and permissions
About this article
Cite this article
Yue, Y., Jiang, L., Li, Z. et al. A glassy carbon electrode modified with a monolayer of zirconium(IV) phosphonate for sensing of methyl-parathion by square wave voltammetry. Microchim Acta 186, 433 (2019). https://doi.org/10.1007/s00604-019-3493-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3493-7