Abstract
We describe a simple solvothermal method for preparation of reduced graphene oxide nanosheets decorated with uniform Cu2O nanoclusters by using poly(vinyl pyrrolidone)-poly(methacrylamide)-poly(vinyl imidazole) triblock co-polymer as a shape-directing agent and L-glutamic acid as a reducing agent. The resulting nanocomposite was deposited on a glassy carbon electrode where it displays improved electrocatalytic activity toward glucose oxidation in 0.5 M NaOH. This observation was exploited to construct a non-enzymatic amperometric sensor for glucose. It has a detection limit as low as 1.0 μM, high sensitivity (23.058 μA mM−1), and a dynamic (analytical) range that extends from 5.0 to 9595 μM at a working potential of 600 mV (vs. SCE).

Uniform Cu2O nanoclusters supported on reduced graphene oxide (Cu2O NCs/r-GO) were prepared by a simple solvothermal method in the presence of a triblock copolymer as a shape-directing agent. The nanocomposite was used to construct a non-enzymatic glucose amperometric sensor with low detection limit, high sensitivity, and wide linear range.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A variety of noble metal nanostructures with desired physical and/or chemical properties has been prepared [1], but their high price and rare storage seriously confine their commercial applications. Therefore, it is essential to search for low-cost and plentiful metals (e.g., Ni, Cu, Co, and Fe) as alternatives.
Cu2O is one of the most investigated semiconductors and has wide applications in CO oxidation, biosensors, solar energy conversion, and photocatalysis [2]. Its catalytic properties are tightly correlated with the respective structural features, size, and morphology [3]. As a result, it is highly desired to prepare novel Cu2O nanocatalysts with fine-controlled structure, dimension, and shape [4]. Accordingly, many shape-controlled Cu2O nanostructures have been prepared, including cages [5], cubes [6], and spheres [7].
Many supports have been introduced to endow the above catalysts with well distribution, especially graphene oxide (GO) nanosheets [7] or reduced graphene oxide (r-GO) [7], owing to their enlarged surface area, high electrical conductivity, better chemical stability, and strong adhesion to the catalysts [8]. To date, many nanocomposites, especially metal oxide-graphenes, have been synthesized, including Cu2O-graphene [9], NiO-graphene [10], TiO2-graphene [11], and MnO2-graphene [12].
Enzyme-based electrochemical biosensors display high sensitivity and selectivity [13]. Nevertheless, they suffer from poor stability and reproducibility. Alternatively, non-enzymatic sensors possess better durability and stability against external environment toward glucose oxidation [14]. For example, Zhou et al. fabricated monodisperse porous Cu2O/r-GO, which showed excellent catalytic activity and good selectivity for glucose detection [15]. In another example, Gao’s group prepared mesocrystalline Cu2O hollow nanocubes for construction of glucose sensors [14]. However, their relatively higher detection limit and narrow linear range are unsatisfied during accurately monitoring glucose.
Polymers have strong interactions and sometimes form coordination complexes with nanocrystals, and thereby influence the growth kinetics of the nanocrystals as capping agents and shape-directing agents [16]. A variety of polymers (e.g., cationic, anionic and nonionic polymers) has been used for shape-controlled synthesis of nanocrystals [17]. Polyvinylpyrrolidone (PVP) is the most frequently used nonionic polymer in this field [18]. Copolymers, containing two or more different monomers, have more functional properties [19]. For example, poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) Pluronic® P123 was used as a shape-directing agent for shape-controlled synthesis of mesoporous silica [20].
Herein, a facile solvothermal method is described for synthesis of uniform Cu2O nanoclusters on r-GO (denoted as Cu2O NCs/r-GO), using poly(vinyl pyrrolidone)-poly(methacrylamide)-poly(vinyl imidazole) triblock copolymer (Fig. S1, Electronic Supplementary Material, ESM) and L-glutamic acid as a shape-directing agent and a reducing agent. The catalytic performance of Cu2O NCs/r-GO was investigated through the fabrication of a non-enzymatic glucose sensor.
Experimental
Reagents and materials
Copper(II) nitrate trihydrate, sodium hydroxide, and glucose were purchased from Shanghai Sigma-Aldrich (Shanghai, China, www.sigmaaldrich.com). Poly(vinyl pyrrolidone)-poly(methacrylamide)-poly(vinyl imidazole) triblock copolymer (LuvisetR Clear), graphite powder (99.95 %, 8000 mesh), L-glutamic acid, ascorbic acid (AA), dopamine (DA), uric acid (UA), lactic acid, glutaric acid, acetaminophen, and NaBH4 were bought from Shanghai Aladdin Chemical Reagent Company (Shanghai, China, www.chemicalbook.com). The other chemicals were of analytical grade and used without further purification. Double distilled-water was used to prepare all of the aqueous solutions throughout the whole experiments.
Preparation of Cu2O NCs/r-GO and of Cu2O nanoparticles
Typically, GO was prepared from natural graphite powder by a modified Hummers’ method, and experimental details are given in the previous work [21].
For typical preparation of Cu2O NCs/r-GO, 0.5 g of poly(vinyl pyrrolidone)-poly(methacrylamide)-poly(vinyl imidazole) triblock copolymer was dissolved into 15 mL of ethanol under stirring. Then, 5 mL of GO (1 mg mL−1), 0.121 g of Cu(NO3)2 · 3H2O, and 0.3 g of L-glutamic acid were put into the mixed solution, respectively. After homogeneously stirring, the mixed solution was transferred into a Teflon lined stainless steel autoclave (25 mL), heated at 160 °C for 6 h, and cooled to 25 °C in air. The final black precipitates were collected by centrifugation, thoroughly washed with water and ethanol for several times, and dried in vacuum at 60 °C for further characterization.
Similarly, Cu2O nanoparticles were fabricated without GO for comparison, and pure r-GO were obtained by reducing GO with NaBH4, while other conditions were kept unchanged.
Characterization
The morphology of the samples was characterized by transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM), which were performed on a JEM-2100 F transmission electron microscope (www.jeol.co.jp/en.com) equipped with the selective area electron diffraction (SAED). The crystal structures were determined by X-ray diffraction (XRD, Rigaku Dmax-2000 diffractometer) using Cu-Kα radiation (Bruker Co., Germany, www.rigaku.com). X-ray photoelectron spectra (XPS) were recorded on a Thermo Scientific Escalab 250 XPS spectrometer (www.pharmaceuticalonline.com) with Al Kα X-ray radiation (1486.6 eV). Fourier transform infrared (FT-IR) analysis was performed on a Nicolet NEXUS670 spectrometer (http://web.ysu.edu/gen/stem). Raman spectra were acquired on a micro-Raman system (Renishaw RM1000 spectrometer) with an excitation wavelength of 633 nm (www.antpedia.com). Thermogravimetric analysis (TGA) was conducted with a simultaneous thermo-gravimetric analyzer (Netzsch, STA 449C, www.brain-power.com). The samples were heated in air from 25 to 800 °C at a heating rate of 10 °C min−1.
Electrochemical measurements
The electrochemical measurements were conducted on a CHI660D electrochemical workstation (Chenhua Instruments Co., Shanghai, China, www.chinstruments.com), and performed with a conventional three-electrode system, including a platinum wire as the counter electrode, a saturated calomel electrode (SCE, saturated KCl) as the reference electrode, and a bare or modified glassy carbon electrode (GCE, 3.0 mm in diameter) as the working electrode.
For typical construction of Cu2O NCs/r-GO modified electrode (Cu2O NCs/r-GO/GCE), 2.0 mg of Cu2O NCs/r-GO was dispersed into 1.0 mL water under ultrasonication to form a homogeneous suspension. Next, 8 μL of the suspension was casted on the clean GCE and dried naturally, followed by casting another layer of Nafion (0.05 wt %) with the volume of 5 μL to fix the deposit on the electrode surface. For comparison, individual r-GO and Cu2O modified electrodes were prepared in a similar way, denoted as r-GO/GCE and Cu2O NCs/GCE, respectively.
The electrocatalytic activity of the above catalysts modified electrodes was determined by linear sweep voltammetry toward glucose oxidation in 0.5 M NaOH at a scan rate of 50 mV s−1. The stability of Cu2O NCs/r-GO/GCE was examined by amperometry at an applied potential of 600 mV in 0.5 M NaOH. The electrochemical experiments were carried out at 25 °C, if not stated otherwise.
Results and discussion
Characterization
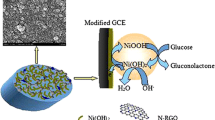
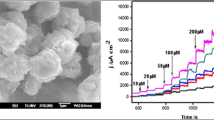
As seen from TEM images (Fig. 1a and b), the product mainly consists of numerous well-defined nanoclusters across the whole section, without any agglomerating, which are uniformly dispersed on r-GO. The nanoclusters have a very narrow size distribution from 1.2 to 3.9 nm (Fig. 1d), with the average size of 2.6 nm. Their polycrystalline nature is demonstrated by the corresponding SAED pattern (inset in Fig. 1b). HRTEM image (Fig. 1c) shows the well-defined lattice fringes with the inter-planar spacing of ca. 0.19 nm, which is in good accordance with the (200) crystal planes of Cu2O.
It is known that surfactants have great effects on the morphology and size of nanocrystals [16, 17]. As expected, only some irregular, agglomerated, and large Cu2O particles (Fig. 2a, b) are obtained without or using smaller amount of the triblock copolymer (0.1 %). When the amounts of the triblock copolymer are 0.5 % (Fig. 2c) and 1.0 % (Fig. 2d), numerous Cu2O nanoparticles with decreased sizes are observed on r-GO. These results indicate that the amounts of the triblock copolymer played the essential role in controlling the morphology and size of Cu2O NCs.
Fig. S2 (ESM) provides the XRD pattern of Cu2O NCs/r-GO, using r-GO as a reference. Clearly, there are four representative diffraction peaks emerged at 36.5, 42.6, 61.5, and 73.6°, which are well assigned to the (111), (200), (220), and (311) planes of the face-centered cubic (fcc) structure of Cu2O [22, 23]. These values are matched well with the Joint Committee Powder Diffraction Standard of pure Cu2O (JCPDS No. 05–0667). The corresponding (111) diffraction peak is stronger and sharper than those of the other planes, showing the preferential growth of Cu2O NCs along the (111) directions [15]. Furthermore, there are no any other peaks of impurities (e.g., CuO and Cu) emerged, indicating high purity of Cu2O NCs. In addition, a new broad peak is observed at 2θ =19.4° for Cu2O NCs/r-GO (Fig. S2, ESM, curve a), which is consistent with that of r-GO (Fig. S2, ESM, curve b, 2θ = 20.9°), suggesting the efficient removal of oxygen-containing functional groups and well dispersion of Cu2O NCs on r-GO [24].
The composition and surface states of Cu2O NCs/r-GO were examined by XPS measurements. The XPS survey spectrum (Fig. 3a) demonstrates the coexistence of Cu, O, N, and C elements in Cu2O NCs/r-GO. As for high-resolution Cu XPS spectrum (Fig. 3b), there are two peaks detected at 933.7 and 953.8 eV, which are assigned to the binding energies of Cu 2p3/2 and Cu 2p1/2 of Cu(I) [25, 26], respectively. A weak peak shows up at 943.7 eV, suggesting that there is a very minimal amount of Cu(II) present in this system, which may be derived from the un-reacted precursor of Cu(NO3)2 [27]. By measuring their relative intensities, Cu(I) is the predominant species, revealing the formation of Cu2O NCs.
High-resolution C 1 s XPS spectrum of Cu2O NCs/r-GO (Fig. 3c) can be well fitted into four peaks at 284.70, 285.70, 287.58 eV, and 288.67 eV, which are attributed to the C-C (sp2), C-O, C = O, and O-C = O groups, respectively [28]. Particularly, the peaks related to the oxygen functionalities are much weaker in comparison with those of GO (Fig. 3d), showing the formation of r-GO under hydrothermal conditions. This assumption is strongly supported by FT-IR spectra of Cu2O NCs/r-GO (Fig. 4A). Notably, the peak intensities at 1046 cm−1 (O–H) and 1730 cm−1 (C = O) are greatly decreased for Cu2O NCs/r-GO (curve a) as compared to those of GO (curve b).
Similarly, Raman spectra (Fig. 4B) display two characteristic peaks at 1362 and 1608 cm−1 for the D and G bands in Cu2O NCs/r-GO (curve a) and GO (curve b), respectively. The integral area ratio of the two bands (denoted as I D /I G ) is 1.5 for Cu2O NCs/r-GO, which is larger than that of GO (0.82), indicating the efficient formation of r-GO [29].
TGA analysis was performed to test the thermal stability and estimate the metal loading of Cu2O NCs/r-GO (Fig. S3, ESM). After heating from 25 to 800 °C, there is no weight left for GO because of the evaporation of water molecules, the removal of some oxygen-containing functional groups, and the decomposition of the carbon skeleton groups [30]. However, Cu2O NCs/r-GO exhibit much lower mass loss and faster achievement of the thermal plateau under the identical conditions, manifesting their better thermal stability and efficient reduction of GO to r-GO [31]. Additionally, the mass loading of Cu2O is 30.7 wt. %.
Electrochemical properties of Cu2O NCs/r-GO/GCE
Firstly, Cu2O NCs/r-GO modified electrode was constructed to examine their electrocatalytic properties, using GCE and pure r-GO/GCE as referenced electrodes. Electrochemical impedance spectroscopy was employed to investigate the electrical conductivity of the nanocomposites to study the synergistic effects between r-GO and Cu2O. Figure 5 shows Nyquist plots obtained on GCE (curve a), r-GO/GCE (curve b), and Cu2O NCs/r-GO/GCE (curve c) in 0.1 M KCl containing 5.0 mM [Fe(CN)6]3–/4–(1:1) solutions. The diameter of a semicircle of Cu2O NCs/r-GO modified electrode in high-frequency zone is larger than those of r-GO/GCE or bare GCE under the identical conditions. It means the larger resistance for electron transfer on Cu2O NCs/r-GO modified electrode as compared to those of the other two cases. This is ascribed to the fact that the decorated Cu2O NCs as a semiconductor increase the resistance in Cu2O NCs/r-GO as contrast to that of r-GO, reflecting the decreased conductivity of Cu2O NCs/r-GO in the present work.
Herein, a non-enzymatic glucose sensor was constructed to investigate the electrocatalytic activity of Cu2O NCs/r-GO (Fig. 6A, curve a) in 0.5 M NaOH containing 5.0 mM glucose, using individual r-GO (curve b) and Cu2O NCs ( curve c) as references. There is almost no anodic current observed at bare GCE. Clearly, Cu2O NCs/r-GO modified electrode shows more negative onset potential and higher anodic currents in contrast to r-GO and Cu2O NCs. It means the enhanced electrocatalytic performance of Cu2O NCs/r-GO. This is attributed to the fact that the present redox pair of Cu(II)/Cu(III) leads to the easy oxidation of glucose in strongly alkaline media [32] and the fast electron transfer provided by r-GO [33].
Figure 6B illustrates a series of linear sweep voltammograms (LSVs) at Cu2O NCs/r-GO modified electrode, displaying the enhanced anodic peak currents with glucose in the range of 10.0 ~ 35.0 mM. This observation confirms the improved catalytic activity of Cu2O NCs/r-GO for glucose oxidation. The maximum catalytic current is observed at the applied potential of 600 mV upon the addition of glucose, which is applied for amperometric determination of glucose.
Determination of glucose
Typical amperometric curves of Cu2O NCs/r-GO modified electrode were recorded at 600 mV with successive addition of glucose into 0.5 M NaOH under constant stirring. As depicted in Fig. 7a, the amperometric response is less than 3 s, indicating rapid responses toward glucose, owing to the improved catalytic activity and fast electron transfer of Cu2O NCs/r-GO with the electrode [4]. The plot shown in Fig. 7b does not fit a conventional linear equation in the whole concentration range. The linear regression equations are I (μA) =1.6301 + 37.5518C (R2 = 0.9950) and I (μA) =37.7961 + 23.0580 C (R2 = 0.9968) for determining glucose over the two linear concentration ranges of 0.005 ~ 2.095 mM and 2.595 ~ 9.595 mM, respectively. The detection limit is 1.0 μΜ at the signal-to-noise ratio (3S/N), which is much lower than most previously reported values [14, 15, 34–37]. It is notable that Cu2O NCs/r-GO modified electrode displays the enhanced catalytic performance for glucose sensing in terms of sensitivity and the limits of detection (LOD), compared to those of Cu2O-based materials previously reported (Table 1) [14, 15, 34–37].
Repeatability and stability
The repeatability of the sensor used in the measurement was obtained by recording the responses to 5.0 mM glucose in 0.5 M NaOH. The relative standard deviations (RSDs) were calculated to be 2.0 % (n = 5) for Cu2O NCs/r-GO/GCE. Additionally, to evaluate the electrode-to-electrode reproducibility, they were independently prepared using the same way and revealed an acceptable reproducibility with low RSD values of 1.4 % (n = 5). The stability of the sensor was examined by intermittently measuring the current responses to 5.0 mM glucose over a 35-day storage period (Fig. S4, ESM). The sensors were stored at 25 °C when not in use. The amperometric responses remained 95.2 % of their initial values after a storage period of 35 days, revealing long-term stability of the resulting sensor. The obtained sensor can be continuously detected without regenerating between each measurement.
Interference study and applications to human serum samples
To evaluate the selectivity of the sensor, potentially interfering biomolecules (i.e., UA, AA, and DA) were detected at Cu2O NCs/r-GO modified electrodes, which usually coexist with glucose in real samples such as human blood. The interference study was investigated in the presence of 5.0 mM glucose. The criterion for interference was a relative error of less than ± 5 %. It was found that 500 folds of CaCl2, MgCl2, KCl, and NaCl, 50 folds of lactic acid and glutaric acid, 15 folds of UA and AA, 10 folds of DA and acetaminophen, and 5-fold concentrations of fructose and maltose had no interference in the determination of glucose. These results demonstrate good selectivity of the resulting sensor for glucose assay.
Glucose in human serum samples were determined by Cu2O NCs/r-GO modified electrode at 600 mV. As shown in Table S1 (ESM), the as-obtained results are consistent with those determined by a hospital-used blood sugar instrument (Accu-Chek Performa), and the RSD values range from 1.23 to 2.37 %. These results display that the nonenzymatic sensor can be employed practically for routine analysis of glucose in real biological samples.
Conclusions
In summary, a facile solvothermal method was developed for preparation of Cu2O NCs/r-GO, with the assistance of the triblock copolymer (LuvisetR Clear) and L-glutamic acid as a shape-directing agent and a reducing agent, respectively. The nanocomposites exhibit better catalytic activity than those of Cu2O nanoparticles and r-GO toward glucose oxidation. The sensor has wide linear range (0.005 to 9.595 mΜ), high sensitivity (23.058 μA mM−1), and selectivity. The improved performances of Cu2O NCs/r-GO make it a promising candidate for the assay of glucose.
References
Guo S, Wang E (2011) Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors. Nano Today 6:240
Li X, Gao H, Murphy CJ, Gou L (2004) Nanoindentation of Cu2O nanocubes. Nano Lett 4:1903
Wang J (2008) Electrochemical glucose biosensors. Chem Rev 108:814
Feng X-X, Guo C-F, Mao L-Y, Ning J-Q, Hu Y (2013) Facile growth of Cu2O nanowires on reduced graphene sheets with high nonenzymatic electrocatalytic activity toward glucose. J Am Ceram Soc 97:811
Lu C-H, Qi L-M, Yang J-H, Wang X-Y, Zhang D-Y, Xie J-L, Ma J (2005) One-pot synthesis of octahedral Cu2O nanocages via a catalytic solution route. Adv Mater 17:2562
Gou L, Murphy CJ (2003) Solution-phase synthesis of Cu2O nanocubes. Nano Lett 3:231
Sinha B, Goswami T, Paul S, Misra A (2014) The impact of surface structure and band gap on the optoelectronic properties of Cu2O nanoclusters of varying size and symmetry. RSC Adv 4:5092
Xia B-Y, Wang B, Wu H-B, Liu Z, Wang X, Lou X-W (2012) Sandwich-structured TiO2–Pt–graphene ternary hybrid electrocatalysts with high efficiency and stability. J Mater Chem 22:16499
Jiang B-B, Wei X-W, Wu F-H, Wu K-L, Chen L, Yuan G-Z, Dong C, Ye Y (2014) A non-enzymatic hydrogen peroxide sensor based on a glassy carbon electrode modified with cuprous oxide and nitrogen-doped graphene in a nafion matrix. Microchim Acta 181:1463
Bajpai R, Roy S, Koratkar N, Misra D (2013) NiO nanoparticles deposited on graphene platelets as a cost-effective counter electrode in a dye sensitized solar cell. Carbon 56:56
Zhang Z-Y, Xiao F, Guo Y-L, Wang S, Liu Y-Q (2013) One-pot self-assembled three-dimensional TiO2-graphene hydrogel with improved adsorption capacities and photocatalytic and electrochemical activities. ACS Appl Mater Interfaces 5:2227
Peng L, Peng X, Liu B, Wu C, Xie Y, Yu G (2013) Ultrathin two-dimensional MnO2/graphene hybrid nanostructures for high-performance, flexible planar supercapacitors. Nano Lett 13:2151
Bhakta SA, Evans E, Benavidez TE, Garcia CD (2015) Protein adsorption onto nanomaterials for the development of biosensors and analytical devices: a review. Anal Chim Acta, ASAP
Gao Z-Y, Liu J-L, Chang J-L, Wu D-P, He J-L, Wang K, Xu F, Jiang K (2012) Mesocrystalline Cu2O hollow nanocubes: synthesis and application in non-enzymatic amperometric detection of hydrogen peroxide and glucose. CrystEngComm 14:6639
Zhou D-L, Feng J-J, Cai L-Y, Fang Q-X, Chen J-R, Wang A-J (2014) Facile synthesis of monodisperse porous Cu2O nanospheres on reduced graphene oxide for non-enzymatic amperometric glucose sensing. Electrochim Acta 115:103
You H, Yang S, Ding B, Yang H (2013) Synthesis of colloidal metal and metal alloy nanoparticles for electrochemical energy applications. Chem Soc Rev 42:2880
Zhang H, Jin M, Xia Y (2012) Noble-metal nanocrystals with concave surfaces: synthesis and applications. Angew Chem Int Ed 51:7656
Wang Y, Choi SI, Zhao X, Xie S, Peng HC, Chi M, Huang CZ, Xia Y (2014) Polyol synthesis of ultrathin Pd nanowires via attachment‐based growth and their enhanced activity towards formic acid oxidation. Adv Funct Mater 24:131
Zhao D, Huo Q, Feng J, Chmelka BF, Stucky GD (1998) Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J Am Chem Soc 120:6024
Zhao D, Feng J, Huo Q, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279:548
Guo Y-Q, Sun X-Y, Liu Y, Wang W, Qiu H-X, Gao J-P (2012) One pot preparation of reduced graphene oxide (RGO) or Au (Ag) nanoparticle-RGO hybrids using chitosan as a reducing and stabilizing agent and their use in methanol electrooxidation. Carbon 50:2513
Liu M-M, Liu R, Chen W (2013) Graphene wrapped Cu2O nanocubes: non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens Bioelectron 45:206
Li B-J, Cao H-Q, Yin G, Lu Y-X, Yin J-F (2011) Cu2O @ reduced graphene oxide composite for removal of contaminants from water and supercapacitors. J Mater Chem 21:10645
Gao Y-J, Ma D, Wang C-L, Guan J, Bao X-H (2011) Reduced graphene oxide as a catalyst for hydrogenation of nitrobenzene at room temperature. Chem Commun 47:2432
Paolella A, Brescia R, Prato M, Povia M, Marras S, De Trizio L, Falqui A, Manna L, George C (2013) Colloidal synthesis of cuprite (Cu2O) octahedral nanocrystals and their electrochemical lithiation. ACS Appl Mater Interfaces 5:2745
Cochell T, Manthiram A (2012) Pt@PdxCuy/C core–shell electrocatalysts for oxygen reduction reaction in fuel cells. Langmuir 28:1579
Wang C, Wang C-X, Xu L, Cheng H, Lin Q, Zhang C (2014) Protein-directed synthesis of pH-responsive red fluorescent copper nanoclusters and their applications in cellular imaging and catalysis. Nanoscale 6:1775
Li Y-J, Gao W, Ci L-J, Wang C-M, Ajayan PM (2010) Catalytic performance of Pt nanoparticles on reduced graphene oxide for methanol electro-oxidation. Carbon 48:1124
Lv J-J, Li S-S, Wang A-J, Mei L-P, Chen J-R, Feng J-J (2014) Monodisperse Au-Pd bimetallic alloyed nanoparticles supported on reduced graphene oxide with enhanced electrocatalytic activity towards oxygen reduction reaction. Electrochim Acta 136:521
Lv J-J, Feng J-X, Li S-S, Wang Y-Y, Wang A-J, Zhang Q-L, Chen J-R, Feng J-J (2014) Ionic liquid crystal-assisted synthesis of PtAg nanoflowers on reduced graphene oxide and their enhanced electrocatalytic activity toward oxygen reduction reaction. Electrochim Acta 133:407
Qiu J-D, Wang G-C, Liang R-P, Xia X-H, Yu H-W (2011) Controllable deposition of platinum nanoparticles on graphene as an electrocatalyst for direct methanol fuel cells. J Phys Chem C 115:15639
Meher SK, Cargnello M, Troiani H, Montini T, Rao GR, Fornasiero P (2013) Alcohol induced ultra-fine dispersion of pt on tuned morphologies of CeO2 for CO oxidation. Appl Catal B 130:121
Luo J, Jiang S-S, Zhang H-Y, Jiang J-Q, Liu X-Y (2012) A novel non-enzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Anal Chim Acta 709:47
Wang G-F, Wei Y, Zhang W, Zhang X, Fang B, Wang L (2010) Enzyme-free amperometric sensing of glucose using Cu-CuO nanowire composites. Microchim Acta 168:87
Wang A-J, Feng J-J, Li Z-H, Liao Q-C, Wang Z-Z, Chen J-R (2012) Solvothermal synthesis of Cu/Cu2O hollow microspheres for non-enzymatic amperometric glucose sensing. CrystEngComm 14:1289
Fu S, Fan G, Yang L, Li F (2015) Non-enzymatic glucose sensor based on au nanoparticles decorated ternary Ni-Al layered double hydroxide/single-walled carbon nanotubes/graphene nanocomposite. Electrochim Acta 152:146
Li M, Liu L, Xiong Y, Liu X, Nsabimana A, Bo X, Guo L (2015) Bimetallic mco (M = Cu, Fe, Ni, and Mn) nanoparticles doped-carbon nanofibers synthetized by electrospinning for nonenzymatic glucose detection. Sens Actuators B 207:61
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (No. 21475118, 21175118 and 21275130), Zhejiang province university young academic leaders of academic climbing project (No. pd2013055), and Zhejiang normal university of undergraduate scientific and technological innovation project (New Talents Program, No. X201414 for LPM).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 363 kb)
Rights and permissions
About this article
Cite this article
Mei, LP., Song, P., Feng, JJ. et al. Nonenzymatic amperometric sensing of glucose using a glassy carbon electrode modified with a nanocomposite consisting of reduced graphene oxide decorated with Cu2O nanoclusters. Microchim Acta 182, 1701–1708 (2015). https://doi.org/10.1007/s00604-015-1501-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1501-0