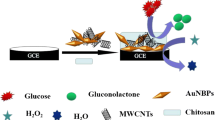
Abstract
Three-dimensional nanoporous copper (NPC) was fabricated by dealloying ribbons of an Al-Cu alloy. NPC possesses a clean metal surface and high electrical conductivity. Subsequently, a non-enzymatic electrochemical sensor was obtained by modifying a glassy carbon electrode with nanocomposites containing nanoporous copper and carbon black (NPC-CB) in a nafion matrix. The sensor, if operated at a working voltage of 0.6 V (vs. SCE) in 50 mM NaOH solution, has a linear analytical range that extends from 6.0 μM to 3.4 mM of glucose, and a 2.6 μM detection limit (at an S/N ratio of 3). It also shows good selectivity over ascorbic acid, uric acid, dopamine and carbohydrates (fructose, saccharose, and maltose). The sensor also has a rapid amperometric response to hydrogen peroxide which can be quantified with a 1.2 μM detection limit.

Nanoporous copper (NPC) was fabricated by dealloying ribbons of an Al-Cu alloy. NPC exhibits three-dimensional nanoporous structure. A nanocomposite made from NPC and carbon black in a nafion matrix shows good sensing performance toward glucose and H2O2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accurate detection of glucose level in blood is essential for clinical diagnostics in diabetes control [1–4]. H2O2 is an essential mediator or a common by-product involved in many chemical and biological processes [5]. Therefore, sensitive detection of glucose and H2O2 is of great importance in the fields of food industry, life science, medical diagnosis, and industrial research, etc. [6, 7]. However, due to the use of enzymes, the traditional electrochemical enzyme-based sensors usually suffer from short device lifetime and decay in activity, and their performances are easily affected by the variation in temperature and pH value during measurement [8]. On the other hand, the application of the GOx-based glucose sensor is still limited due to the inherent drawbacks associated with enzyme purification, immobilization and its protection from denaturing. To this end, nonenzymatic, direct electrocatalytic detection of glucose has garnered interest as it paves the way to electron-transfer-shuttle-free sensor and thus a high sensitivity and repeatability. Therefore, it is critical to develop non-enzymatic sensors with high sensitivity and repeatability, fast response and stability to replace the conventional electrochemical enzyme-based sensors for the detection of glucose and H2O2 [9]. Moreover, for successful nonenzymatic electrocatalytic detection of glucose and H2O2 to work, high conductivity and catalytic activity is required.
With the development of nanotechnology, nanomaterials have become excellent candidates for the electrocatalyst in glucose and H2O2 detection due to their highly specific surface area and some unique properties [10]. Among them, copper-based nanomaterials have been widely studied because of their low cost and high electroactivity, especially good poisoning tolerance [11]. Various copper-based nanomaterials with different morphologies, such as nanowires [12], nanoplatelets [11], nanospheres [13], and nanofibers [14], have been described. They are of particular interest and have been widely used in alkaline media by the constant potential method to determine glucose. Zhang and his coworkers have reported the fabrication of one-dimensional copper nanowires (Cu NWs) by a modified method and their electrocatalytic activity towards glucose [15]. Liu et al. prepared CuO nanofibers (NFs) by electrospinning and calcination technologies, finding high glucose sensing performance [16].
Dealloying is a promising strategy for the fabrication of nanoporous metals owing to its advantages of low cost, simple preparation and good control to structure uniformity [17]. Nanoporous metals and their sensing performances have attracted great attention due to their high surface area and interconnected porous channels [18, 19], which are preferable for unblocked mass and electron transport. Among them, nanoporous copper (NPC), emerging as an interesting three-dimensional (3D) material, has captured considerable attention [17, 20]. NPC fabricated by dealloying Al/Mg from Al/Mg-Cu alloy in an alkaline/acidic solution can be prepared in large scale [21, 22]. The glucose and H2O2 sensors based on the NP-PtM(M = Au,Co,Ni) and NPG by dealloying method have been successfully constructed [23–25]. However, there are few reports by using NPC as electrocatalyst in glucose and H2O2 detection.
Based on the above considerations, we first prepared Al75Cu25 alloy ribbons by a melt spinning method, then fabricated NPC via selectively etching Al in 1 M NaOH solution for 6 h at 60 °C. The NPC show bicontinuous nanoporous structure with ligaments of ~3 nm. Subsequently, we designed a hybrid composite based electrocatalyst consisting of nanoporous copper (NPC) and carbon black (CB) by a simple mixing method, showing two major advantages as follows: (i) in addition to the low cost of copper, the preparation nanoporous metals(NPC)is usually simple and environmental protection by dealloying; and (ii) a good synergetic effect between NPC and CB was confirmed, thus resulting in a good selectivity. By taking advantages of the synergic effect of NPC and CB, the fabricated NPC-CB nanocomposites presented good sensitivity, wide linear range, improved stability, attractive selectivity against common interfering species, as well as outstanding feasibility for real sample analysis. It is believed that the NPC-CB nanocomposites will be a promising material for sensor application.
Experimental
Reagents
Al75Cu25 alloy was prepared by refining high-purity (>99.9 %) Al and Cu metals in an argon-protected arc furnace, followed by melt-spinning into foils with typical thickness at 50 μm. Carbon black (Vulcan XC-72) was obtained from Shanghai Cabot Chemical Co., Ltd. (Shanghai, China, http://www.cabotcorp.com/).Glucose and H2O2 solution (30 %) were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China (http://www.sinoreagent.com/). Ascorbic acid (AA), uric acid (UA), and dopamine (Purity ≥ 98 %, Mw = 189.64) were purchased from Yuanye biotechnology Co., Ltd., Shanghai, China (http://www.shyuanye.com/). All other reagents were purchased from Jingchun biochemical technology Co., Ltd., Shanghai, China (http://www.aladdin-reagent.com/).The phosphate buffer (0.1 M) was prepared by mixing the stock solutions of NaH2PO4 and Na2HPO4, and then adjusting the pH with 0.1 M NaOH and 0.1 M H3PO4. The 18.2 MΩ·cm Milli-Q water was used through the whole experiment.
Modification of the electrode with nanoporous copper and carbon black
Nanoporous copper (NPC) was prepared by dealloying the Al75Cu25 alloy in 1 M NaOH solution for 6 h at 60 °C. The sample were washed several times with ultrapure water (18.2 MΩ·cm) and dried at room temperature in air. Catalyst ink was prepared by mixing 2.0 mg NPC, 1.0 mg carbon black (CB), 200 μL ethanol, and 200 μL nafion solutions (0.5 wt%) under sonicating for 1 h to form a hybrid composite. The working electrode (Nafion/NPC-CB/GCE) was made by placing 4.0 μL catalyst ink on a polished glassy carbon electrode. After being dried under ambient conditions then drop 3 μL of 0.05 wt% Nafion on it and stored in a clean environment. A Nafion coated GCE (Nafion/GCE), carbon black modified GCE (Nafion/CB/GCE), and NPC modified GCE (Nafion/NPC/GCE) were also prepared as control electrodes in a similar way.
Instruments
The X-ray diffraction (XRD) patterns were acquired on a Rigaku D/max 2200/PC diffractometer equipped with graphite-monochromatized Cu Kα radiation (λ = 1.54060 Å) in 2θ ranging from 20° to 80°. The microstructure and chemical composition were characterized by a Zeiss Sigma field emission scanning electronic microscopy (FESEM) equipped with an Oxford INCA energy-dispersive X-ray spectrometer (EDS). The transmission electron microscopy (TEM) images were taken on a Tecnai G220S-TWIN (Shanghai FEI, China, http://www.feicompany.cn) with an accelerating voltage of 200 kV. All electrochemical measurements were performed on a CHI 660D electrochemical work station (Shanghai CH Instruments Co., China, http://chi.instrument.com.cn). For electrochemical measurements, a conventional three electrode system was used. The glassy carbon electrodes acted as the working electrodes (GCE, 3 mm in electrode diameter). A Pt foil and a saturated calomel electrode (SCE) were used as the counter and reference electrodes, respectively.
Results and discussion
Characterization of NPC
The phase characteristics of Al-Cu precursor as well as the dealloyed sample (NPC) were first identified from the X-ray diffraction (XRD) patterns as presented in Fig. 1. The diffraction peaks of the Al-Cu precursor is composed of two phases:α-Al solid solution and Al2Cu intermetallic compound, which is consist with previous report [21, 22]. After dealloying in the 1 M NaOH solution, the XRD patterns of the alloy ribbons are also shown in Fig. 1. There are three broad diffraction peaks observed with 2θ values of 42.34, 49.42, and 73.46o, which can be assigned to the (111), (200), and (220) diffractions of Cu structure. The XRD analysis indicates that Al atoms in α-Al and Al2Cu have been fully etched off. In addition, there are some weak diffractions (located at ca.37 o) existed, which can be indexed to the Cu2O species. The formation of Cu oxides can be attributed to the oxidation of the exposed Cu during the drying process.
Figure 2a shows the scanning electron microscopy (SEM) images of the NPC. The surface exhibits a porous structure with channel sizes of hundreds of nanometers. It is clear that these large-sized channels are not cracks but runs through the whole sample surface. From high magnification SEM image (Fig. 2b), the large-sized channel wall have an open three-dimensional bicontinuous spongy morphology with the typical ligament size around 3 nm. TEM images provide more details for this structure. As illustrated in Fig. 2c, this demonstrates a uniform distribution of dark metallic ligaments and bright pores. The high-resolution TEM (HRTEM) image (Fig. 2d) shows that continuous lattice fringes were resolved across several pores; the lattice space was calculated to be approximately 0.238 nm, which corresponds to the (111) plane spacing for the NPC. Obviously, the resulted sample exhibits bimodal pore/ligament size distributions, which is beneficial for the mass and electron transport during electrochemical sensing [24].
Electrooxidation of glucose on the Nafion/NPC-CB/GCE
The electrocatalytic activities of the Nafion/GCE (d), Nafion/NPC/GCE (c), Nafion/CB/GCE (b), and the Nafion/NPC-CB/GCE (a) were investigated with a cyclic voltammetric (CV) method in 50 mM NaOH solution with 5 mM glucose at 100 mV·s−1 (Fig. 3a). All CVs were carried out in the potential range from 0 V to +0.8 V which covers the glucose electrooxidation range in alkaline electrolyte. As shown in Fig. 3a, in the absence of NPC, neither the Nafion/CB/GCE nor the Nafion/GCE shows oxidation peaks. This suggests that NPC played a major role in the oxidation of glucose with its catalytic activity against glucose. In contrast, the Nafion/NPC-CB /GCE exhibited substantially higher current, which was more prominent than that obtained at the Nafion/NPC/GCE. The enhanced performance of the Nafion/NPC-CB /GCE hybrid composite can be attributed to the result of a large surface area, high conductivity and fast electron transfer provided by carbon black, as well as the porous network structure and excellent electrocatalytic properties for glucose in alkaline media of the NPC.
a Cyclic voltammograms of Nafion/NPC-CB/GCE (a), Nafion/CB/GCE (b), Nafion/NPC/GCE (c) and Nafion /GCE (d) in 5 mM glucose +50 mM NaOH solution, scan rate: 100 mV·s−1. b Cyclic voltammograms of 2 mM glucose +50 mM NaOH solution on Nafion/NPC-CB/GCE at different scan rates of 5, 25, 50, 100, 150, 200, and 250 mV·s−1. c Current vs. scan rate. d Cyclic voltammograms of Nafion/NPC-CB/GCE in various glucose concentrations (a: 5 mM, b: 2 mM, c: 1 mM d: 0 Mm) with scan rate of 100 mV·s−1 in 50 mM NaOH solution. Inset: Cyclic voltammograms of Nafion/GCE in (f) 50 mM NaOH solution and (e) 5 mM glucose +50 mM NaOH solution, scan rate: 100 mV·s−1
The CVs responses of the Nafion/NPC-CB/GCE at different scan rate were investigated (Fig.3b) to confirm the redox reaction model. As presented in Fig. 3c, the anodic peak currents varied linearly with potential scan rate in the range of 5 to 250 mV·s−1, which suggested that the redox reaction is a surface-confined process [26, 27], and the glucose molecules were direct oxidized on the surface of composite electrode and the electron were directly transferred, without other mediators.
In order to investigate the applicability of Nafion/NPC-CB/GCE in glucose electrooxidation and sensing, more CVs studies were carried out. Figure 3d shows the effect of glucose concentration on electrooxidation of glucose in alkaline medium. NaOH concentration was maintained at 50 mM and the glucose concentration was varied from 0 to 5 mM. Clearly, with an increase in glucose concentration, the anodic current increased, peaked at 0.6 V vs. SCE before declining thereafter. As a result, the potential of 0.6 V vs. SCE was selected as sensing voltage for subsequent amperometric tests so to optimize the electrocatalytic response as well as obtain the best sensitivity.
In addition, the effect of NaOH concentration on the Nafion/NPC-CB/GCE was also studied in the range from 5 mM to 50 mM (Fig. S1). The anodic currents increased from 5 mM to 50 mM, and decreased above 50 mM. The following experiments were performed at 50 mM NaOH.
Amperometric response of the Nafion/NPC-CB/GCE towards glucose
Figure 4a exhibits the amperometric response of the Nafion/NPC-CB/GCE at 0.6 V upon successive addition of glucose. It is interesting to find that the electrode responds rapidly and sensitively to each addition of glucose to the stirred NaOH solution. This is attributed to the fact that glucose can diffuse freely into the three dimensional bicontinuous nanoporous structure to be oxidized on the surface of NPC [25]. As plotted in Fig. S2, the calibration plot is linear over a broad concentration range of 0.006–3.369 mM (linear equation: y = 33.75× + 7.922) with a correlation coefficient of 0.998. The detection limit is thus estimated to be 2.6 μM (signal-to-noise ratio = 3) and the sensitivity is obtained to be 33.75 μA•cm−2•mM−1. The present electrode was compared with those previously reported electrodes for glucose non-enzymatic detection, which are summarized in Table 1. Clearly, the NPC-CB electrode exhibits lower detection limit, wide linear range and satisfied sensitivity compared to those earlier reports. These results indicate that NPC-CB holds great potential for the fabrication of glucose sensor. One possible reason is that NPC are entangled with the carbon black and thus a special structure of NPC-CB was formed with good conductivity. Therefore, carbon black offer numerous conducting channels to transfer electrons and the structure of NPC-CB provide desirable electrical contact between NPC and CB, resulting in effective electron transfer between the active sites of NPC and electrodes.
Selectivity and stability of Nafion/NPC-CB/GCE
Selectivity is another essential parameter for nonenzymatic glucose sensor as a good selectivity ensures high accuracy. The selectivity of the NPC-CB sensor was tested with various potentially interfering reagents. As shown in Fig. 5a, the addition of 0.1 mM of glucose resulted in a quick and significant current increase, whereas an addition of 0.05 mM uric acid (UA), ascorbic acid (AA), dopamine (DA) and carbohydrate (0.05 mM fructose, saccharose, and maltose) did not cause obvious current changes. These results indicate that the NPC-CB sensor possesses a very favorable selectivity toward glucose detection.
In addition, the long-term sensing stability of the NPC-CB for glucose sensing was also evaluated by continuous detecting glucose every day for a period of 12 days. From Fig. 5b, the recorded amperometric response of the electrode has a not very obvious decline over a period of 12-day, demonstrating that the NPC-CB sensor is relatively stable and repeatable to glucose detection. Based on the measurement results in the period of 12 days, the relative standard deviation (RSD) on the NPC-CB modified electrode for 5 mM glucose is 12.86 %, suggesting the reproducibility of the NPC-CB electrodes as sensing electrocatalysts requiring further exploration.
It should be noted that the NPC-CB has examined in alkaline solution only, but not in buffer of pH = 7. As we all know, strong NaOH solution is etching and a risk to any unskilled person. Despite its major limitation, the sensor exhibits superior electrocatalytic activity, high sensitivity and low detection limit toward the oxidation of glucose.
Amperometric response of the Nafion/NPC-CB/GCE towards H2O2
In addition to remarkably enhanced electrocatalytic activities towards glucose detection, the electrocatalytic activity of the NPC-CB towards H2O2 was also explored.
Figure 6a shows the amperometric responses of NPC-CB catalysts on successive addition of H2O2 into the stirred phosphate buffer solution at an applied potential of 0.75 V. The electrode exhibit a sensitive response even for 3 μM H2O2 (inset of Fig. 6a), which can be attributed to the synergetic effect between NPC and CB. As plotted in Fig. S3, the calibration plot is linear over a broad concentration range of 0.003–2.338 mM (linear equation: y = 3.914× +0.761, R = 0.993) with a sensitivity of 3.914 μA•cm−2•mM−1 and a good detection limit of 1.2 μM. These results are also compared with other sensors used H2O2 electroanalysis in Table S1. As is compared, the NPC-CB electrode exhibits superior H2O2 sensing performance, which has a comparable sensitivity, larger linear range, and the lower practical detection limit.
Real sample analysis
In order to illustrate the feasibility of Nafion/NPC-CB/GCE nonenzymatic sensor in practical applications, amperometric measurements were employed to measure the glucose content in some commercial beverage glucose. The samples were diluted 10 times by 50 mM NaOH before testing and the results are listed in Table 2. For H2O2, 1 mL of the different contact lens solution samples was added to 20.0 mL of 0.1 M phosphate buffer (pH =7.0). As shown in Table S2, the results were compared with those determined by classical potassium permanganate titration method [35]. These results indicate that the sensor we prepared is sensitive in real samples.
Conclusions
In conclusion, we have successfully synthesized a 3D nanoporous copper (NPC) by a simple one-step dealloying process. In particular, the NPC-CB nanocomposites modified electrode exhibits superior sensing performance with superior sensitivity and low detection limit toward glucose and H2O2. The synergistic effects between NPC and CB may account for the improvement of electrocatalytic activity and high sensitivity. This has been used in the determination of real sample with satisfactory result. In addition, the NPC-CB hybrid composite also shows a long-term sensing durability toward glucose as well little interference from DA, AA, and UA. Overall, on the basis of these attractive features, NPC-CB exhibits a great potential for use as nonenzymatic glucose and H2O2 sensor.
References
Heller A, Feldman B (2008) Electrochemical glucose sensors and their applications in diabetes management. Chem Rev 108:2482–2505
Chen XM, Wu GH, Cai ZX, Oyama M, Chen X (2014) Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim Acta 181:689–705
Zhao L, Wu GH, Cai ZX, Zhao TT, Yao QH, Chen X (2015) Ultrasensitive non-enzymatic glucose sensing at near-neutral pH values via anodic stripping voltammetry using a glassy carbon electrode modified with Pt3Pd nanoparticles and reduced graphene oxide. Microchim Acta 182:2055–2060
Mei H, Wu WQ, Yu BB, Li YB, Wu HM, Wang SG, Xia QH (2015) Non-enzymatic sensing of glucose at neutral pH values using a glassy carbon electrode modified with carbon supported Co@ Pt core-shell nanoparticles. Microchim Acta 182:2055–2060
Rui Q, Komori K, Tian Y, Liu HQ, Luo YP, Sakai Y (2010) Electrochemical biosensor for the detection of H2O2 from living cancer cells based on ZnO nanosheets. Anal Chim Acta 670:57–62
Zhai DY et al. (2013) Highly sensitive glucose sensor based on Pt nanoparticle/polyaniline hydrogel heterostructures. ACS Nano 4:3540–3546
Wang GF, He XP, Wang LL, Gu AX, Huang Y, Fang B, Geng BY, Zhang XJ (2013) Non-enzymatic electrochemical sensing of glucose. Microchim Acta 180:161–186
Wang J (2008) Electrochemical glucose biosensors. Chem Rev 108:814–825
Guo C, Huo H, Han X, Xu C, Li H (2014) Ni/CdS bifunctional Ti@TiO2 core-shell nanowire electrode for high-performance nonenzymatic glucose sensing. Anal Chem 86:876–883
Chen XM, Tian XT, Zhao LM, Huang ZY (2014) Nonenzymatic sensing of glucose at neutral pH values using a glassy carbon electrode modified with graphene nanosheets and Pt-Pd bimetallic nanocubes. Microchim Acta 181:783–789
Wang J, Zhang WD (2011) Fabrication of CuO nanoplatelets for highly sensitive enzyme-free determination of glucose. Electrochim Acta 56:7510–7516
Zhuang ZJ, Su XD, Yuan HY, Sun Q, Xiao D, Choi MMF (2008) An improved sensitivity non-enzymatic glucose sensor based on a CuO nanowire modified Cu electrode. Analyst 133:126–132
Reitz E, Jia W, Gentile M, Wang Y, Lei Y (2008) CuO nanospheres based nonenzymatic glucose sensor. Electroanalysis 20:2482–2486
Sahay R, Sundaramurthy J, Kumar PS, Thavasi V, Mhaisalkar SG, Ramakrishna S (2012) Synthesis and characterization of CuO nanofibers, and investigation for its suitability as blocking layer in ZnO NPs based dye sensitized solar cell and as photocatalyst in organic dye degradation. J Solid State Chem 186:261–267
Zhang YC, Su L, Dan M (2012) Ultrasensitive and selective non-enzymatic glucose detection using copper nanowires. Biosens Bioelectron 31:426–432
Liu GY, Zheng BZ, Jiang YS, Cai YQ (2012) Improvement of sensitive CuO NFs-ITO nonenzymatic glucose sensor based on in situ electrospun fiber. Talanta 101:24–31
Xu CX, Wang LQ, Wang RY, Wang K, Zhang Y, Tian F, Ding Y (2009) Nanotubular mesoporous bimetallic nanostructures with enhanced electrocatalytic performance. Adv Mater 21:2165–2169
Yu F, Ahl S, Caminade AM, Majoral JP, Knoll W, Erlebacher J (2006) Simultaneous excitation of propagating and localized surface plasmon resonance in nanoporous gold membranes. Anal Chem 78:7346–7350
Huang JF, Sun IW (2005) Fabrication and surface functionalization of nanoporous gold by electrochemical alloying/dealloying of Au-Zn in an ionic liquid, and the self-assembly of L-Cysteine monolayers. Adv Funct Mater 15:989–994
Smith AJ, Tran T, Wainwright MS (1999) Kinetics and mechanism of the preparation of Raney® copper. J Appl Electrochem 29:1085–1094
Liu W, Zhang S, Li N, Zheng J, Xing Y (2012) Fabrication and dealloying behavior of monolithic nanoporous copper ribbons with bimodal channel size distributions. J Mater Sci Technol 8:693–699
Li M, Zhou YZ, Geng HR (2012) Fabrication of nanoporous copper ribbons by dealloying of Al-Cu alloys. J Porous Mater 19:791–796
Xu CX, Wang JP, Zhou JH (2013) Nanoporous PtNi alloy as an electrochemical sensor for ethanol and H2O2. Sensors Actuators B 182:408–415
Xu CX, Sun FL, Gao H, Wang JP (2013) Nanoporous platinum-cobalt alloy for electrochemical sensing for ethanol, hydrogen peroxide, and glucose. Anal Chim Acta 780:20–27
Wang JP, Gao H, Sun FL, Xu CX (2014) Nanoporous PtAu alloy as an electrochemical sensor for glucose and hydrogen peroxide. Sensors Actuators B 191:612–618
Bai YF, Xu TB, Luong JHT, Cui HF (2014) Direct electron transfer of glucose oxidase-boron doped diamond interface: A new solution for a classical problem. Anal Chem 86:4910–4918
Zhou J, Liao C, Zhang L, Wang Q, Tian Y (2014) Molecular hydrogel-stabilized enzyme with facilitated electron transfer for determination of H2O2 Released from live cells. Anal Chem 86:4395–4401
Male KB, Hrapovic S, Liu YL, Wang DS, Luong JHT (2004) Electrochemical detection of carbohydrates using copper nanoparticles and carbon nanotubes. Anal Chim Acta 516:35–41
Huang T, Lin K, Tung S, Cheng T, Chang I, Hsieh Y, Lee C, Chiu H (2009) Glucose sensing by electrochemically grown copper nanobelt electrode. J Electroanal Chem 636:123–127
Kang XH, Mai ZB, Zou XY, Cai PX, Mo JY (2007) A sensitive nonenzymatic glucose sensor in alkaline media with a copper nanocluster/multiwall carbon nanotube-modified glassy carbon electrode. Anal Biochem 363:43–150
Liu MM, Liu R, Chen W (2013) Graphene wrapped Cu2O nanocubes: Non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens Bioelectron 45:206–212
Guo SJ, Wen D, Dong SJ, Wang E (2009) Gold nanowire assembling architecture for H2O2 electrochemical sensor. Talanta 77:1510–1517
Zhang KY, Zhang N, Cai H, Wang C (2012) A novel non-enzyme hydrogen peroxide sensor based on an electrode modified with carbon nanotube-wired CuO nanoflowers. Microchim Acta 176:137–142
Gu AX, Wang GF, Zhang XJ, Fang B (2010) Synthesis of CuO nanoflower and its application as a H2O2 sensor. Bull Mater Sci 33:17–20
Vogel AI (1989) Textbook of Quantitative Chemical Analysis. Longman United Kingdom
Acknowledgments
We greatly appreciate the support of the National Natural Science Foundation of China (20905010), Jiangsu Provincial Natural Science Foundation (BK20131191 and BK20140416), Qing Lan Project (SZ2014005) and Suzhou Science and Technology Project (SYN201302, SYN201515). The work is also supported by Hebei Provincial Natural Science Foundation (B2011205079).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests
Electronic supplementary materials
ESM 1
(DOC 142 kb)
Rights and permissions
About this article
Cite this article
Mei, L., Zhang, P., Chen, J. et al. Non-enzymatic sensing of glucose and hydrogen peroxide using a glassy carbon electrode modified with a nanocomposite consisting of nanoporous copper, carbon black and nafion. Microchim Acta 183, 1359–1365 (2016). https://doi.org/10.1007/s00604-016-1764-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1764-0