Abstract
Purpose
Sarcopenia is common in elderly gastrectomized patients and a known risk factor for postoperative complications and poor overall survival. However, the long-term outcomes of skeletal muscle loss after gastrectomy and the differences in outcomes of different gastrectomy procedures remain unclear.
Methods
The subjects of this retrospective study were 136 patients who underwent various gastrectomy procedures for early gastric cancer, namely: total gastrectomy (TG; n = 20), proximal gastrectomy (PG; n = 16), distal gastrectomy (DG; n = 60), and pylorus-preserving gastrectomy (PPG; n = 40). Skeletal muscle volume (SMV), calculated as the skeletal muscle index (SMI), was measured using cross-sectional computed tomography (CT) scans preoperatively and then 1, 2, and 3 years after gastrectomy.
Results
Sarcopenia developed from 2 years onwards in all the patients who underwent TG. The SMI and sarcopenia prevalence after gastrectomy deteriorated over time. Multivariate analysis revealed that TG and PG were significant risk factors for skeletal muscle loss in postoperative years 1 and 3. A decrease in the SMI after TG or PG was most remarkable in elderly patients.
Conclusions
The type of gastrectomy affects skeletal muscle loss in the long term. Elderly patients who undergo TG or PG are at high risk of severe skeletal muscle loss.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, high response rates of chemotherapy have been reported, but radical gastrectomy remains the standard treatment for gastric cancer [1]. Studies have found that nutritional status impacts patient survival [2, 3] and quality of life [4]; therefore, nutritional therapy is critical for patients receiving oncological therapy after gastrectomy.
Rosenberg [5] defined sarcopenia as “low muscle mass plus low muscle strength and/or low physical performance in the elderly”. Recent studies have shown that preoperative sarcopenia is a risk factor for perioperative complications [6,7,8], chemotherapy intolerance [9], and impaired overall survival after gastric cancer surgery [10]. In contrast, few reports have investigated secondary sarcopenia following gastrectomy, even though gastrectomy causes malabsorption and skeletal muscle loss. Yamaoka et al. reported that after total gastrectomy (TG), the skeletal muscle index (SMI) decreased by 6.2% (± 6.8%) from the preoperative value [11]. Yet, long-term postoperative changes in skeletal muscle volume (SMV) and perioperative risk factors for sarcopenia secondary to gastrectomy remain unclear.
In this study, we investigated the patterns of skeletal muscle loss following gastrectomy for gastric cancer and tried to identify the patients and treatment-specific factors that are associated with long-term skeletal muscle loss contributing to a worse prognosis. We evaluated the SMV and subcutaneous and visceral fat compartments based on the findings of computed tomography (CT), performed annually for 3 years in patients who underwent one of four types of gastrectomy for early-stage gastric cancer: TG, proximal gastrectomy (PG), distal gastrectomy (DG), or pylorus-preserving gastrectomy (PPG).
Methods
Study population
This retrospective study included 202 consecutive patients who underwent gastrectomy (TG, PG, DG, or PPG) for pathological T1-classified gastric cancer (mucosa and submucosa), according to the Japanese classification of gastric cancer [12], between January, 2012 and December, 2014, at the University of Tokyo Hospital in Tokyo, Japan. 22 patients with previous or concomitant cancers and 6 patients with recurrent disease were excluded from the study. The skeletal muscle area could not be measured at all in 38 patients whose CT scans were lost during follow-up and the skeletal muscle mass could not be measured at the level of the third lumbar vertebra (L3) on the CT scans of 12 patients. 15 patients withdrew from follow-up, 3 changed hospitals, and 8 died of diseases other than cancer. Finally, 136 patients were included in the analysis (Fig. 1). None of the patients received adjuvant or neoadjuvant chemotherapy. This retrospective study was approved by the University of Tokyo, Faculty of Medicine Ethics Committee (ID: 3962) and complies with the ethical standards of the Declaration of Helsinki.
CT image analysis of body composition
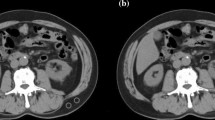
We reviewed CT scans taken preoperatively and then 1, 2, and 3 years postoperatively, which were required for the evaluation of tumor staging and screening for recurrence, for each of the patients who underwent gastrectomy. The cross-sectional abdominal CT inferior aspect of L3 was assessed to estimate the muscle mass. Images were analyzed using OsiriX (ver. 8.5.1, Pixmeo, Switzerland), which is open-source software that enables the examination of specific tissues using Hounsfield units (HUs). Measurements were recorded in a semi-automated fashion by setting the tissue of interest threshold at − 29 to + 150 HUs for skeletal muscle, − 190 to − 30 for subcutaneous adipose tissue [13], and − 150 to − 50 for visceral adipose tissue [11]. Skeletal muscle areas (SMA; cm2) in the L3 region computed from each image were normalized for height squared (m2) to obtain the SMI at the level of the inferior aspect of L3. Sarcopenia was defined as L3 SMI < 52.4 cm2/m2 in men and < 38.5 cm2/m2 in women [14]. At the same time, subcutaneous and visceral fat compartments for the subcutaneous fat area (SFA) and visceral fat area (VFA) were evaluated at the equivalent L3 level as the skeletal muscle area. Figure 2 shows typical transverse CT images at L3.
Statistical analyses
Statistical analyses were performed using JMP software (Pro 14, SAS Institute, Cary, NC, USA). Data are expressed as the mean ± standard error. Categorical variables were compared using the χ2 test or Fisher’s exact test. Multivariate logistic regression was performed. A P < 0.05 was considered significant. The following perioperative factors were analyzed: sex, age, preoperative sarcopenia, comorbidity based on the Clavien–Dindo (C–D) classification (C–D ≥ III) [15], surgical procedure (DG or PPG versus PG or TG), and lymphadenectomy (D1 or D1 + versus D2). In accordance with previous reports, we defined severe skeletal muscle loss (SSML) as a 10% decrease in SMI from the preoperative value [11, 16]. Operative time and blood loss were divided into two groups, using their average value as the cut-off point.
Results
Patient characteristics
Table 1 summarizes the clinical characteristics of the 136 patients in the study cohort. The study population comprised 94 men and 42 women, with a mean age of 66.06 (± 10.76) years. The preoperative mean SMI was 47.84 (± 8.10) cm2/m2 and the prevalence of preoperative sarcopenia was 54.4%. Table 1 lists the clinical characteristics of the patients, who were divided into four groups according to the gastrectomy procedure they underwent. The average operative blood loss was higher in the TG group than in the other groups, and the average operative time was longer for the DG group than for the PG or TG groups.
Changes in SMI, VFA, and SFA
Change in SMI was calculated by the rate of change from preoperative values. Because the values for VFA and SFA were close to 0 in lean patients and the rate of change was an outlier, VFA and SFA were calculated based on the amount of change. Figure 3 shows the rate of change in SMI and changes in VFA and SFA for each group in each postoperative year. The SMI was significantly lower than the preoperative value for every group in postoperative years 1, 2, and 3. The rate of change in SMI for the DG group decreased mainly in the first 2 years after gastrectomy and stabilized in postoperative year 3. The rate of change in SMI for the PPG group decreased mainly in postoperative year 1, then stabilized in postoperative years 2 and 3. The rate of change in SMI for the TG and PG groups decreased continually until postoperative year 3. There were significant differences in the rate of change in SMI between the DG and the TG groups in postoperative year 1 (P = 0.0223), the DG and the PG groups in postoperative years 1, 2, and 3 (P = 0.0020, P = 0.00400, P = 0.0214), and the PPG and the PG groups in postoperative year 3 (P = 0.0361). The rate of change in SMI for the PPG and PG groups were not significantly different from those for the DG and TG groups. VFA and SFA decreased significantly from the preoperative values in both groups every year; mainly in postoperative years 1 and 2 for VFA and mainly in postoperative year 1 for SFA.
Prevalence of sarcopenia evaluated using SMI after the different types of gastrectomy
Figure 4 shows changes in the prevalence of sarcopenia evaluated using SMI over time for each of the four groups. The numbers of patients with sarcopenia identified in each group preoperatively and then 1, 2, and 3 years postoperatively were as follows: 32, 37, 40, and 40 in the DG group (53.3%, 61.7%, 66.7%, 66.7%); 21, 25, 25, and 25 (52.5%, 62.5%, 62.5%, 62.5%) in the PPG group; 14, 16, 20 and 20 (70.0%, 80.0%, 100%, 100%) in the TG group; and 7, 9, 9, and 10 (43.8%, 56.3%, 56.3%, 62.5%) in the PG group. The prevalence of sarcopenia increased by approximately 10%–20% in the DG, PPG, and PG groups. However, in the TG group, the prevalence of sarcopenia was significantly higher than in any of the other groups, increasing from 71.4% to 100% in postoperative years 2 and 3.
Risk factors for postoperative SSML
Severe skeletal muscle loss of more than 10% from the preoperative value was seen 1, 2, and 3 years after surgery in 20 patients (14%), 46 patients (33%), and 53 patients (38%), respectively. Table 2 summarizes the results of multivariate analyses of six clinical risk factors for SSML: sex, age, preoperative sarcopenia, comorbidities, TG or PG, and lymphadenectomy. Multivariate analysis identified TG and PG as independent risk factors for SSML in postoperative years 1 and 3 (odds ratio [OR] 4.08, 95% confidence interval [CI] 1.44–12.05; OR 2.32, 95% CI 1.06–5.19). Age was an independent risk factor for SSML in postoperative year 2 (OR 2.42, 95% CI 1.51–5.34), and this tendency was also observed in postoperative years 1 and 3.
Rate of change in SMI as stratified by age
Sarcopenia is associated with compromised outcomes in the elderly, so we subdivided patients into an elderly group (age ≥ 65 years [n = 79]) and a younger group (under 65 years old [n = 57]) for comparison. The preoperative SMI of the elderly patients was 46.89 (± 7.87) cm2/m2 and that of the younger patients was 48.52 (± 8.25) cm2/m2. There was no significant difference in preoperative SMI values between the elderly and younger patients. Figure 5 shows the rate of change in SMI in the elderly and younger patients according to the four types of gastrectomy. The surgical procedure had a greater impact on the elderly patients than on the younger patients. In postoperative year 3, the rate of change in SMI of the TG and PG patients in the elderly group decreased prominently to − 19.3% and − 12.4%, whereas the rate of change in SMI of the TG and PG patients in the younger group returned to − 2.9% and − 4.4%, respectively.
Discussion
We reviewed the long-term SMI changes after four types of gastrectomy in patients with early gastric cancer and sought to identify the perioperative risk factors. The results of this study revealed annual progressive skeletal muscle loss and exacerbation of the prevalence of sarcopenia. TG and PG were both risk factors for a significant loss of skeletal muscle in postoperative years 1 and 3. The type of gastrectomy, especially in elderly patients, appeared to have a major impact on the SMI decrease.
Many reports have demonstrated that preoperative sarcopenia is a risk factor for perioperative complications [7, 8], impaired overall survival of patients with gastrointestinal and hepatopancreatobiliary malignancies [10], and increased postoperative morbidity [6]; yet only a few reports have described the progression of postoperative sarcopenia. It was reported that 57.7% of patients have postoperative sarcopenia after gastrectomy [17], and that 25.5% of patients showed significant skeletal muscle loss after TG [11]. Kugimiya et al. also reported that skeletal muscle loss after gastrectomy is an independent predictor of poor prognosis [18]. However, these reports were limited to TG and/or DG. To our knowledge, the present study is the first to examine long-term skeletal muscle loss after each of the four major types of gastrectomy.
Limited resection approaches, such as PPG and PG, are now being used more frequently to treat early gastric cancer in the middle-third and upper-third sections of the stomach, for their potential to maintain postoperative function and quality of life. PG resulted in better outcomes than TG in relation to postoperative morbidity and nutrition [19, 20] and body weight loss [21, 22]. Moreover, PPG is superior to DG because it ameliorates post-gastrectomy syndrome and maintains quality of life [23]. However, our study indicates that neither PG nor PPG has any advantage over TG or DG for skeletal muscle loss management. Further studies on large numbers of gastrectomy patients, with due consideration of the reconstructive techniques used and other relevant factors, are needed.
In this study, the SMI in the PG and TG groups continued to decrease throughout the observation period, in contrast to the pattern exhibited by the DG and PPG groups, in which the SMI decreased mainly in postoperative years 1 and 2. Recent studies show the nutritional benefit of subtotal gastrectomy, in which part of the stomach is left, even if it is small, over TG and PG [24, 25]. Since our DG group had less SMI reduction than the TG and PG groups, we consider subtotal gastrectomy the procedure of choice for upper gastric cancer.
Heneghan et al. reported that the prevalence of sarcopenia was 81.4%, 18 to 24 months after esophagectomy and total or subtotal gastrectomy [26]. This does not conflict with our findings, which showed that all patients who underwent TG had sarcopenia from 2 years postoperatively onward. Although the PG patients had the same rate of SMI reduction as the TG patients, the prevalence of sarcopenia in the TG patients might have been higher due to lower preoperative SMI values or variations in skeletal muscle loss in the PG patients. The postoperative SMI after every type of gastrectomy decreased each year, while the SFA and VFA began to increase from 2 years onward. A previous study noted that body weight decreased 6 months postoperatively, but then gradually increased [21]. The present study found that the SMI decreased over 3 years postoperatively, which is a different trend from the change in body weight. This finding suggests that bodyweight recovery reflects fat tissue recovery.
The elderly patients in the TG and the PG groups had severe skeletal muscle loss, which is associated with a worse prognosis [18, 27]. Takeshita and Liang et al. reported that elderly patients had a poorer overall survival rate than younger patients, although the disease-specific survival rates of the two groups were similar [28, 29]. TG and PG, which are selected based on the tumor location, cannot be avoided when performing curative gastrectomy. To improve the prognosis of elderly patients after TG and PG, we must evaluate the efficacy of surgical techniques and postoperative supportive care, such as oral nutritional support with training intervention. It is thought that gastrectomy-induced malnutrition can be compensated through nutritional support. The estimates of the amount of such support needed are based on body weight [30, 31]. We reviewed our patients’ CT scans, but records of their body weight were unreliable. In a study on upper-third gastric cancer, Yoo et al. reported that food intake and body weight decreased for 6 months after TG or PG, but then increased gradually thereafter [21]. In our study, the SMI continued to decrease in all groups from 1 to 3 years postoperatively, while the SFA and VFA began to increase from 2 years onward. The apparent discrepancy between food intake and fat tissue changes and the persistence of the SMI decrease suggests that skeletal muscle loss is related to additional factors. Hatao et al. reported that oral nutritional supplements after TG diminish postoperative weight loss but not skeletal muscle loss. Interventions such as progressive resistance training, where participants exercise against an increasing load [30, 31], offer potential protection against skeletal muscle loss after gastrectomy and should be evaluated in future studies.
This study has several limitations. First, it was a single-center retrospective observational study with a small sample size, especially for the TG group (20 patients) and PG group (16 patients). Second, it aimed to evaluate skeletal muscle loss but could not assess sufficient data such as albumin and pre-albumin, body weight, or the amount of food intake during the perioperative period. Third, we estimated skeletal muscle only from the CT images, which cannot always specifically diagnose sarcopenia, defined as low muscle mass plus reduced muscle function. The literature contains many studies of CT-based assessment of muscle mass and studies that measure the total cross-sectional area of muscle mass use distinct sex-specific cut-off values [32,33,34,35,36,37]. The cut-off values for sarcopenia, based on the study of sarcopenic obesity in patients with solid tumors by Prado et al. [14] from a Canadian institution, might overestimate those values for Eastern patients. Therefore, we should consider appropriate cut-off values for different ethnic groups. Prospective and larger-scale studies that include muscle-function evaluation and nutritional data are needed to establish the prevalence of postoperative sarcopenia and its influence on the overall survival of gastric cancer patients.
The findings of this long-term retrospective study of CT images showed that all patients who underwent TG had sarcopenia from 2 years postoperatively onward. TG and PG were risk factors for long-term severe skeletal muscle loss, especially in the elderly.
References
Kodera Y, Sano T. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20(1):1–19.
Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, et al. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: a meta-analysis. Eur J Surg Oncol. 2016;42(8):1176–82.
Saito H, Kono Y, Murakami Y, Shishido Y, Kuroda H, Matsunaga T, et al. Postoperative serum albumin is a potential prognostic factor for older patients with gastric cancer. Yonago Acta Med. 2018;61(1):72–8.
Carey S, Storey D, Biankin AV, Martin D, Young J, Allman-Farinelli M. Long term nutritional status and quality of life following major upper gastrointestinal surgery—a cross-sectional study. Clin Nutr. 2011;30(6):774–9.
Rosenberg I. Symposium: sarcopenia: diagnosis and mechanisms. J Nutr. 1997;127:990–1.
Peng PD, Van Vledder MG, Tsai S, De Jong MC, Makary M, Ng J, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB. 2011;13(7):439–46.
Otsuji H, Yokoyama Y, Ebata T, Igami T, Sugawara G, Mizuno T, et al. Preoperative sarcopenia negatively impacts postoperative outcomes following major hepatectomy with extrahepatic bile duct resection. World J Surg. 2015;39(6):1494–500.
Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Nagatsuma Y, Nakayama T, et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer. 2016;19(3):986–93.
Tan BHL, Brammer K, Randhawa N, Welch NT, Parsons SL, James EJ, et al. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol. 2015;41(3):333–8.
Levolger S, van Vugt JLA, de Bruin RWF, IJzermans JNM,. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015;102(12):1448–58.
Yamaoka Y, Fujitani K, Tsujinaka T, Yamamoto K, Hirao M, Sekimoto M. Skeletal muscle loss after total gastrectomy, exacerbated by adjuvant chemotherapy. Gastric Cancer. 2015;18(2):382–9.
Sano T, Kodera Y. Japanese classification of gastric carcinoma: 3rd english edition. Gastric Cancer. 2011;14(2):101–12.
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85(1):115–22.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35.
Clavien PA, Barkun J, De Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The clavien-dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96.
Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA J Am Med Assoc. 2013;310(15):1591–600.
Tegels JJW, van Vugt JLA, Reisinger KW, Hulsewé KWE, Hoofwijk AGM, Derikx JPM, et al. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J Surg Oncol. 2015;112(4):403–7.
Kugimiya N, Harada E, Oka K, Kawamura D, Suehiro Y, Takemoto Y, et al. Loss of skeletal muscle mass after curative gastrectomy is a poor prognostic factor. Oncol Lett. 2018;16(1):1341–7.
Huh YJ, Lee HJ, Oh SY, Lee KG, Yang JY, Ahn HS, et al. Clinical outcome of modified laparoscopy-assisted proximal gastrectomy compared to conventional proximal gastrectomy or total gastrectomy for upper-third early gastric cancer with special references to postoperative reflux esophagitis. J Gastric Cancer. 2015;15(3):191–200.
Kosuga T, Ichikawa D, Komatsu S, Okamoto K, Konishi H, Shiozaki A, et al. Feasibility and nutritional benefits of laparoscopic proximal gastrectomy for early gastric cancer in the upper stomach. Ann Surg Oncol. 2015;22:929–35.
Yoo CH, Sohn BH, Han WK, Pae WK. Proximal gastrectomy reconstructed by jejunal pouch interposition for upper third gastric cancer: prospective randomized study. World J Surg. 2005;29(12):1592–9.
Asaoka R, Irino T, Makuuchi R, Tanizawa Y, Bando E, Kawamura T, et al. Changes in body weight, skeletal muscle and adipose tissue after gastrectomy: a comparison between proximal gastrectomy and total gastrectomy. ANZ J Surg. 2019;89(1–2):79–83.
Hosoda K, Yamashita K, Sakuramoto S, Katada N. Postoperative quality of life after laparoscopy-assisted pylorus-preserving gastrectomy compared with laparoscopy-assisted distal gastrectomy: a cross-sectional postal questionnaire survey. Am J Surg. 2016;213(4):763–70.
Kosuga T, Hiki N, Nunobe S, Noma H. Feasibility and nutritional impact of laparoscopy-assisted subtotal gastrectomy for early gastric cancer in the upper stomach. Ann Surg Oncol. 2014;21(6):2028–35.
Jiang X, Hiki N, Nunobe S. Laparoscopy-assisted subtotal gastrectomy with very small remnant stomach : a novel surgical procedure for selected early gastric cancer in the upper stomach. Gastric Cancer. 2011;14(2):194–9.
Heneghan HM, Zaborowski A, Fanning M, Mchugh A, Doyle S, Moore J, et al. Prospective study of malabsorption and malnutrition after esophageal and gastric cancer surgery. Ann Surg. 2015;262(5):803–8.
Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol. 2007;36(1):228–35.
Takeshita H, Ichikawa D, Komatsu S, Kubota T, Okamoto K, Shiozaki A, et al. Surgical outcomes of gastrectomy for elderly patients with gastric cancer. World J Surg. 2013;37(12):2891–8.
Liang YX, Deng JY, Guo HH, Ding XW, Wang XN, Wang BG, et al. Characteristics and prognosis of gastric cancer in patients aged ≥ 70 years. World J Gastroenterol. 2013;19(39):6568–78.
Hatao F, Chen KY, Wu JM, Wang MY, Aikou S, Onoyama H, et al. Randomized controlled clinical trial assessing the effects of oral nutritional supplements in postoperative gastric cancer patients. Langenbeck’s Arch Surg. 2017;402(2):203–11.
Rogers C. Postgastrectomy nutrition. Nutr Clin Pract. 2011;26(2):126–36.
Gholami S, Janson L, Worhunsky DJ, Tran TB, Jin LX, Spolverato G, et al. Number of lymph nodes removed and survival after gastric cancer resection: an analysis from the US Gastric Cancer Collaborative. J Am Coll Surg. 2016;221(2):291–9.
Taguchi S, Akamatsu N, Nakagawa T, Gonoi W, Kanatani A, Miyazaki H, et al. Sarcopenia evaluated using the skeletal muscle index is a significant prognostic factor for metastatic urothelial carcinoma. Clin Genitourin Cancer. 2016;14(3):237–43.
Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yagi S, et al. Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation. 2017;101(3):565–74.
Huang DD, Zhou CJ, Wang SL, Mao ST, Zhou XY, Lou N, et al. Impact of different sarcopenia stages on the postoperative outcomes after radical gastrectomy for gastric cancer. Surgery. 2017;161(3):680–93.
Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer. Medicine. 2016;95(13):e3164.
Ninomiya G, Fujii T, Yamada S, Yabusaki N, Suzuki K, Iwata N, et al. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: a retrospective cohort study. Int J Surg. 2017;39:45–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yasuyuki Seto received research grants from Taiho Pharmaceutical Co., Ltd and Chugai Pharma Manufacturing CO., Ltd. Asami Okamoto, Susumu Aikou, Ryohei Iwata, Shuichiro Oya, Koichiro Kawasaki, Yasuhiro Okumura, Kotaro Wakamatsu, Masayuki Takegami, Koichi Yagi, Masato Nishida, Hiroharu Yamashita, and Sachiyo Nomura have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Okamoto, A., Aikou, S., Iwata, R. et al. The type of gastrectomy affects skeletal muscle loss and the long-term outcomes of elderly patients with gastric cancer: a retrospective study using computed tomography images. Surg Today 52, 812–821 (2022). https://doi.org/10.1007/s00595-021-02414-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-021-02414-2