Abstract
We report on an enzymatic electrochemical glucose biosensor based on the use of zinc oxide nanorods (ZnO NRs) modified with gold nanoparticles (Au-NPs) and arranged in the form of an array. The nanorods were obtained via a hydrothermal method, and the Au-NPs were synthesized by photo-reduction. The surface of the nanoarrays was then modified with Au-NPs via electrostatic self-assembly. GOx was immobilized onto the surface of Au-NPs modified ZnO nanoarrays by electrostatic adsorption. The performance of the resulting glucose biosensors were investigated by cyclic voltammetry and amperometry. Compared to biosensors based on ZnO nanoarrays without Au-NPs, the ones described here exhibit a higher sensitivity (43.7 nA cm−2 mM−1) and a lower K app,M (0.78 mM). It is obvious, therefore, that the Au-NPs play an important role with respect to the performance of this biosensor.

An enzymatic electrochemical glucose biosensor was constructed by using Au nanoparticles modified ZnO nanoarrays. Via the modification of Au nanoparticles onto the ZnO surface, the performance of glucose biosensor has been considerably improved, applicable to the determination of tear and blood glucose levels in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucose biosensors always play a significant role in the field of clinical diagnosis, biological detection and food production. Due to high sensitivity and fast response to glucose molecules, enzyme (glucose oxidase, GOx) coated amperometric biosensor is one of the most reliable ways for glucose detection in vitro or in vivo.
During the past decades, inorganic nanomaterials, such as Si, MnO2, TiO2, metal oxide NPs and noble metal NPs [1–5], were studied for the construction of biosensors since they can provide large surface areas for enzyme coating and compatible microenvironment to maintain the bio-activity of enzyme. Among these nanomaterials, one-dimensional ZnO has attracted great attention due to its outstanding properties including favorable biocompatibility, excellent bio-safety, fine electron transport property and easy preparation [6]. Furthermore, ZnO nanomaterials with a high isoelectric point of 9.5 are suitable for adsorbing proteins or enzymes with low isoelectric point, such as GOx with isoelectric point 4.2 at a physiological pH value of 7.4 by electrostatic attraction [7, 8].
Recently, the application investigation of nano-composites was one of the most remarkable research areas to combine and integrate the superiorities of each component [9–12]. To enhance electron communication properties of inorganic nanomaterials, attempts have been made by doping or modifying with metal NPs [10, 13].
In this work, we constructed a glucose biosensor by using Au NPs modified ZnO nanoarrays. Via the modification of Au NPs onto the surface of ZnO, a considerable improvement in the performance of the sensor has been presented. This work also indicates that the Au nanoparticles modified ZnO nanoarrays can offer a promising immobilization material for biosensor design.
Experimental
Reagents and chemicals
Glucose (99 %) and hydrogen peroxide (H2O2, 30 %) was purchased from Beijing Chemical Reagent Company (http://www.crc-bj.com/). Nafion solution (5 wt.%) and GOx (113 U mg−1) were both obtained from Sigma-Aldrich (http://www.sigmaaldrich.com/). All the reagents were of analytical grade and used without further purification.
Preparation and characterization of Au NPs modified ZnO NR arrays
Aligned ZnO NR arrays on Fluorine-doped tin oxide (FTO) glass were prepared via hydrothermal approach for 16 h [14]. Au NPs were synthesized by photo-reduction method [15] and deposited directly onto the ZnO NR arrays by the following procedures. First, 0.05 % HAuCl4 aqueous solution, 1 % polyvinyl alcohol solution and methanol were mixed together with a proper ratio. The pH value of the mixed solution was adjusted to 8 by NaOH when Au NPs protected by polyvinyl alcohol are negatively charged while ZnO NR arrays are positively charged. The FTO glass with ZnO NR arrays was then immersed in the photo-reduction solution and an ultra-vitalux lamp (OSRAM, 300 W) was used to irradiate 15 min for the photo-reduction. Next, the obtained FTO glass was annealed in air at 350 °C for 3 h to remove the capped polyvinyl alcohol. The resulting substrate was applied to construct the glucose biosensor.
The products were characterized by field emission scanning electron microscopy (FE-SEM, Zeiss, SUPRA-55), energy dispersive spectrometer (EDS) and X-ray photoelectron spectrum analyzer (XPS, AXIS ULTRADLD, KRATOS).
Construction and performance test of glucose biosensor
ZnO NR arrays on FTO glass and Au NPs/ZnO NR arrays on FTO glass were firstly wetted by phosphate buffer saline (PBS, 0.01 M, pH = 7.4) respectively to generate a hydrophilic surface, and the effective surface area of the modified electrode is 0.5 cm * 0.4 cm. Then, 2 μL GOx (50 g L−1) was dropped onto the surface of nanoarrays respectively and dried in air. The above procedure was repeated twice. 3 μL 0.5 wt.% Nafion solution was further dropped onto both of the substrate’s surface respectively to form a protective film. The fabricated Nafion/GOx/ZnO NR arrays/FTO and Nafion/GOx/Au NPs/ZnO NR arrays/FTO electrodes were stored at 4 °C overnight for further measurements.
All the electrochemical measurements were then carried out at room temperature utilizing an electrochemical workstation (SI 1287, Solartron Analytical) with a three-electrode system, in which the constructed electrode was used as working electrode, a columnar platinum electrode as auxiliary electrode and Ag/AgCl electrode as reference electrode.
Results and discussion
Characterization of the products
The vertically aligned ZnO NR arrays are found to be uniformly distributed with an average diameter of 50 ~ 100 nm and a length of about 2.5 μm (Fig. 1a). As depicted in Fig. 1b and c, the average diameter of Au NPs was 8 ~ 10 nm and the distribution of them over ZnO NRs is fairly uniform. EDS patterns shown in the insets of Fig. 1a and b indicate that ZnO NR arrays are composed of the elements Zn with an atomic ratio 55.75 % and O with 44.25 %, and Au NPs/ZnO NR arrays are composed of the elements Au with an atomic ratio 4.05 %, Zn with 38.84 % and O with 57.11 %. Fig. 1d displays the XPS spectrum of the Au 4f region. Au peaks are located at 83.6 and 87.3 eV, corresponding to the binding energy of metallic Au [16].
Electrochemical characterization
The sensing mechanism of GOx/Au NPs/ZnO NR arrays modified electrode in glucose solution is illustrated in Fig. S1 (Electronic Supplementary Material, ESM). GOx can selectively catalyze the oxidation of glucose to gluconolactone and H2O2 in the presence of O2, and the accumulation of H2O2 generated by the oxidation of increasing glucose results in continuous enhancement of the response current. Hence, the concentration of glucose can be indirectly examined by quantification of the produced H2O2 under O2 saturation. The electro-catalytic ability of GOx/Au NPs/ZnO NR arrays based electrode towards H2O2 was firstly investigated at +0.6 V vs. Ag/AgCl, and the real-time current response was presented in Fig. S2. It can be seen that the response current was enhanced obviously as the concentration (1 μM ~ 4 mM) of H2O2 increased.
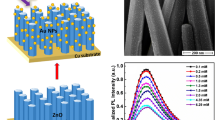
To preliminarily evaluate the performance of Au NPs/ZnO NR arrays based electrode towards glucose, cyclic voltammetry characteristics of the two fabricated electrodes at a scan rate of 50 mV s−1 were examined, as shown in Fig. 2a. Compared to the electrode based on ZnO NR arrays (black line), the current of Au NPs/ZnO NR arrays based electrode in PBS (blue line) is several times enhanced, which could be ascribed to the larger electrode surface area and increased electro-catalytic ability induced by Au NPs. A pair of current peaks (Epa at 0.164 V, Epc at 0.11 V) appear when the GOx/Au NPs/ZnO NR arrays based electrode was immersed in the 1 mM glucose solution (red line), which can be attributed to the formation of H2O2 [17]. In Fig. 2b and the insert, the Ipa and Ipc are sequentially increased and linearly proportional to the square root of scan rate respectively at the scan rates from 20 to 120 mV s−1 in 1 mM glucose, indicating a diffusion-controlled process [18].
a Cyclic voltammetry characteristics of GOx/ZnO NR arrays based electrode in PBS (black line), GOx/Au NPs/ZnO NR arrays based electrode in PBS (blue line) and in 1 mM glucose (red line); b Cyclic voltammetry curves of GOx/Au NPs/ZnO NR arrays based electrode at various scan rates in 1 mM glucose, and the inset shows the peak current vs. the square root of scan rate
The amperometric responses of biosensors based on ZnO NR arrays (black curve) and Au NPs/ZnO NR arrays (red curve) to different concentration glucose were examined under air saturation at an applied potential of +0.6 V vs. Ag/AgCl, as shown in Fig. 3. Both of the biosensors presented well-defined steady-state current responses to increasing glucose concentration. For the GOx/Au NPs/ZnO NR arrays modified biosensor, the response current to 3 μM glucose is 4 times as much as that when 1 μM glucose was added, as shown in the inset of Fig. 3. The detection limit is 3 μM at a signal-to-noise ratio larger than 3 (S/N > 3).
After analysis and calculation, it can be found that the current response to glucose concentration was enhanced obviously in the linear range of 3 μM ~ 3 mM for both of the two biosensors mentioned above. The linear equation for the biosensor based on ZnO NR arrays is y = 0.0243 x + 0.0084 (R2 = 0.9971), where y and x stand for the current density (μA cm−2) and the glucose concentration (mM), respectively. The equation slope demonstrates that the sensitivity of the biosensor is 24.3 nA cm−2 mM−1. For the biosensor based on Au NPs/ZnO NR arrays, the linear equation is y = 0.0437 x + 0.0402 (R2 = 0.9899), indicating a higher sensitivity of 43.7 nA cm−2 mM−1. This is because of the increased electrode surface area to immobilize more GOx and fine electro-catalytic ability to facilitate the aforementioned enzymic catalytic reaction induced by Au NPs that were adsorbed on ZnO NRs surface [16, 19].
The apparent Michaelis-Menten constant (K app,M ), a measure of the enzyme-substrate kinetics of the glucose biosensor, can be calculated from the Lineweaver-Burk equation [18]:.
where Imax is the saturation current. For GOx/Au NPs/ZnO NR arrays and GOx/ZnO NR arrays modified biosensors, the K app,M can be determined to be 0.78 mM and 1.58 mM, respectively. The smaller K app, M of GOx/Au NPs/ZnO NR arrays based biosensor means that the immobilized GOx possesses a higher enzymatic activity and improved affinity for substrate glucose [18, 20].
Cross sensitivity, reproducibility and stability of GOx/Au NPs/ZnO NR arrays modified biosensor
The cross sensitivity of the GOx/Au NPs/ZnO NR arrays based biosensor was then evaluated, as demonstrated in Fig. 4. Several most possible interfering compounds, uric acid (UA), ascorbic acid (AA) and dopamine (DA) [21] were examined. It can be found that UA, AA and DA do not bring obvious interference to the detection of 2 mM glucose.
The reproducibility of the GOx/Au NPs/ZnO NR arrays based electrode was also investigated by measuring the current response to 1 mM glucose in 0.01 M PBS. Three bio-electrodes, prepared independently under the same condition, showed a relative standard deviation (R.S.D.) of 4.39 %.
The storage stability of the GOx/Au NPs/ZnO NR arrays modified electrode was examined by comparing the response currents to 1 mM glucose in 0.01 M PBS. Dry stored at 4 °C for a week, the same electrode still maintains about 87 % of its original activity.
Real sample analysis
To evaluate the reliability of the fabricated biosensor based on Au NPs/ZnO NR arrays, the glucose concentration in real blood samples provided by a local hospital was analyzed here. The amperometric experiment was implemented by adding 500 μL of the blood sample into 10 mL PBS (0.01 M, pH 7.4). The blood glucose concentration was calculated according to the linear equation for the biosensor, and the results agree well with those learned from hospital, as shown in Table 1, demonstrating the high reliability of this method for detecting glucose in real samples.
The performance of glucose biosensor in this work was compared with those reported earlier in Table 2. The relatively lower detection limit of 3 μM and the linear range of 3 μM ~ 3 mM of the sensor make it practical in the determination of tear glucose levels in humans (the average normal value 138 μM) [22].
Conclusions
In summary, ZnO NR arrays have been successfully prepared and modified by Au NPs via photo-reduction method combined with electrostatic self-assembly technique. The Au NPs/ZnO NR arrays based glucose biosensor performed better performance in comparison to that based on ZnO NR arrays, which can be ascribed to larger surface-to-volume ratio and excellent electro-catalytic ability of the Au NPs modified ZnO NR arrays. It is expected that Au NPs modified ZnO NR arrays will have promising application prospects for the detection of glucose and other biological species.
References
Cui Y, Wei Q, Park H, Lieber CM (2001) Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 293:1289–1292
Zhang L, Yuan SM, Yang LM et al (2013) An enzyma tic glucose biosensor based on a glassy carbon electrode modified with manganese dioxide nanowires. Microchim Acta 180:627–633
Wang W, Xie YB, Wang Y et al (2014) Glucose biosensor based on glucose oxidase immobilized on unhybridized titanium dioxide nanotube arrays. Microchim Acta 181:381–387
Shi XH, Gu W, Li BY et al (2014) Enzymatic biosensors based on the use of metal oxide nanoparticles. Microchim Acta 181:1–22
Wang J (2012) Electrochemical biosensing based on noble metal nanoparticles. Microchim Acta 177:245–270
Zhao YG, Yan XQ, Kang Z et al (2013) Highly sensitive uric acid biosensor based on individual zinc oxide micro/nanowires. Microchim Acta 180:759–766
Lei Y, Yan XQ, Luo N et al (2012) ZnO nanotetrapod network as the adsorption layer for the improvement of glucose detection via multiterminal electron-exchange. Colloids Surf A 361:169–173
Wei A, Sun XW, Wang JX et al (2006) Enzymatic glucose biosensor based on ZnO nanorod array grown by hydrothermal decomposition. Appl Phys Lett 89:123902
Xiang C, Zou Y, Qiu S et al (2013) Bienzymatic glucose biosensor based on direct electrochemistry of cytochrome c on gold nanoparticles/polyaniline nanospheres composite. Talanta 110:96–100
Giannoudakos A, Agelakopoulou T, Asteriadis I et al (2008) Development and characterization of ZnO, Au/ZnO and Pd/ZnO thin films through their adsorptive and catalytic properties. J Chromatogr A 1187:216–225
Mao S, Long Y, Li W et al (2013) Core-shell structured Ag@C for direct electrochemistry and hydrogen peroxide biosensor applications. Biosens Bioelectron 48:258–262
Kurita R, Hayashi K, Fan X et al (2002) Microfluidic device integrated with pre-reactor and dual enzyme-modified microelectrodes for monitoring in vivo glucose and lactate. Sens Actuators B 87:296–303
Wen D, Guo S, Wang Y, Dong S (2010) Bifunctional nanocatalyst of bimetallic nanoparticle/TiO2 with enhanced performance in electrochemical and photoelectrochemical applications. Langmuir 26:11401–11406
Liao QL, Yang Y, Xia L et al (2008) High intensity, plasma-induced emission from large area ZnO nanorod array cathodes. Phys Plasmas 15:114505
Sau TK, Pal A, Jana NR et al (2008) Size controlled synthesis of gold nanoparticles using photochemically prepared seed particles. J Nanopart Res 3:257–261
Wang C, Tan X, Chen S et al (2012) Highly-sensitive cholesterol biosensor based on platinum-gold hybrid functionalized ZnO nanorods. Talanta 94:263–270
Zhang XH, Liao QL, Chu MM et al (2014) Structure effect on graphene-modified enzyme electrode glucose sensors. Biosens Bioelectron 52:281–287
Che X, Yuan R, Chai Y et al (2011) A glucose biosensor based on chitosan–Prussian blue–multiwall carbon nanotubes–hollow PtCo nanochains formed by one-step electrodeposition. Colloids Surf B 84:454–461
Xian Y, Hu Y, Liu F et al (2006) Glucose biosensor based on Au nanoparticles–conductive polyaniline nanocomposite. Biosens Bioelectron 21:1996–2000
Kong T, Chen Y, Ye Y et al (2009) An amperometric glucose biosensor based on the immobilization of glucose oxidase on the ZnO nanotubes. Sens Actuators B 138:344–350
Yang X, Feng B, He X et al (2013) Carbon nanomaterial based electrochemical sensors for biogenic amines. Microchim Acta 180:935–956
Yan QY, Peng B, Su G et al (2011) Measurement of tear glucose levels with amperometric glucose biosensor/capillary tube configuration. Anal Chem 83:8341–8346
Pang PF, Yang WY, Huang SJ et al (2007) Measurement of glucose concentration in blood plasma based on a wireless magnetoelastic biosensor. Anal Lett 40:897–906
Li YS, Du YD, Chen TM et al (2010) A novel immobilization multienzyme glucose fluorescence capillary biosensor. Biosens Bioelectron 25:1382–1388
Wu BL, Zhang GM, Shuang SM, Choi MMF (2004) Biosensors for determination of glucose with glucose oxidase immobilized on an eggshell membrane. Talanta 64:546–553
Nan CF, Zhang Y, Zhang GM et al (2009) Activation of nylon net and its application to a biosensor for determination of glucose in human serum. Enzym Microbiol Technol 44:249–253
Kim HJ, Yoon SH, Choi HN et al (2006) Amperometric glucose biosensor based on sol–gel-derived Zirconia/Nafion composite film as encapsulation matrix. Bull Korean Chem Soc 27:65–70
Li JP, Wei XP, Yuan YH (2009) Synthesis of magnetic nanoparticles composed by Prussian blue and glucose oxidase for preparing highly sensitive and selective glucose biosensor. Sens Actuators B 139:400–406
Zheng BZ, Xie SP, Qian L et al (2011) Gold nanoparticles-coated eggshell membrane with immobilized glucose oxidase for fabrication of glucose biosensor. Sens Actuators B 152:49–55
Acknowledgments
This work was supported by the National Major Research Program of China (2013CB932600), the Major Project of International Cooperation and Exchanges (2012DFA50990), the Program of Introducing Talents of Discipline to Universities, the National Natural Science Foundation of China (51232001, 51172022, 51372023, 31371203), the Research Fund of Co-construction Program from Beijing Municipal Commission of Education, the Fundamental Research Funds for the Central Universities, the Program for Changjiang Scholars and Innovative Research Team in University.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 256 kb)
Rights and permissions
About this article
Cite this article
Zhao, Y., Fang, X., Yan, X. et al. Nanorod arrays composed of zinc oxide modified with gold nanoparticles and glucose oxidase for enzymatic sensing of glucose. Microchim Acta 182, 605–610 (2015). https://doi.org/10.1007/s00604-014-1364-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1364-9