Abstract
Cuprous oxide (Cu2O) thin films have been deposited onto fluorine doped tin oxide (FTO) glass substrates by using electrochemical route. The structural, morphological, and chemical composition of the deposited films have been studied by using X-ray diffraction (XRD), Scanning electron microscopy (SEM) and Energy dispersive x-ray spectroscopy (EDAX) techniques respectively. The optical studies have been carried out by using UV-Vis spectroscopy. The effect of potential, pH and bath temperature onto absorption and band gap of Cu2O thin films have been studied. The highest sensitivity 6.25 mA·mM·cm- 2 is observed for the thin films which shows glucose concentration 7 mM in 0.1 M NaOH solution. The results indicates Cu2O is promising material for glucose sensor with high sensitivity, high stability, and repeatability.

The surface morphology of Cu2O thin films was found to be tip-truncated octahedral. The films were prepared by electrodeposition. The Cu2O thin films were used to construct low cost, highly sensitive and stable glucose sensor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tremendous work is carried out to develop electrochemical glucose sensors because of their applications in sports, medicine, environment monitoring, food quality control and pharmaceutical industries [1]. There are two types of glucose sensors, viz. enzymatic and nonenzymatic. The disadvantage of glucose sensing with enzymatic sensors is poor stability. The boron doped diamond electrode (BDDE) is alternative material for electrochemical sensors but it shows a wide band gap of 5.5 eV, so it is normally electrically insulating. The specific conditions and expensive instrumentation needs for synthesis of BDDE. The Cu2O electrode shows narrow band gap so it carries more charges and can be easily deposited via low cost methods with high stability, reproducibility and repeatability of glucose sensing than BDDE electrode. There are large number of materials are used for electrochemical sensing, such as ruthenium oxide [2], zinc oxide [3], polyglutamic acid [4], copper oxide [5], carbon nanotubes [6], titanium dioxide [7], gold nanoparticles [8] and graphene oxide [9, 10]. Copper is an important material in many industrial fields because of its high catalytic activity, low growth temperature and good electrical conductivity. Cuprous oxide or cuprite (Cu2O) is a p-type semiconducting material having direct band gap of nearly 2.17 eV [11]. Semiconducting Cu2O thin films find applications in solar cell [13], glucose sensor [14], photocatalyst [15], hybrid diode [16], biosensor [17], solar water splitting [18], gas sensor [19], chemiluminescence [20], polymer memory devices [21], transistors [22] and lithium ion batteries [23]. In recent years electrodeposition method is most attracted towards the deposition of Cu2O thin films. Although there are various techniques for deposition of Cu2O material and each technique has self-advantages and disadvantages. The deposition of Cu2O is carried out by using electrodeposition [24], up scaling [25], radio frequency magnetron sputtering [26], solvothermal [27], co-precipitation [28], sol gel method [29] and etc. The deposition of Cu2O as n-type, p-type and p-n- homojunction semiconductor is also possible by changing pH of the bath [30]. The formation of Cu2O with different morphology and size is very critical method. The variation of potential, bath pH, chemical concentration and bath temperature of Cu2O gives various types of morphology like complete octahedra, tip-truncated octahedra, truncated octahedra, cuboctahedra, tip-truncated cubes, cubes, nanowires, nanotubes and star [31, 32] etc. The deposition of Cu2O is possible onto various substrates like fluorine doped tin oxide (FTO), indium doped tin oxide (ITO), silicon foil (Si), titanium foil (Ti), copper foil (Cu), anodic aluminum oxide (AAO) membranes, etc.
In this investigation, we report on the deposition of Cu2O thin films onto FTO substrates by using electrodeposition. The variation in band gap is observed for changing its potential, bath pH value and bath temperature. The variation of temperature of the solution changes the absorption of visible light with respect to wavelength. The superior results are obtained for sensing in bath contains glucose and NaOH as electrolytes. The Cu2O thin films are more sensitive to glucose by addition of NaOH. This work is useful to produce highly sensitive glucose sensor by low cost electrodeposition method.
Experimental
Preparation of Cu2O thin films
Cuprous oxide (Cu2O) thin films were electrodeposited onto FTO substrates. Initially FTO substrates were washed with laboline and double distilled water. The three electrode system was used for electrodeposition technique. The platinum was used as counter electrode and Ag/AgCl was used as reference electrode. The FTO substrate was used as working electrode. The electrolytes were containing all analytical reagent chemicals such as 1 M CuSO4.5H2O and 1 M C3H6O3 (lactic acid). A 5 M NaOH was used for balancing the pH. The 50 ml solution was stirred for 1 h and heated upto 40 °C. The cathodic electrodeposition potential was kept constant at −0.20 V (vs. Ag/AgCl) for 30 min. The electrodeposited Cu2O thin films were washed with double distilled water and dried by natural way.
Characterizations
The electrodeposition, cyclic voltammograms and current time response were carried out by using Autolab PGSTAT 101 instrument with Nov. 1.11 software. The crystal structure of electrodeposited Cu2O thin films was studied by using x-ray diffraction (XRD) technique with model Bruker D8 Advance with Cu Kα radiation (λ = 1.54Ao) in the range (2θ) of 20° - 80°. The surface morphology and chemical composition inside the morphology of deposited films were analyzed by using scanning electron microscope (SEM) with energy dispersive x-ray spectroscopy (EDAX) of model JEOL JSM-6360 having operating bias voltage 20 kV. To study the interaction between thin film surface and liquid, the contact angel measurement was studied by using Rame-hert USA instrument with CCD camera. The optical measurements within the wavelength range 485–800 nm were carried out using UV-VIS spectrophotometer SYSTRONICS 119. The Raman spectra was studied by using micro-Raman Renishaw spectrometer of wavelength 532 nm at room temperature.
Electrochemical measurements
The electrochemical measurement were performed by using three electrode system on Autolab PGSTAT 101 instrument with Nov. 1.11 software. The Cu2O thin films was used as working electrode, platinum foil as counter electrode and Ag/AgCl as reference electrode. Freshly prepared 100 ml 0.1 M NaOH solution in different concentrations of glucose was used as electrolyte. All electrochemical experiments were carried out at room temperature.
Results and discussion
Structural and morphological study
The phenomenon of X-ray diffraction governed by the Bragg’s diffraction law is
where d is lattice spacing, λ is the wavelength of the monochromatic X-ray, ‘n’ is order of diffraction and θ is diffraction angle.
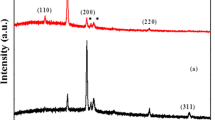
The XRD pattern of electrodeposited Cu2O thin films onto FTO substrates is as shown in Fig. 1. The XRD results of Cu2O thin films shows polycrystalline in nature. The peaks of FTO onto Cu2O thin films marked by asterisks. The bath temperature for electrodeposition was kept constant at 40 °C. At this temperature more H+ ions are formed in the bath, because of this there is no possibility of formation of Cu (OH)2. The XRD results shows the hkl plane (111) is more intensive having 2θ = 36.52o Liau et al. reported lowest intensity of maximum peak than these reported results [24]. The results indicates crystallinity of Cu2O thin films is more for this plane. The 2θ (degree) and d values of electrodeposited Cu2O thin films are in good agreement with standard values of JCPDS card no. 75–1531 and it is as shown in Table 1.
The crystallite size (D) for the more intensive peaks (111), (200) having bath pH 9 of electrodeposited Cu2O thin films are estimated by full width at half maximum (FWHM) by Debye- Scharrer’s formula:
where D is crystallite size, λ is wavelength of X-ray used, θ is the Bragg’s angle, β is full width at half maximum of the peak in radian and K is a constant. The Crystallite size for (111) plane is observed to be 18.60 nm and for (200) plane it is 19.38 nm.
The surface morphology study for Cu2O thin films deposited onto FTO substrates having bath pH 9 is shown in Fig. 2. The morphology of deposited thin films is tip -truncated octahedra type shape which is good in accordance with previous results [31]. The Fig. 2(a) provide lower X 3000 whereas Fig. 2(b) provide higher X 10,000 magnified images of Cu2O thin films. The change in morphology also observed for change in various parameters as per literature survey [32].
Chemical composition and contact angle study
The chemical composition of Cu2O is measured by energy dispersive x-ray spectroscopy (EDAX) measurement as shown in Fig. S1 (Electronics Supplementary Material, ESM). The results show that the presence of Sn in EDAX is attributed due to Cu2O deposited onto SnO2: F (FTO) surface. It also affects more atomic weight percent of oxygen as compared to Cu. The Table 2 shows that the exact element, weight percent and atomic percent of Cu2O material on FTO thin films. The energy drink glucose served as sample for electrochemical sensing of Cu2O electrode, which contains dextrose monohydrate 99.4 % and calcium phosphate 0.6 % (99.9 % pure glucose).
The interaction between liquid and thin film surface of Cu2O is measured by contact angle method as shown in Fig. 3. The deposited thin films according to contact angle are divided into two forms hydrophilic and hydrophobic. The angle is formed for film deposited at pH 9 is found to be 52o. The contact angle of electrodeposited thin film is higher than reported earlier [33].
Optical measurements
The UV-VIS absorption spectroscopy of Cu2O thin films in the range of 485–800 nm is shown in Fig. S2 (A) (ESM). The optical band gap (Eg) of Cu2O thin films by using absorption spectra is determined by using the equation.
where Eg stands for the band gap, hν for incident photon energy, A constant, α coefficient of absorption and n determines the electronic transition causing absorption. The Fig. S2 (A) (ESM) shows that the optical absorption of Cu2O thin films at potential −0.20 V (vs. Ag/AgCl) (curve (a)) and −0.80 V (vs. Ag/AgCl) (curve (b)). Due to high working potential the oxidation of Cu (I) to Cu (II) changes the particle characteristics. The Fig. S2 (A) (ESM) shows the change in absorption with potential. In Fig. S2 (B) (ESM) the decrease in band gaps are observed when potential changes from lower to higher state. Tauc plot detects the value of band gap of Cu2O thin films. The Fig. S2 (B) (ESM) shows the variation in band gaps with deposition potentials. For the potential −0.20 V/ (Ag/AgCl) (curve (a)) the band gap is 2.16 eV while at potential −0.80 V (vs. Ag/AgCl) (curve (b)) it is 1.94 eV. The band gap decreases as the potential increases due to co-existence of Cu2O to Cu2O and Cu for redshift absorption edge [34]. X. Jiang et al. reports different results than these reported values [35]. In Fig. S2 (C) (ESM) it is seen that at pH 9 (a), pH 10 (b) and pH 11 (c) the absorption spectra of electrodeposited Cu2O thin films vary with change in bath pH. The change in band gaps with pH of the bath is shown in Fig. S2 (D) (ESM). The band gaps for pH 9 (a), pH 10 (b) and pH 11 (c) are 2.10, 2.16 and 2.23 eV respectively. The change in pH results change in H+ ion concentration and it causes increase in crystallite size. The shifts of band gap are due to change in particle size and increase in concentration reported by previous results [28]. The optical absorption intensity is more than 50 % in visible region for Cu2O thin films of tip truncated octahedra. The thin films deposited at different bath temperatures shows the optical absorption spectra in Fig. S2 (E) (ESM). The thin films of Cu2O electrodeposited at various bath temperatures are a (40°), b (45°), c (50°), d (55°), e (60°), f (65°), g (70°), h (750) and i (80 °C) as shown in Fig. S2 (E) (ESM). The bath temperature differs the band gap of deposited thin films, because the crystal structure and surface morphology affecting on it [36].
Raman spectra of thin films
The Raman spectroscopy in the range of 100 to 1000 cm−1 is shown in Fig. 4. It indicates that only Cu2O phase is formed, no other impurities like Cu and CuO are observed. The strongest peak is observed at 110 cm−1, other peaks are shown at 148, 201, 220, 416, 525 and 635 cm−1. The peaks at 148, 220, 416 and 635 cm−1 are corresponding to second order overtone Γ15 (1), 2Γ12, 2Γ15 (1) and Γ15 (2) vibrations of the Cu2O thin films respectively [29, 36]. The absence of 299 and 346 cm−1 peaks shows that CuO impurity is not presence in prepared films.
Electrochemical sensor measurements
In order to study the glucose sensing property of Cu2O thin films the cyclic voltammetry is one the good preferential option. The cyclic voltammetry of Cu2O thin films in absence of glucose and in presence of glucose is shown in Fig. 5(a). The scan rate 10 mVs−1 and potential window for cyclic voltammetry is −0.20 to +0.80 V. In Fig. 5(a) 0.1 M NaOH was used as electrolyte for plot a (10 mV·s−1) scan rate and in presence of 1 mM glucose for plot b (10 mVs−1) scan rate. It was observed that the current suddenly increases with addition of glucose in NaOH solution. In presence of glucose no oxidation peaks were observed in plot b, but strong reduction peak was observed at 0.55 V. In absence of glucose there was reduction peak observed at 0.58 V. It shows the formation of Cu (III)/Cu (II) couple [37]. The following reaction explains conversion of glucose to gluconic acid [38].
Cyclic voltammetry of Cu2O thin films (a) in absence and presence of glucose at scan rate 10 mVs−1, (b) in presence of 1 mM glucose in 0.1 M NaOH from scan rates 10 to 90 mVs−1, (c) linearly fitted curve of Cu2O thin films for current increase with scan rate. (d) current-time measurement of Cu2O thin films in presence of glucose from potential 0.20 to 0.70 V, (e) cyclic voltammetry of Cu2O thin films from concentration 1 to 7 mM of glucose, and (f) linearly fitted curve of Cu2O thin films for current with increase in glucose concentration
The production of Cu (III) is on the surface of Cu2O thin film and glucose gets oxidized into gluconic acid. Cu (III) acts as a mediator for travel of electrons with catalytic activity. The cyclic voltammetry of Cu2O thin films in presence of 1 mM glucose in 0.1 M NaOH solution with scan rate 10 to 90 mV·s−1 is shown in Fig. 5(b). The results show that there is increase in current with increase in scan rate from 10 to 90 mV·s−1. The increase in scan rate affects the position of redox peak and redox peak current. The Fig. 5(c) shows the linearly fitted curve of Cu2O thin films for increase in current with rise in scan rate. The equation y = 0.0091× + 2.0778 for Cu2O electrode with the correlation coefficient R2 = 0.995. L. Mei et al. reports current linearly rises with increase in scan rate for the electrode of nanoporous copper composite with carbon black and nafion [39]. The Fig. 5(d) shows the current-time response of Cu2O thin films in the range of 0 to 400 s. from potential 0.20 to 0.70 V. From the results it was found that the current increases with potential of Cu2O electrode. At lower potentials 0.20 to 0.35 V very low current response was observed and after 0.45 V current increases suddenly with potential. M. Liu et al. reports different results than theses reported values [40]. In Fig. 5(e) the concentration of glucose in 0.1 M NaOH is varied from 1 mM to 7 mM. The cyclic voltammetry measurement shows that the increase in current with concentration of glucose. These results indicates the good electrocatalytic activity of nearer to glucose oxidation and it is used to non-enzymatic glucose sensor [5]. As the percentage of glucose increases the line width between the cycle’s decreases. The change in current with respect to concentration of glucose were observed in linearly fitted curve at Fig. 5(f). The result shows that as concentration increase from 1 mM to 7 mM the current rises suddenly but after 7 mM glucose the current remains constant. H. Heidari et al. reports there is linear relation between concentration of glucose and current of the electrode and it gives perfect electrical conduction [41]. The equation y = 0.6709× + 1.5673 with correlation coefficient R2 = 0.9655 shows the effect of glucose on conduction of Cu2O electrode. The highest current sensitivity 6.25 mA/mM cm2 was observed for 7 mM glucose and after that slightly decrease in current was observed. These results are attractive and show higher electrochemical sensing than previously reported materials results by J. Ping et al. [42] and N. Xu et al. [43]. The above all results shows that Cu2O thin films are very good for glucose sensing. The stability of these glucose sensors is stable for 40 days with repetitive results. In feature these films will be used for detection of glucose in the human body, milk and other related industries.
Conclusions
The crystallite size is found to be 18.60 nm for (111) plane and for (200) plane it is 19.38 nm. This novel work describes the variation of potential, pH, concentration and bath temperature and its effects on absorption and band gap of Cu2O thin films. The highest band gap 2.23 eV is observed for the thin films deposited at pH 11. The Raman spectroscopy shows single phase Cu2O thin films, in absence of other impurities. This work represents synthesis of high sensitive Cu2O thin films for glucose sensor. The Cu2O glucose sensor shows high sensitivity in presence of NaOH electrolyte. The Cu2O thin films shows good linear range of detection of glucose, and highest current sensitivity 6.25 mA/mM cm2 for 7 mM glucose. The electrodeposited Cu2O thin films gives high sensitive, and repeatable glucose sensor.
References
Nikolaus N, Strehlitz B (2008) Amperometric lactate biosensors and their application in (sports) medicine, for life quality and wellbeing. Microchim Acta 160:15
Shim JH, Kang M, Lee Y, Lee C (2012) A nanoporous ruthenium oxide framework for amperometric sensing of glucose and potentiometric sensing of pH. Microchim Acta 177:211
Zhao Y, Fang X, Yan X, Zhang X, Kang Z, Zhang G, Zhang Y (2015) Nanorod arrays composed of zinc oxide modified with gold nanoparticles and glucose oxidase for enzymatic sensing of glucose. Microchim Acta 182:605
Zhang R, Xu S, Luo J, Liu X (2015) Molecularly imprinted photo-sensitive polyglutamic acid nanoparticles for electrochemical sensing of hemoglobin. Microchim Acta 182:175
Wang G, Wei Y, Zhang W, Zhang X, Fang B, Wang L (2010) Enzyme-free amperometric sensing of glucose using Cu-CuO nanowire composites. Microchim Acta 168:87
Tu X, Zhao Y, Luo S, Luo X, Feng L (2012) Direct electrochemical sensing of glucose using glucose oxidase immobilized on functionalized carbon nanotubes via a novel metal chelate- based affinity method. Microchim Acta 177:159
Li J, Kuang D, Feng Y, Zhang F, Liu M (2012) Glucose biosensor based on glucose oxidase immobilized on a nanofilm composed of mesoporous hydroxyapatite, titanium dioxide, and modified with multi-walled carbon nanotubes. Microchim Acta 176:73
Li S-J, Deng D-H, Shi Q, Liu S-R (2012) Electrochemical synthesis of a graphene sheet and gold nanoparticle-based nanocomposite, and its application to amperometric sensing of dopamine. Microchim Acta 177:325
Gong H, Sun M, Fan R, Qian L (2013) One-step preparation of a composite consisting of graphene oxide, Prussian blue and chitosan for electrochemical sensing of hydrogen peroxide. Microchim Acta 180:295
Luo J, Zhang H, Jiang S, Jiang J, Liu X (2012) Facile one-step electrochemical fabrication of a non-enzymatic glucose-selective glassy carbon electrode modified with copper nanoparticles and graphene. Microchim Acta 177:485
Mei L-P, Feng J-J, Wu L, Chen J-R, Shen L, Xie Y, Wang A-J (2016) A glassy carbon electrode modified with porous Cu2O nanospheres on reduced graphene oxide support for simultaneous sensing of uric acid and dopamine with high selectivity over ascorbic acid. Microchim Acta 183:2039
Kim J, Kim HS, Choi JH, Jeon H, Yoon Y, Liu J, Park J-G, Braun PV (2014) Epitaxial growth of three-dimensionally Mesostructured single-crystalline Cu2O via Templated electrodeposition. Chem Mater 26:7051
Vequizo JJM, Zhang C, Ichimura M (2015) Fabrication of Cu2O/Fe–O heterojunction solar cells by electrodeposition. Thin Solid Films 597:83
Mei L-P, Song P, Feng J-J, Shen J-H, Wang W, Wang A-J, Weng X (2015) Nonenzymatic amperometric sensing of glucose using a glassy carbon electrode modified with a nanocomposite consisting of reduced graphene oxide decorated with Cu2O nanoclusters. Microchim Acta 182:1701
Luo Y, Huang Q, Li B, Dong L, Fan M, Zhang F (2015) Synthesis and characterization of Cu2O–modified Bi2O3 nanospheres with enhanced visible light photocatalytic activity. Appl Surf Sci 357:1072
Izaki M, Saito T, Ohata T, Murata K, Fariza BM, Sasano J, Shinagawa T, Watase S (2012) Hybrid Cu2O diode with orientation-controlled C60 Polycrystal. ACS Appl Mater Interfaces 4:3558
Zhang Z, Zhang S, Liu S, Wang M, Fu G, He L, Yang Y, Fang S (2015) Electrochemical aptasensor based on one-step synthesis of Cu2O@aptamer nanospheres for sensitive thrombin detection. Sensors Actuators B 220:184
Ma Q-B, Hofmann JP, Litke A, Hensen EJM (2015) Cu2O photoelectrodes for solar water splitting: tuning photoelectrochemical performance by controlled faceting. Sol Energy Mater Sol Cells 141:178
Hsu C-L, Tsai J-Y, Hsueh T-J (2016) Ethanol gas and humidity sensors of CuO/Cu2O composite nanowires based on a Cu through-silicon via approach. Sensors Actuators B 224:95
Kaviyarasan K, Anandan S, Mangalaraja RV, Sivasankar T, Ashokkumar M (2016) Sonochemical synthesis of Cu2O nanocubes for enhanced chemiluminescence applications. Ultrasonics. Sonochemistry 29:388
Choi DJ, Kim J-K, Seong H, Jang M-S, Kim Y-H (2015) The formation of Cu2O nanoparticles in polyimide using Cu electrodes via chemical curing, and their application in flexible polymer memory devices. Org Electron 27
Al-Jawhari HA (2015) A review of recent advances intransparent p-type Cu2O-based thin film transistors. Mater Sci Semicond Process 40:241
Valvo M, Rehnlund D, Lafont U, Hahlin M, Edströma K, Nyholm L (2014) The impact of size effects on the electrochemical behaviour of Cu2O-coated Cu nanopillars for advanced Li-ion microbatteries. J Mater Chem A 2:9574
Liau LC-K, Tseng P-C (2015) Effect of Current Pulse on Electronic Properties of Cu2O Films Fabricated by Electrochemical Deposition Process. Electrochim Acta 182:781
Carbó-Argibay E, Bao X-Q, Rodríguez-Abreu C, Cerqueira MF, Petrovykh DY, Liu L, Kolen’ko YV (2015) Up-scaling the synthesis of Cu2O submicron particles with controlled morphologies for solar H2 evolution from water. J Colloid Interface Sci 456:219
Poulopoulos P, Baskoutas S, Pappas SD, Garoufalis CS, Droulias SA, Zamani A, Kapaklis V (2011) Intense quantum confinement effects in Cu2O thin films. J Phys Chem C 115:14839
Luo X-L, Wang M-J, Yun L, Yang J, Chen Y-S (2016) Structure-dependent activities of Cu2O cubes in thermal decomposition of ammonium perchlorate. J Phys Chem Solids 90:1
Sekhar H, Rao DN (2012) Preparation, characterization and nonlinear absorption studies of cuprous oxide nanoclusters, micro-cubesand micro-particles. J Nanopart Res 14:976
WL Y, Lin YZ, Zhu XW, ZG H, Han MJ, Cai SS, Chen LL, Shao HH (2015) Diversity of electronic transitions and photoluminescence properties of p-type cuprous oxide films: a temperature-dependent spectral transmittance study. J Appl Phys 117:045701
Liau LC-K, Lin Y-C, Peng Y-J (2013) Fabrication Pathways of p-n Cu2O Homojunction Films by Electrochemical Deposition Processing. J Phys Chem C 117:26426
Susman MD, Feldman Y, Vaskevich A, Rubinstein I (2014) Chemical deposition of Cu2O Nanocrystals with precise morphology control. ACS Nano 8:162
Choi K-S (2008) Shape control of inorganic materials via electrodeposition. Dalton Trans 40:5432
Eskandari A, Sangpour A, Vaezi MR (2014) Hydrophilic Cu2O nanostructured thin films prepared by facile spin coating method: investigation of surface energy and roughness. Mater Chem Phys 147:1204
Jiang X, Zhang M, Shi S, He G, Song X, Sun Z (2014) Influence of applied potential on the band gap of Cu/Cu2O thin films. J. Electrochem. Society 161:D640
Jiang X, Zhang M, Shi S, He G, Song X, Sun Z (2014) Microstructure and optical properties of nanocrystalline Cu2O thin films prepared by electrodeposition. Nanoscale Res Lett 9:219
Huang M-C, Wang TH, Chang W-S, Lin JC, C-C W, Chen I-C, Peng K-C, Lee S-W (2014) Temperature dependence on p-Cu2O thin film electrochemically deposited onto copper substrate. Appl Surf Sci 301:369
Lu N, Shao C, Li X, Shen T, Zhang M, Miao F, Zhang P, Zhang X, Wang K, Zhang Y, Liu Y (2014) CuO/Cu2O nanofibers as electrode materials for non-enzymatic glucose sensors with improved sensitivity. RSC Adv 4:31056
Khan R, Ahmad R, Rai P, Jang L-W, Yun J-H, Y-T Y, Hahn Y-B, Lee I-H (2014) Glucose- assisted synthesis of Cu2O shuriken-like nanostructures and their application as nonenzymatic glucose biosensors. Sensors Actuators B 203:471
Mei L, Zhang P, Chen J, Chen D, Quan Y, Gu N, Zhang G, Cui R (2016) Non-enzymatic sensing of glucose and hydrogen peroxide using a glassy carbon electrode modified with a nanocomposites consisting of nanoporous copper, carbon black and nafion. Microchim Acta 183:1359
Liu M, Liu R, Chen W (2013) Graphene wrapped Cu2O nanocubes: non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens Bioelectron 45:206
Heidari H, Habibi E (2016) Amperometric enzyme-free glucose sensor based on the use of a reduced graphene oxide paste electrode modified with electrodeposited cobalt oxide nanoparticles. Microchim Acta 183:2259
Ping J, Ru S, Fan K, Wu J, Ying Y (2010) Copper oxide nanoparticles and ionic liquid modified carbon electrode for the non-enzymatic electrochemical sensing of hydrogen peroxide. Microchim Acta 171:117
Xu N, Ding Y, Ai H, Fei J (2010) Acetylene black-ionic liquids composite electrode: a novel platform for electrochemical sensing. Microchim Acta 170:165
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic Supplementary Materials
ESM 1
(DOCX 207 kb)
Rights and permissions
About this article
Cite this article
Pagare, P.K., Torane, A.P. Band gap varied cuprous oxide (Cu2O) thin films as a tool for glucose sensing. Microchim Acta 183, 2983–2989 (2016). https://doi.org/10.1007/s00604-016-1949-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1949-6