Abstract
Ordered mesoporous carbon (OMC) was synthesized and used to modify the surface of a glassy carbon (GC) electrode. Due to the unique properties of OMC, a decrease in the overvoltage of the reduction potential of methyl parathion (MP) (to ca. 219 mV) and a 76-fold increase in the peak current are observed (compared with a bare GC electrode). The absorption capacity of the surface of the electrode for MP was determined by chronocoulometry. The results show that the Г value of the modified electrode (2.34 × 10–9 mol cm–2) is 9.5 times as large as that of the GC electrode (2.47 × 10–10 mol cm–2). The new electrode exhibits synergistic electrocatalytic and accumulative effects on MP. MP can be determined by linear sweep voltammetry (LSV) which displays a linear relationship between peak current and MP concentration in the range from 0.09 to 61 μM, with a detection limit as low as 7.6 nM (at an S/N of 3) and after an accumulation at 0 V for 5 min. The electrode was successfully applied to the determination of MP in spiked lake water samples.

A glassy carbon (GC) electrode modified with ordered mesoporous carbon (OMC) performed an enhanced electrocatalytic activity and accumulative effect towards methyl parathion (MP). The electrode also exhibited wider linear range, lower detection limit, better electrochemical stability and utilization for MP determination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorus pesticides (OPs) are widely used in agriculture [1] to enhance production and quality currently. However, even trace contamination of organophosphorus pesticides in the natural aquatic systems creates a lot of pollution problems due to their toxicity and bioaccumulation property [2]. Methyl parathion (MP) is an effective OP insecticide against many pests in important crops [3, 4]. But it may be converted in vivo into the active metabolite, methyl-paraoxon, which acts as an acetylcholinesterase inhibitor [5] and can be harmful and fatal. Therefore it is considered extremely toxic [6]. Due to high toxicity of OPs, quantitative analysis of these toxicants is the persistent purpose for environment control and food security [7].
Many methods for organophosphorus pesticide determination have been developed in recent years, for example, spectrometry, gas chromatography, high-performance liquid chromatography and chromatography-mass spectrometry [8–11], etc. Although these methods are sensitive and accurate, these analytical instruments are available only in centralized laboratories [12]. Moreover, these analytical techniques are difficult to be applied under field conditions or on-line monitoring. Some drawbacks of these methods mainly are complex separation processes in the samples pretreatment, expensive equipments [6], time consuming, complex and using toxic organic solvent, etc. So, we need convenient, fast, sensitive and accurate methods for their detection.
Electroanalytical sensors are alternative and viable methods because of their compact nature, easy handling in field trials, low cost and low power requirements. They can also produce selective responses within a very short time [13] with respect to those methods described above. In recent years, various enzyme-based electrochemical biosensors have been fabricated for detecting OPs. For instance, Gong et al. [14] reported a nanomaterial based acetylcholinesterase (AChE) biosensor, where they immobilized AChE onto Au nanoparticles (AuNPs)-polypyrrole nanowires composite film modified GC electrode (labeled as AChE-AuNPs-PPy/GC electrode). Though these enzyme-based biosensors usually exhibit high sensitivity, the stability of enzyme is limited since they tend to denature during immobilization and storage [15].
Nitroaromatic organophosphorus pesticides, such as methyl parathion (MP) exhibit good redox activities at the electrode surface [16] and thus very suitable for electrochemical detection. Recently, in order to improve the performance of the electrode for methyl parathion sensing, e.g. their electrocatalysis properties, selectivity, sensitivity and foul resistance with respect to non-modified electrodes, some work has been done by applying suitable modifiers such as nanosilver/nafion composite [17], mesoporous zirconia [2], ex-situ depositing bismuth-film [18] on the electrode surface.
Over the past decades, carbon-based materials, e.g. carbon nanotubes (CNTs) have been exploited for the electrochemical determination of methyl parathion [19]. The experiment results indicate the electrocatalytic activity of CNTs. Besides CNTs mentioned above, there has been significant interest in the development of ordered mesoporous carbon (OMC). Since the discovery of OMC in 1999 [20], the OMC has been receiving much attention owing to its large surface area, well defined pore size, high thermal stability, flexible framework composition and chemical inertness [20–24]. For these reasons, OMC is a particularly promising carbon material and can be widely applied in the field of molecule separation, adsorption, catalysis, sensors, energy storage and capacitors [25]. It has recently been reported that, compared to CNTs, OMC exhibits remarkably strong and stable electrocatalytic response towards some compounds [26]. To verify that OMC can promote the electron-transfer reactions, electrocatalysis of some important molecules by OMC have been investigated, such as morphine, hydroquinone, nitrobenzene [27–29], which has made OMC be a good model for constructing various electrochemical sensors for further electrochemical detection of other molecules.
Nevertheless, to the best of our knowledge, the detection of methyl parathion at an OMC modified electrode has not yet been reported. In this work, an OMC/GC electrode was fabricated in purpose of improving the analytical characteristics of detecting MP, e.g. preconcentration capacity, linear range, detection limit and stability. An obvious decrease in the overvoltage and a marked enhancement in the current response of the MP reduction reaction (compared with the GC electrode) were observed. This OMC/GC electrode also exhibited excellent accumulative effect on MP. These make OMC/GC electrode a promising candidate for efficient electrochemical sensors for the detection of MP.
Experimental
Reagents
OMC was synthesized according to the previous reported work [24]. Methyl parathion (MP) was purchased from Dr. Ehrenstorfer Company (Germany, http://www.ehrenstorfer.com/). MP stock solutions of 0.01 M were prepared in ethanol and kept at 4 °C until used. Other reagents not mentioned here are of analytical grade and used as received. Double distilled water was prepared through a quartz apparatus and used throughout. The 0.1 M phosphate buffer solution, which was made up from Na2HPO4, NaH2PO4 and H3PO4, was employed as a supporting electrolyte.
Apparatus
All electrochemical measurements were performed on a CHI830b electrochemical workstation (Shanghai Chenhua Instrument Co., China, http://www.chistruments.com/). The working electrode is a modified GC electrode (3 mm diameter), whereas an Ag/AgCl (in saturated KCl solution) and a platinum wire served as the reference and counter electrodes, respectively. All solutions were purged with high-purity nitrogen gas before the experiments. All experiments were carried out at room temperature.
Small angle X-ray diffraction (XRD) patterns were obtained on an X-ray D/max-2200vpc (Rigaku Corporation, Japan, http://www.rigaku.com/) instrument operated at 40 kV and 20 mA and using Cu Kα radiation (λ = 0.15406 nm). TEM images were obtained using a JEM-2100 F transmission electron microscope (JEOL, Japan, http://www.jeol.cn/) operating at 200 V.
Preparation of OMC modified electrode
SBA-15, as the template, was prepared as discussed in the literature [30] and detailed information on the preparation and properties of OMC can be found in [24, 30]. Prior to the modification, the GC electrode was hand polished with 1, 0.3 and 0.05 μm alumina powder, respectively, rinsed with water and then sonicated successively in alcohol and water. Approximately 60 min of ultrasonication was necessary to disperse 1.5 mg OMC into 3 mL N, N-dimethylformamide (DMF). Then 5 μL of OMC suspensions was placed onto the surface of GC electrode, and the solvent was evaporated under an infrared lamp. Thus, OMC/GC electrode was obtained.
Results and discussion
Characterization of OMC
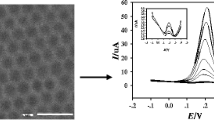
The morphology of OMC was characterized using TEM. TEM images of OMC clearly show highly ordered carbon nanowires viewed from the [0 0 1] (Fig. 1a) and [1 0 0] (Fig. 1b) directions. The structure of OMC is exactly an inverse replica of their parent templates SBA-15. The ordered arrangement gives rise to the well-resolved XRD peaks, as shown in inset of Fig. 1a, which can be assigned to three (1 0 0), (1 1 0) and (2 0 0) diffractions of the 2D hexagonal space group p6mm.
Electrochemical catalysis of MP at the OMC/GC electrode
The successive cyclic voltammogram (CV) of 10 μM methyl parathion in 0.1 M phosphate buffer solution at pH 6.0 at the OMC/GC electrode is shown in Fig. 2. In the first cycle, a sharp irreversible reduction peak (E pc1 , –0.531 V) can be observed, which corresponds to the reduction of the nitro group to hydroxylamine group via a four-electron process [31] (reaction 1). Then it was oxidized to the nitroso group (E pa , 0.064 V, reaction 2). In the following second cycle, the appearance of another reduction peak (E pc , –0.002 V) is ascribed to reverse process (reaction 3) of reaction 2. The pair of reversible redox peaks should be attributed to a two-electron-transfer redox process. The reduction peak Pc1 almost disappears, which should be due to the reaction exhaustion of methyl parathion at the surface of OMC/GC electrode. The mechanism above accords with the described electrochemical reactions of nitroaromatic compounds [6, 18].

However, at the bare GC electrode (inset of Fig. 2), the redox peaks (E pa , 0.144 V and E pc , 0.007 V) are not obvious, and a shoulder peak with low current response is observed for the reduction of methyl parathion at –0.750 V (E pc1 ). Whereas at the OMC/GC electrode, it was interesting to note that the peak potential (E pc1 ) shifts 219 mV positively and the peak current (i pc1 ) increases by 76 times, when compares with the response of MP at the bare GC electrode under identical conditions. So far as we know, there has been no report for such a drastic current increase for the reduction of methyl parathion.
The experimental results indicate the presence of OMC makes the electron transfer easier. We think the unique structure of the OMC may offer a favorite microenvironment which is beneficial for accelerating electron transfer between the electrode and species in solution [26]. Additionally, OMC is hydrophobic and can extract MP via hydrophobic interaction [32], also making the response of MP increase. Accordingly, OMC has excellent electrocatalytic towards methyl parathion.
Influence of scan rate on electrochemical behavior
For further discussion of redox process of MP at the OMC/GC electrode, we investigated the relationship between the peak currents of the second cycle and scan rate by cyclic voltammetry.
As can be known from the CVs (Fig. 3a), both I pa and I pc become larger with the increase of scan rate. It is also found that the anodic and cathodic peak currents of the OMC/GC electrode in MP solution are both linearly proportional to the scan rates ranging from 10 to 130 mV·s–1 (Fig. S1a, Electronic Supplementary Material), and the linear regression equations are I pa (μA) = –6.85 – 0.48 v (mV·s–1) (R2 = 0.9928) and I pc (μA) = 7.01 + 0.49 v (mV·s–1) (R2 = 0.9910). It manifests that the redox reaction of MP occurring at the OMC/GC electrode is a surface-controlled process in the range from 10 to 130 mV·s–1. Furthermore, we have already observed the peak currents of MP at the OMC/GC electrode become larger with the increase of dipping time in the electrolyte, indicating the occurrence of adsorption at the OMC/GC electrode. At higher scan rates, the peak currents increases linearly with the square root of the scan rates from 140 to 600 mV·s–1 (Fig. S1b, Electronic Supplementary Material), which indicates a diffusion-controlled behavior. The linear regression equations, I pa (μA) = 6.80 – 6.78 v 1/2 (mV·s–1) and I pc (μA) = –22.51 + 8.03 v 1/2 (mV·s–1), with correlation coefficient (R2) of 0.9948 and 0.9992 were obtained, respectively.
(a) Cyclic voltammograms of 10 μM MP at the OMC/GC electrode in 0.1 M phosphate buffer solution (pH 6.0) at scan rates of 10, 50, 100, 150, 200, 240, 300, 400, 500, 600 mV·s–1 (from inter to outer). (b) Plots of peak potential as function of lnν (ν: V s–1). Inset figure: the plots of E p versus lnν at high scan rate
The dependence of the anodic peak potential (E pa ) and the cathodic peak potential (E pc ) on the lnv in pH 6.0 phosphate buffer solution is represented in Fig. 3b. At lower scan rates, E pa and E pc remain practically unchanged with the increase of the scan rate, but E pa and E pc shift slightly to positive and negative potential at higher scan rates, respectively. The peak separation (ΔE p ) is increased, suggesting the electrode reaction becomes electrochemically irreversible. At higher scan rates, the plots of E p versus lnv were linear (inset of Fig. 3b), and obeys the following Nicholson’s equation [33]:
Where α is the electron transfer coefficient, n is the electron transfer number, v is the scan rate. The calibration equations are as follows: E pa (V) = 0.122 + 0.069 lnv (V·s–1) (R2 = 0.9975) (anodic peak is used to study), E pc (V) = –0.171 – 0.074 lnv (V·s–1) (R2 = 0.9962). According to the literature, we know that n = 2 [2] (reaction 2 and 3), and from the equations above and the slope of linear curve of E p -lnv, α was calculated to be 0.82 at 288 K.
Optimization of some experimental conditions
In Fig. 4 is represented the evolution of the CV curve when solution pH was varied from 3.0 to 8.0. As one can notice that the anodic peak current of 10 μM MP first reaches maximum at pH 6.0. It indicates that OMC has the maximum adsorption to methyl parathion at mild acid environment. In the acidic medium, the substantial quantity of H+ may somewhat prevent the access of MP to the adsorption sites of the OMC, and whereas basic medium can result in the hydrolyzation of OP compounds [18].
And it is also found that the increase of the solution pH leads to the negative shift of both peak potentials, which is a consequence of the deprotonation involved in the oxidation process that is facilitated at higher pH values [28]. The peak potential and the pH display a linear relationship (Fig. S2, Electronic Supplementary Material) with the regression equation E pa (V) = 0.350 – 0.056 pH (R2 = 0.9925), which is approximately consistent with that given by the Nernstian equation for a two protons, two electrons process [34]. CVs of three support electrolytes (inset of Fig. S2) indicate that there is the biggest current response in phosphate buffer solution. To sum up, a pH value of 6.0 phosphate buffer solution was employed for further measurements.
The anodic peak current of MP is almost independent of accumulation potential. However, the peak current increases remarkably within 5 min and scarcely changes with longer accumulation time (Fig. S3, Electronic Supplementary Material), meaning that the adsorption of MP on the OMC/GC electrode became saturated. Thus, 5 min of accumulation time under open-circuit conditions was selected.
Chronocoulometric response of MP
Chronocoulometry was used to determine the saturating adsorption capacity for MP on the surface of the electrode. The GC or OMC/GC electrode was immersed in a 10 μM MP solution under stirring for 5 min to achieve saturated adsorption. Then, a step potential from –0.6 V to 0.4 V was applied. The typical Q-t curves are recorded in Fig. 5. For the OMC/GC electrode, the charge amount increases rapidly and then slows down (curve b). After subtracting the background charge, we obtained the plot of Q versus t 1/2 (inset of Fig. 5). The linear regression equations are as follows: Q (C) = 8.02 × 10–6 + 1.07 × 10–6 t 1/2 (s) (R2 = 0.9947) (curve c) and Q (C) = 1.24 × 10–4 + 7.19 × 10–6 t 1/2 (s) (R2 = 0.9952) (curve d).
Chronocoulometric response of bare GC electrode (a) and OMC/GC electrode (b) in 0.1 M phosphate buffer solution (pH 6.0) containing 10 μM MP. Inset figure: The plots of Q versus t 1/2, bare GC electrode (c) and OMC/GC electrode (d) in 0.1 M phosphate buffer solution (pH 6.0) containing 10 μM MP (background charge subtracted)
According to the Cottrell equation [35]:
Q dl is the double-layer charge, and Q ads is the adsorption charge of the electrochemical reaction of adsorbed species. The corresponding apparent diffusion coefficients (D) were calculated 2.92 × 10−6 cm2·s−1 (for the GC electrode) and 1.20 × 10−5 cm2·s−1 (for the OMC/GC electrode), respectively. The difference between them may be due to the slightly faster diffusion of MP in the OMC film around the GC electrode.
Using Laviron’s [36] theory of Q ads = nFAГ and intercepts for curves c and d, a Г (surface coverage) value of 2.34 × 10−9 mol·cm−2 (for the OMC/GC electrode) was calculated, which was almost 8.5 times larger than 2.47 × 10−10 mol·cm−2 (for the GC electrode).
Obviously, OMC has excellent accumulative ability towards methyl parathion. The bulky adsorption of MP on the surface of the OMC/GC electrode is attributed to the unique properties of OMC, especially the presence of the oxygen-containing functional groups on the surface of OMC, e.g. –OH、–COOH and C = O [21, 27], which can attract pesticides molecules containing special groups. On the other hand, MP is slightly soluble, and has the higher interfacial activity, which enables MP to move to the electrode surface easily and adsorb largely.
Analytical performance
LSV was recorded for MP determination with the OMC/GC electrode under optimized conditions. As shown in Fig. 6, the anodic peak currents (I pa ) increase with the concentration of MP. It can be observed that I pa is linearly related to MP concentration ranging from 0.09 to 61 μM with an equation of I pa (μA) = –18.74 – 1.31c (μM) (R2 = 0.9936), and the detection limit is 7.6 nM (S/N = 3). A comparison of linear range, detection limit for OMC modified electrode with other MP sensors reported in the literature [7, 12, 19, 37] is shown in Table S1 (Electronic Supplementary Material). All the data reveal the analytical parameters for OMC/GC electrode are comparable and even better than those obtained at several electrodes reported recently.
Interferences from electroactive aromatic compounds and inorganic ions were tested. The results show voltammetric determination of 10 μM MP is not affected by 10-fold of phenol, nitrophenol and nitrobenzene, 100-fold of \( {\hbox{CO}}_3^{{2 - }} \) and \( {\hbox{C}}{{\hbox{H}}_{{3}}}{\hbox{CO}}{{\hbox{O}}^{ - }} \), 500-fold of Mg2+, Ca2+, \( {\hbox{PO}}_4^{{3 - }} \) and \( {\hbox{SO}}_4^{{2 - }} \), 1000-fold of \( {\hbox{NH}}_4^{ + } \), \( {\hbox{NO}}_3^{ - } \) and Cl-.
We also investigated the stability and reproducibility of the OMC/GC electrode. By performing eight parallel experiments with the same electrode, the relative standard deviation (RSD) 1.36% was obtained. After the OMC/GC electrode was stored at 277 K for 2 weeks, the oxidation peak current remained 91.3% of the initial response, suggesting a good stability of the electrode.
Application to real sample
The practicality of the method was evaluated by applying it to the determination of MP in water sample collected from Jing Lake. The real water samples were filtered with a 0.45 μm filter to eliminate particulate matters. No current response of MP was observed in the pretreated sample solution. Thus, in recovery tests, we adopted the standard-additions approach to estimate the reliability. Different amounts of MP were spiked into the lake water samples respectively, and adjusted to a certain concentration. The results obtained were summarized in Table 1. The recoveries were from 98.5% to 100.6%. So the method outlined in this work for MP sensing is promising for direct analysis of environmental samples.
Conclusion
In summary, we have confirmed that the OMC/GC electrode performed an enhanced electrocatalytic activity and accumulative effect towards MP. Furthermore, the electrode also exhibited wider linear range, lower detection limit and better electrochemical stability. Therefore this electrochemical sensing methodology is promising for determing OPs in environment samples.
References
Russo MV, Campanella L, Avino P (2002) Determination of organophosphorus pesticide residues in human tissues by capillary gas chromatography-negative chemical ionization mass spectrometry analysis. J Chromatogr B 780:431–441
Tan XH, Li BH, Zhan GQ, Li CY (2010) Sensitive Voltammetric Determination of Methyl Parathion Using a Carbon Paste Electrode Modified with Mesoporous Zirconia. Electroanalysis 22:151–154
Castanho GM, Vaz CMP, Machado SAS (2003) Electroanalytical procedure for the determination of methylparathion in soil suspensions and its application for sorption studies with Brazilian soils. J Braz Chem Soc 14:594–600
De Souza D, Machado SAS (2006) Study of the electrochemical behavior and sensitive detection of pesticides using microelectrodes allied to square-wave voltammetry. Electroanalysis 18:862–872
Parham H, Rahbar N (2010) Square wave voltammetric determination of methyl parathion using ZrO2-nanoparticles modified carbon paste electrode. Journal of Hazardous Materials 177:1077–1084
Sbaï M, Essis-Tome H, Gombert U (2007) Electrochemical stripping analysis of methyl-parathion (MPT) using carbon fiber microelectrodes (CFME) modified with combinations of poly-NiTSPc and Nafion films. Sensors and Actuators B 124:368–375
Zhang Y, Kang TF, Wan YW, Chen SY (2009) Gold nanoparticles-carbon nanotubes modified sensor for electrochemical determination of organophosphate pesticides. Microchim Acta 165:307–311
Zourob M, Ong KG, Zeng K, Mouffouk F (2007) A wireless magnetoelastic biosensor for the direct detection of organophosphorus pesticides. Analyst 132:338–343
Berijani S, Assadi Y, Anbia M (2006) Dispersive liquid-liquid microextraction combined with gas chromatography-flame photometric detection. Very simple, rapid and sensitive method for the determination of organophosphorus pesticides in water. J Chromatogr A 1123:1–9
Cappielo A, Famiglini G, Palma P (2003) Electron Ionization for LC/MS. Anal Chem 75:496A–503A
Lacorte S, Barceló D (1996) Determination of parts per trillion Levels of organophosphorus pesticides in groundwater by automated on-line liquid-solid extraction followed by liquid chromatography/atmospheric pressure chemical ionization mass spectrometry using positive and negative ion modes of operation. Anal Chem 68:2464–2470
Nirmala JN, Kumaravel A (2010) tearic acid modified glassy carbon electrode for electrochemical sensing of parathion and methyl parathion. J Appl Electrochem 40:1571–1574
Brett CMA (2001) Electrochemical sensors for environmental monitoring. Strategy and examples. Pure Appl Chem 73:1969–1977
Gong JM, Wang LY, Zhang LZ (2009) Electrochemical biosensing of methyl parathion pesticide based on acetylcholinesterase immobilized onto Au-polypyrrole interlaced network-like nanocomposite. Biosens Bioelectron 24:2285–2288
Shoji E, Freund MS (2001) Potentiometric sensors based on the inductive effect on the pKa of poly(aniline): A nonenzymatic glucose sensor. J Am Chem Soc 123:3383–3384
Wang J, Pumera M, Chatrathi MP (2002) Single-channel microchip for fast screening and detailed identification of nitroaromatic explosives or organophosphate nerve agents. Anal Chem 74:1187–1191
Kumaravel A, Chandrasekaran M (2010) A novel nanosilver/nafion composite electrode for electrochemical sensing of methyl parathion and parathion. Journal of Electroanalytical Chemistry 638:231–235
Du D, Ye XP, Zhang JD (2008) Cathodic electrochemical analysis of methyl parathion at bismuth-film-modified glassy carbon electrode. Electrochimica Acta 53:4478–4484
Li CY, Mo WY, Zhan GQ (2008) Sensitive Determination of Methyl-Parathion in Contaminated Water Using Single-Walled Carbon Nano-Tube Film Electrode. Chem Anal (Warsaw) 53:201–214
Ryoo R, Joo SH, Jun S (1999) Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J Phys Chem B 103:7743–7746
Jia NQ, Wang ZY, Yang GF (2007) Electrochemical properties of ordered mesoporous carbon and its electroanalytical application for selective determination of dopamine. Electrochem Commun 9:233–238
Liang C, Dai S (2006) Synthesis of mesoporous carbon materials via enhanced hydrogen-bonding interaction. J Am Chem Soc 128:5316–5317
Zhou M, Guo JD, Guo LP, Bai J (2008) Electrochemical Sensing Platform Based on the Highly Ordered Mesoporous Carbon-Fullerene System. Anal Chem 80:4642–4650
Jun S, Joo SH, Ryoo R, Kruk M (2000) Synthesis of new nanoporous carbon with hexagonally ordered mesostructure. J Am Chem Soc 122:10712–10713
Ndamanisha JC, Guo LP, Wang G (2008) Mesoporous carbon functionalized with ferrocenecarboxylic acid and its electrocatalytic properties. Micropor Mesopor Mater 113:114–121
Zhou M, Shang L, Li BL, Dong SJ (2008) The characteristics of highly ordered mesoporous carbons as electrode material for electrochemical sensing as compared with carbon nanotubes. Electrochem Commun 10:859–863
Li F, Song JX, Niu L (2010) Electrochemical determination of morphine at ordered mesoporous carbon modified glassy carbon electrode. Biosensors and Bioelectronics 25:1408–1413
Hou Y, Guo LP, Wang G (2008) Synthesis and electrochemical performance of ordered mesoporous carbons with different pore characteristics for electrocatalytic oxidation of hydroquinone. Journal of Electroanalytical Chemistry 617:211–217
Qi B, Lin FY, Bai J, Liu L, Guo LP (2008) An ordered mesoporous carbon/didodecyldimethylammonium bromide composite and its application in the electro-catalytic reduction of nitrobenzene. Materials Letters 62:3670–3672
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279:548–552
Liu GD, Lin YH (2005) Electrochemical Sensor for Organophosphate Pesticides and Nerve Agents Using Zirconia Nanoparticles as Selective Sorbents. Anal Chem 77:5894–5901
Holbrey JD, López-Martin I, Rothenberg G (2008) Desulfurisation of oils using ionic liquids: Selection of cationic and anionic components to enhance extraction efficiency. Green Chemistry 10:87–92
Zhang Z, Wang E (2000) Electrochemical Principles and Methods. Science Press, Beijing
Vieira DC, Fatibillo-Filho O, Angnes L (1999) Zucchini crude extract palladium modified carbon paste electrode for the determination of hydroquinone in photographic developers. Anal Chim Acta 398:145–151
Wang B, Cao X (1991) Anodic oxidation of hydrazine on glassy carbon modified by macrocyclic transition metal complexes: part 1. Cobalt protoporphyrin dimethyl ester modified electrode. J Electroanal Chem 309:147–158
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28
Kang TF, Wang F, Lu LP (2010) Methyl parathion sensors based on gold nanoparticles and Nafion film modified glassy carbon electrodes. Sensors and Actuators B 145:104–109
Acknowledgements
The authors gratefully acknowledge the financial support by the National Natural Science Foundation of China (No. 20875012).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Table S1
Comparison of linear range, detection limit of MP at different electrodes (DOC 33 kb)
Fig. S1
(a) The relationship between the peak currents and scan rates ranging from 10 to 130 mV·s–1. (b) The relationship between the peak currents and the square root of scan rates ranging from 140 to 600 mV·s–1 (DOC 55 kb)
Fig. S2
The plots of the peak potential E pa versus solution (0.1M phosphate buffer solution) pH. Scan rate: 100 mV·s–1. Inset figure shows the comparison of three different types of buffer solutions: phosphate buffer solution (a), acetate buffer solution (b) and B-R buffer solution (c) (DOC 43 kb)
Fig. S3
The effect of accumulation time on the peak current intensity of MP voltammograms (0.1 M phosphate buffer solution pH 6.0, other variables are same as those in Fig. 2) (DOC 37 kb)
Rights and permissions
About this article
Cite this article
Pan, D., Ma, S., Bo, X. et al. Electrochemical behavior of methyl parathion and its sensitive determination at a glassy carbon electrode modified with ordered mesoporous carbon. Microchim Acta 173, 215–221 (2011). https://doi.org/10.1007/s00604-011-0551-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0551-1