Abstract
Application of malachite green (MG) and leucomalachite green (LMG) in fish farm water causes an environmental problem. This study proposes for the first time a sensitive and convenient electrochemical impedance spectroscopy (EIS) method for determining MG and LMG by a bovine serum albumin-decorated gold nanocluster (BSA-AuNC)/antibody composite film-based immunosensor. In order to improve the analytical performance, the glassy carbon electrode (GCE) was modified with 1, 4-phenylenediamine to form a stable layer, and then, BSA-AuNCs were covalently bound to the GCE. An adequate quantity of the polyclonal antibody of LMG was immobilized onto the surface of the BSA-AuNCs by the chemical reaction of EDC/NHS. The sensors can respond to the specific target based on specific covalent bonding. The experimental parameters, such as the pH, incubating concentration, and time, have been investigated and optimized. The calibration curve for LMG was linear in the range of 0.1~10.0 ng/mL with the limit of detection (LOD) 0.03 ng/mL. Furthermore, the sum of MG and LMG was detected in fish farm water by MG reduction. The recovery was between 89.7 % and 99.2 % in spiked samples. The EC sensor method was also compared with the ELISA method and validated by the LC–MS/MS method, which proves its great promise as a field instrument for the rapid monitoring of MG and LMG pollution.

1, 4-Phenylenediamine and BSA-AuNC/antibody-decorated glassy carbon electrodes have been used for the impedimetric detection of the sum of malachite green and leucomalachite green via specific immuno-binding
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Malachite green (MG) has been used as the cheap and effective biocide in the aquaculture industry worldwide [1]. MG is easily absorbed by fish tissues and is extensively metabolized to the reduced form, leucomalachite green (LMG), when it enters water cycles. Because of potential hazards to the human immune system and the reproductive system and to be potential carcinogens, MG and LMG have been prohibited to be used in fish farm water in many countries [2, 3]. A report prepared by the Water Research Centre for the Department of the Environment, Transport, and the Regions of the United Kingdom recommended an annual average environmental quality standard of 500 ng/L LMG and MG for the protection of freshwater aquatic life [3]. Numerous methods have been proposed for the detection of MG and LMG in a variety of matrices, including enzyme-linked immunosorbent assay (ELISA) [4, 5], surface-enhanced resonance Raman scattering (SERS) [6], liquid chromatography–tandem mass spectrometry (LC–MS/MS) [2], fluorometry method [7], and so on [8]. Besides this, development of a rapid and highly sensitive method for MG and LMG analyses in fish farms is always required in order to achieve on-site supervision.
Electrochemical immunosensors have been widely used for the determination of chemical contaminants because of its high sensitivity, low detection limit, and rapid analysis [9–13]. Among these, the electrochemical impedance-based immunosensor (EIS) [13, 14] is one of the most efficient and sensitive methods. An impedimetric immunosensor is based on the detection of the binding between an immobilized antibody and an antigen [15] and is more amenable for miniaturization and economization [16]. Some electrochemical impedimetric immunosensors have been built to detect contaminants, but the detection of MG and LMG has not been reported.
In this work, an impedimetric immunosensor was proposed for the determination of MG and LMG based on the bovine serum albumin-decorated gold nanocluster (BSA-AuNC)-modified glassy carbon electrode (GCE). In order to improve the BSA-AuNCs’ stability and to get better performance on the GCE surface, a diazotization method was used. Moreover, the antibody against LMG was chemically connected by optimization of concentration, incubation reaction time, and pH. Electron transfer resistance values were directly detected by the EIS after the immunoreactions. The developed EIS sensor was further compared with an ELISA method and a LC–MS/MS method for validation.
Materials and methods
Chemicals and reagents
MG and LMG standards were purchased from J&K Scientific (J&K Scientific Ltd., China). The molecular structures of MG and LMG are shown in Fig. 1. Methanol, acetonitrile, and formic acid (HPLC grade) were purchased from Tedia Company (Fairfield, USA). Ammonium formate, p-phenylenediamine, sodium nitrite, hydrochloric acid, potassium chloride, disodium, trisodium, sodium o-phosphate, sodium hydroxide and o-phosphoric acid, potassium borohydride (PBH), and hydrogen tetra-chloroaurate (HAuCl4·4H2O) were guaranteed reagents from Sinopharm Chemical Reagent Co., Ltd, China. Bovine serum albumin (BSA, 99 %), 1-(3-(dimethylamino)-propyl)-3-ethylcarbodiimide hydrochloride (EDC), and N-hydroxysulfosuccinimide (NHS) were all purchased from Sigma-Aldrich (Sigma-Aldrich Co. LLC., USA). The antibody against LMG was prepared in our lab. Ultrapure water was obtained from a Milli-Q Academic System (Millipore, USA). All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Instrumentation
Cyclic voltammetry was performed using an Autolab potentiostat interfaced to a PC for data acquisition and instrument control via the Nova operating system (Autolab, The Netherlands). The voltammetric cell contained a platinum counter electrode, a silver/silver chloride reference electrode (Ag/AgCl), and a 3-mm-diameter glassy carbon electrode as the working electrode (Chen Hua Instruments, Shanghai, China). A field emission scanning electron microscope (FE-SEM, Helios Nanolab 600i, FEI Company, USA) and a FT-IR spectrometer (PerkinElmer Spectrum 100, PerkinElmer, Inc., USA) were used to characterize the morphology. A LC–MS/MS instrument from Applied Biosystems (API 5000, USA) was used for method validation.
Preparation of the immunosensors
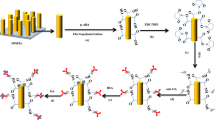
The p-phenylenediamine-modified GCE (GCE-Ph-NH2) was prepared as reported previously [17–19] with the following modification. A clean GCE was dipped in HCl solution (0.5 mol/L) containing p-phenylenediamine (6.0 × 10−3 mol/L). NaNO2 (10.0 mg) was added to the reaction mixture and left to react overnight. The modified GCE was characterized by the FT-IR spectrometer after washing with ultrapure water and acetonitrile to remove adsorbed molecules.
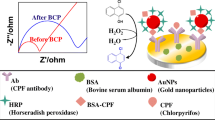
The BSA-decorated gold nanoclusters (BSA-AuNCs) were prepared following the procedure described previously with modification (see Electronic Supplementary Material, ESM) [20]. The morphology of BSA-AuNCs was measured by TEM. For the formation of the AuNC-modified electrode, the GC-Ph-NH2 surface was immersed in a solution containing 5.0 mmol/L NaNO2 and 0.5 mol/L HCl for 20 min. Then 20 μL of the BSA-AuNCs was placed on the GCE surface and incubated for 60 min. For attachment of the antibody, 30 μL of freshly prepared EDC (500 mmol/L) and NHS (150 mmol/L) in water was placed onto the modified electrode surface for 10 min [21], then reacted with antibody solution (0.5 mg/mL, 20 μL) for 1 h. The modified immunosensor was incubated in a PBS buffer (pH = 7.5) containing 5 % of BSA for 1 h at room temperature, washed, and stored at 4 °C until use. The scheme of BSA-AuNC/Ab/LMG immunosensor preparation is illustrated in Fig. 2.
Electrochemical measurement
All electrochemical measurements were carried out in 0.1 mol/L PBS solution containing 0.1 mol/L KCl and 5 mmol/L Fe(CN)6 4−/3− (1:1) as the electrochemical active probe [22]. The cyclic voltammetric scan (−0.3~0.6 V, 50 mV/s) and EIS investigation (0.1~10,000 Hz) were applied to characterize the surface change in the modification process. The Nyquist plot was used to present the electrochemical impedance output, i.e., the imaginary component (capacitive reactance, Z″) versus the real component (ohmic resistance, Z′) at each excitation frequency [23–25].
Sample collection and pre-treatment
Water samples were obtained from the Badu River channel, Fangshan District, Beijing, at a depth of 0.5 m. A volume of 10 μL of PBH (0.02 mol/L)/mL water solution was added to induce the MG reduction reaction [26]. The HCl solution (0.1 mol/L) was used to neutralize water samples after the reaction, and the samples were passed through 0.22-μm filter paper (Jinteng company, Tianjin, China) prior to voltammetric analysis. Moreover, the samples were proportionated and analyzed by ELISA and LC–MS/MS for method comparison. The ELISA and the LC–MS/MS analytical procedures were described in the ESM.
Results and discussion
Characterization of the GCE-Ph-NH2 and BSA-AuNCs
The sensitivity of the EIS immunosensor was mainly determined by the amount of immobilized antibody which is the key step to generate a stable, reproducible, and selective immunosensor. Thus, the GCE surface was modified by the BSA-AuNCs via a diazotization method [27–29]. As characterized by FT-IR, there were no absorption peaks for the bare GCE, whereas the absorption peaks were observed for the GCE-Ph-NH2 (Fig. 3a). The absorption bands at 1500~1600 and 3000~3100 cm−1 demonstrated the C=C skeletal vibration and the C–H stretching vibration of the benzene ring, respectively. The reaction scheme of GCE in situ derivatization by diazonium reduction is delineated in the insert of Fig. 3a. In conclusion, the GCE has been modified successfully by p-phenylenediamine.
Alternative materials have been used for developing stable immunosensors in order to meet the growing demands of on-site environmental monitoring of trace pollutants, including the gold nanoparticles [27], carbon nanotubes [15, 30], and AuNCs, which have attracted special attention due to the facile synthesis protocol, subnanometer size, and low toxicity [31]. Indeed, these AuNCs are excellent for quantitative studies of nanoparticle–protein interactions. However, only little experimental work has been performed [32, 33]. In our study, the morphologies and microstructures of the synthetic BSA-AuNCs were observed by TEM. As presented in Fig. 3b, most of the water-soluble AuNCs ranged between 2 and 3 nm in diameter. The BSA-AuNCs could offer a biocompatible microenvironment for encapsulating the antibodies and sustaining their bioactivity.
Characterization of the immunosensor assembly
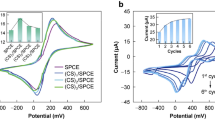
Electrochemical characteristics of the immunosensor were investigated by CV and EIS. A pair of well-defined oxidation and reduction peaks was observed at the bare GCE (Fig. 4a, curve a); this is due to the high electron transfer between Fe(CN)6 4−/3− solution and the electrode. Further immobilization of AuNCs-Ab on the GCE decreased the current of the redox couple (Fig. 4a, curve b), which was mainly attributed to the protein layer acting as an inert electron transfer blocking layer. Incubation with LMG solution at a different concentration, the peak current of the redox couple was further reduced. It shows that the Ab-LMG complex layer blocked the electron communication between the redox couple Fe(CN)6 4−/3− and the electrode.
Characterization of different electrodes by a cyclic voltammograms and b electrochemical impedance spectroscopy; a bare GCE, b AuNC-modified GCE and modified GCE fabricated based on the AuNC/Ab composite films incubated in PBS (at pH 7.4) containing LMG of c 0.0 ng/mL, d 1.0 ng/mL, and e 10.0 ng/mL for 25 min in 0.1 mol/L PBS solution containing 0.1 mol/L KCl and 5 mmol/L Fe(CN)6 4−/3−. Points were raw data and lines presented fit the model in c using the NOVA software. c An equivalent circuit representing the immunosensor-electrolyte solution interface. (R s solution-phase resistance; CPE double-layer capacitance; R et electron transfer resistance; Z w Warburg impedance) d Resistance to charge transfer for different sensor fabrication steps using the equivalent circuit model in c
Electrochemical impedance spectroscopy is a highly effective technique to investigate the electron transfer properties of the modified electrodes. The R et values were estimated from the EIS Nyquist curves according to the equivalent circuit model using the NOVA software [21, 22] (Fig. 4c). A constant phase element (CPE) was selected since the surface of the AuNCs-GCE was very rough and had a larger real surface area [22]. A good agreement with the circuit model and the measurement system was observed over the entire measurement frequency range (Fig. 4b), indicating the elements were chosen properly. Besides, R s and Z w represent the diffusion features of the redox probe in solution and the bulk properties of the electrolyte solution, respectively. Negligible changes were observed at the values of R s (95 Ω) and Z w (1.45 mMho) at every impedance scan, showing that the values of R s and Z w were not affected by the modification of the electrode surface. Therefore, R et was chosen as a suitable signal for sensing the interfacial properties during the assembly procedures. The increase of R et was mainly caused by electrically insulating bioconjugates produced from specific interaction of LMG and the antibody (Fig. 4d), which would block the electron transfer process of the redox probe. Therefore, the results of the EIS assays were in correspondence with those of the above CV measurements, indicating that the anti-LMG was successfully immobilized on the surface of the electrode.
Optimization of the conditions during the assembly process
The electrochemical signal was related to the preparation process of the modified film, containing the concentration and incubation time of the antibody. The tested antibody was immobilized on the nanoscale electrode with concentration between 0 and 100 μg/mL and incubation time between 30 and 90 min at 37 °C. The optimal antibody concentration and incubation time were at 60 μg/mL and 60 min, respectively (Fig. 5a, b) and therefore were selected for BSA-AuNC-modified GCE immobilization.
Effect of a concentration (0~100 μg/mL, incubation time 60 min), b incubation time (30~90 min, the concentration of the LMG antibody 60 μg/mL) of the LMG antibody immobilized on the surface of the immunosensor, c incubation time (10~60 min, pH 7.5) on the specific binding of LMG (10.0 ng/mL) and the antibody immobilized on the surface of the immunosensor, and d pH (6.0~8.5, incubation time 40 min) of PBS on the specific binding of LMG (10.0 ng/mL) and the antibody immobilized on the surface of the immunosensor
The incubation time and the pH of the solution directly affect the EIS analysis as antibody activity was highly time and pH dependent [15]. For optimization of the antigen incubation time and pH value of the reaction solution, the antibody-modified electrode was immersed in LMG solution (10 ng/mL) at the range of the pH (6.0~8.5) and incubation time (10~60 min). The optimal analytical condition was obtained when incubating at pH 7.5 for 40 min (Fig. 5c, d).
Calibration curve of the immunosensor
The EIS response to various LMG concentrations was investigated under the following conditions: amplitude 5 mV, frequency range 0.1 Hz~100 kHz, and potential 0.29 V. The potential was detected under the condition of open circuit and the analysis time was about 10 min, which was much faster than the ELISA method (2 h). The R et value increased in proportion with the increment of the LMG concentration. A calibration curve was plotted using the relative resistance versus the LMG concentration. Good linearity was obtained for LMG concentration at the range of 0.1~10.0 ng/mL (Fig. 6b, curve a). The linear equation could be depicted as R et (Ω) = 113.95C + 1470.8 (R 2 = 0.9923) and the limit of detection was 0.03 ng/mL. The change of electron transfer resistance tended to be relatively steady when the concentration of LMG exceeds 10.0 ng/mL. In order to eliminate the influence of nonspecific adsorption, a control experiment was carried out without the LMG antibody. The increase in resistance was almost negligible when the GCE modified only with BSA (Fig. 6b, curve b), indicating that the influence of the nonspecific absorption could be ignored.
a Nyquist diagrams of AuNC/Ab/LMG-modified GCE after incubation at 37 °C with a 0.1 ng/mL, b 0.5 ng/mL L, c 1 ng/mL, d 4 ng/mL, e 7 ng/mL, f 10 ng/mL, g 15 ng/mL, and h 20 ng/mL of LMG in PBS (pH = 7.5) containing 5.0 mmol/L Fe(CN)6 4−/3− for 100 min. b Linear relationship between electron transfer resistance and LMG concentration in PBS at AuNC/Ab/LMG-modified GCE (curve a) and AuNC/BSA/LMG-modified GCE (curve b)
Repeatability and stability of the immunosensor
Repeatability of the EIS was measured with the LMG concentration at 10.0 ng/mL and analyzed five times [34]. Relative standard deviation (RSD) was 5.6 %. In addition, the immunosensors were stored at 4 °C and tested once a day to evaluate their stability. No significant reduction in its performance was observed in 2 weeks. The impedance remained 90 % of the initial value after 2 weeks, indicating that the repeatability and stability of the immunosensor were acceptable.
Analysis application
Five fish farm water samples were spiked with LMG at concentrations 1.0 and 5.0 ng/mL, respectively, and analyzed by both EIS and ELISA methods for comparison. The spiked samples were further analyzed by LC–MS/MS for validation. Good recoveries and precision were obtained for the EIS method (Table 1). Moreover, the analytical results from the three methods were comparable. This shows that the electrochemical sensor was adequate for practical application for detecting MG and LMG in a water environment.
Conclusions
An EIS immunosensor was developed to detect the sum of the MG and LMG. This method immobilized the antibody onto the BSA-AuNC-GCE surface. In addition, an optimization process for all of the conditions was performed to achieve the highest performance. The experimental results demonstrated that the EIS immunosensor has a low detection limit and high stability. This EIS immunosensor exhibited a linear range from 0.1 to 10.0 ng/mL, and the limit of detection was at 0.03 ng/mL. This sensitive and simple immunosensor has potential applications in the detection of MG and LMG for further field monitoring in a water environment.
References
Yang MC, Fang JM, Kuo TF, Wang DM, Huang YL, Liu LY, et al. Production of antibodies for selective detection of malachite green and the related triphenylmethane dyes in fish and fishpond water. J Agric Food Chem. 2007;55(22):8851–6.
Mitrowska K, Posyniak A, Zmudzki J. Determination of malachite green and leucomalachite green residues in water using liquid chromatography with visible and fluorescence detection and confirmation by tandem mass spectrometry. J Chromatogr A. 2008;1207(1–2):94–100.
Burchmore S, Wilkinson M. Proposed environmental quality standards for malachite green in water (DWE 9026). UK Department of the Environment, Transport, and the Regions Report No. 3167/2, prepared by the Water Research Centre, Marlow, Buckinghamshire, England. 1993.
Jiang Y, Chen L, Hu K, Yu W, Yang X, Lu L. Development of a fast ELISA for the specific detection of both leucomalachite green and malachite green. J Ocean Univ China. 2014;14(2):340–4.
Xing W, He L, Yang H, Sun C, Li D, Yang X, et al. Development of a sensitive and group-specific polyclonal antibody-based enzyme-linked immunosorbent assay (ELISA) for detection of malachite green and leucomalachite green in water and fish samples. J Sci Food Agric. 2009;89(13):2165–73.
Zhang Y, Yu W, Pei L, Lai K, Rasco BA, Huang Y. Rapid analysis of malachite green and leucomalachite green in fish muscles with surface-enhanced resonance Raman scattering. Food Chem. 2015;169:80–4.
Ju S, Deng J, Cheng J, Xiao N, Huang K, Hu C, et al. Determination of leucomalachite green, leucocrystal violet and their chromic forms using excitation-emission matrix fluorescence coupled with second-order calibration after dispersive liquid-liquid microextraction. Food Chem. 2015;185:479–87.
Dong JX, Xu C, Wang H, Xiao ZL, Gee SJ, Li ZF, et al. Enhanced sensitive immunoassay: noncompetitive phage anti-immune complex assay for the determination of malachite green and leucomalachite green. J Agric Food Chem. 2014;62(34):8752–8.
Privett BJ, Shin JH, Schoenfisc MH. Electrochemical sensors. Anal Chem. 2010;82(12):4723–41.
Eric B. Electrochemical sensors. Anal Chem. 2004;76(12):3285–98.
Vasudev A, Kaushik A, Bhansali S. Electrochemical immunosensor for label free epidermal growth factor receptor (EGFR) detection. Biosens Bioelectron. 2013;39(1):300–5.
Zhu C, Yang G, Li H, Du D, Lin Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal Chem. 2015;87(1):230–49.
Pejcic B, De Marco R. Impedance spectroscopy: over 35 years of electrochemical sensor optimization. Electrochim Acta. 2006;51(28):6217–29.
Hayat A, Barthelmebs L, Marty J-L. Electrochemical impedimetric immunosensor for the detection of okadaic acid in mussel sample. Sensors Actuators B Chem. 2012;171–172:810–5.
Yu L, Zhang Y, Hu C, Wu H, Yang Y, Huang C, et al. Highly sensitive electrochemical impedance spectroscopy immunosensor for the detection of AFB1 in olive oil. Food Chem. 2015;176:22–6.
Yang P, Zheng Q, Xu H, Liu J, Jin L. A highly sensitive electrochemical impedance spectroscopy immunosensor for determination of 1-pyrenebutyric acid based on the bifunctionality of Nafion/gold nanoparticles composite electrode. Chin J Chem. 2012;30(5):1155–62.
Sun X, Ma Z. Highly stable electrochemical immunosensor for carcinoembryonic antigen. Biosens Bioelectron. 2012;35(1):470–4.
Zhang Y, Li B, Yan C, Fu L. One-pot fluorescence detection of multiple analytes in homogenous solution based on noncovalent assembly of single-walled carbon nanotubes and aptamers. Biosens Bioelectron. 2011;26(8):3505–10.
Anjum SQW, Gao W, Zhao J, Hanif S, Aziz-ur-Rehman, Xu G. Fabrication of biomembrane-like films on carbon electrodes using alkanethiol and diazonium salt and their application for direct electrochemistry of myoglobin. Biosens Bioelectron. 2015;65:159–65.
Xie J, Zheng Y, Jackie YY. Protein-directed synthesis of highly fluorescent gold nanoclusters. J Am Chem Soc. 2009;131(3):888–9.
Liu H, Malhotra R, Peczuh MW, Rusling JF. Electrochemical immunosensors for antibodies to peanut allergen ara h2 using gold nanoparticle-peptide films. Anal Chem. 2010;82(13):5865–71.
Wang M, Kang H, Xu D, Wang C, Liu S, Hu X. Label-free impedimetric immunosensor for sensitive detection of fenvalerate in tea. Food Chem. 2013;141(1):84–90.
Mashazi P, Tetyana P, Vilakazi S, Nyokong T. Electrochemical impedimetric immunosensor for the detection of measles-specific IgG antibodies after measles infections. Biosens Bioelectron. 2013;49:32–8.
Chen Y, Yang Y, Tu Y. An electrochemical impedimetric immunosensor for ultrasensitive determination of ketamine hydrochloride. Sensors Actuators B Chem. 2013;183:150–6.
Ahmed A, Rushworth JV, Wright JD, Millner PA. Novel impedimetric immunosensor for detection of pathogenic bacteria Streptococcus pyogenes in human saliva. Anal Chem. 2013;85(24):12118–25.
Deng J, Li L, Yang X, Cen J, Xin S, Wei Y, et al. Determination of malachite green in water using solid phase extraction-high performance liquid chromatography with fluorescence detection. Food Sci. 2012;33(14):150–3.
Han S, Yuan Y, Hu L, Xu G. Electrochemical derivatization of carbon surface by reduction of diazonium salts in situ generated from nitro precursors in aqueous solutions and electrocatalytic ability of the modified electrode toward hydrogen peroxide. Electrochem Commun. 2010;12(12):1746–8.
Cougnon C, Gohier F, Belanger D, Mauzeroll J. In situ formation of diazonium salts from nitro precursors for scanning electrochemical microscopy patterning of surfaces. Angew Chem. 2009;48(22):4006–8.
Cougnon C, Mauzeroll J, Belanger D. Patterning of surfaces by oxidation of amine-containing compounds using scanning electrochemical microscopy. Angew Chem. 2009;48(40):7395–7.
Zhang K, Ji J, Fang X, Yan L, Liu B. Carbon nanotube/gold nanoparticle composite-coated membrane as a facile plasmon-enhanced interface for sensitive SERS sensing. Analyst. 2015;140:134–9.
Santhosh M, Chinnadayyala SR, Kakoti A, Goswami P. Selective and sensitive detection of free bilirubin in blood serum using human serum albumin stabilized gold nanoclusters as fluorometric and colorimetric probe. Biosens Bioelectron. 2014;59:370–6.
Shang L, Yang L, Seiter J, Heinle M, Brenner-Weiss G, Gerthsen D et al. Nanoparticles interacting with proteins and cells: a systematic study of protein surface charge effects. Adv Mater Interfaces. 2014;1(2).
Marradi M, Di Gianvincenzo P, Enriquez-Navas PM, Martinez-Avila OM, Chiodo F, Yuste E, et al. Gold nanoparticles coated with oligomannosides of HIV-1 glycoprotein gp120 mimic the carbohydrate epitope of antibody 2G12. J Mol Biol. 2011;410(5):798–810.
Rezaei B, Askarpour N, Ensafi AA. A novel sensitive doxorubicin impedimetric immunosensor based on a specific monoclonal antibody-gold nanoparticle-sol-gel modified electrode. Talanta. 2014;119:164–9.
Acknowledgments
This work was supported by the Natural Science Foundation of China (31371779) and the International Science and Technology Cooperation Program of China (2015DFG31890).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 455 kb)
Rights and permissions
About this article
Cite this article
Zhu, D., Li, Q., Pang, X. et al. A sensitive electrochemical impedance immunosensor for determination of malachite green and leucomalachite green in the aqueous environment. Anal Bioanal Chem 408, 5593–5600 (2016). https://doi.org/10.1007/s00216-016-9660-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9660-3