Abstract
Purpose
Sarcopenia is a risk factor of severe surgical complications, short-term outcomes, and long-term outcomes for patients with gastric cancer. Several computed tomography (CT) measurements have been performed to diagnose sarcopenia. However, the optimal CT measurements for determining long-term outcomes have not been revealed.
Methods
A retrospective review of gastric cancer patients with clinical stage I, II, or III who underwent gastrectomy at age 75 years or more at Shizuoka General Hospital from 2007 to 2015 was performed. Using preoperative CT, skeletal muscle index (SMI), total psoas area, intramuscular adipose tissue content in multifidus muscle, morphologic change of psoas muscle, and visceral-to-subcutaneous adipose tissue area ratio (VSR) were measured in the third lumbar section. A Cox regression analysis was used to explore prognostic factors for overall survival.
Results
A total of 257 patients were reviewed. There were 171, 53, and 33 patients with clinical stages I, II, and III, respectively. A multivariate analysis indicated that, in addition to age, performance status, clinical stage, and types of resection, which are known prognostic factors, SMI and VSR are prognostic factors (p = 0.016, 0.046, respectively). The prognostic score, which was the frequency of positive SMI and VSR values within the cutoff, also indicates overall survival. The five-year OS rates of patients with prognostic scores of 0, 1, and 2 were 90.9%, 62.3%, and 52%, respectively (p < 0.001).
Conclusion
Preoperative SMI and VSR were prognostic factors for the overall survival of elderly patients with gastric cancer after gastrectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcopenia was first proposed by Rosenberg in 1989 [1]. In 2018, its definition was revised as “a muscle disease rooted in adverse muscle changes that accrue across a lifetime” [2].
Sarcopenia is attributable to aging, physical inactivity, and systemic diseases, especially those that may invoke inflammatory processes such as malignancy or organ failure. Because sarcopenia impairs the ability to perform activities of daily living and is a risk factor for fractures, morbidity disorders, and death, screening for and diagnosis of sarcopenia is essential.
Gastric cancer is the third most common cause of cancer-related death worldwide [3]. The main curative treatment for clinical stages I, II, and III gastric cancer is resection with or without adjuvant chemotherapy [4]. After gastrectomy, patients often experience appetite loss, diminished food intake, and weight loss. For patients with gastric cancer, sarcopenia is a risk factor for severe surgical complications [5]. It is also associated with short-term and long-term outcomes of gastric cancer patients [6].
Sarcopenia is screened using questionnaires as well as muscle strength based on the handgrip or chair stand test and is confirmed by measurements of muscle quality or quantity [2]. Computed tomography (CT) is the gold standard for diagnosing sarcopenia [2]; however, this exposes patients to X-rays. Because patients routinely undergo CT before gastrectomy to have their clinical stage determined, sarcopenia can be diagnosed without additional radiation exposure. There are numerous measurements that can be performed to determine sarcopenia. The total muscle area of the third lumbar vertebra (L3) level has been measured during many studies [6,7,8]. Other studies have measured the total psoas area (TPA) [9], intramuscular adipose tissue content of the multifidus muscle (IMAC) [10], and morphologic change of the psoas muscle (MPM) [11] to evaluate muscle mass and quality. The visceral-to-subcutaneous adipose tissue area ratio (VSR) has also been reported as a long-term prognostic factor [12]. However, to the best of our knowledge, no report has compared CT measurements to diagnose sarcopenia. This study aimed to explore the optimal preoperative CT measurements for determining sarcopenia and predicting long-term outcomes of gastric cancer patients who underwent gastrectomy.
Materials and methods
Study design and patient population
The study protocol was approved by the institutional review board of Shizuoka General Hospital (SGHIRB #2018061). The requirement of patient consent was waived because of the retrospective nature of the study. The records of patients with a diagnosis of clinical stage I, II, or III gastric cancer who underwent gastrectomy and were aged 75 or older between January 2007 and December 2015 at Shizuoka General Hospital were retrospectively identified from a prospectively maintained database. Patients with urgent conditions such as bleeding or stenosis, double primary cancers, or no CT before treatment at our institution were excluded from this study.
We investigated the clinical findings, clinical stage, laboratory data, surgical outcomes, and postoperative complications using prospectively collected data and individual patient medical records. Postoperative follow-up was performed routinely every 6 months or every year for 5 years at our institution. Patients who had transferred to other hospitals or stopped attending hospital visits were investigated using hospital referral forms or telephoning the patients.
The modified Charlson comorbidity index [13], excluding gastric cancer, was calculated. Postoperative complications were graded according to the Clavien–Dindo (CD) classification system [14]. CD grade II or higher complications were assessed during this study. Cancer staging was evaluated according to the 7th edition of the Union for International Cancer Control TNM classification system [15]. The indication for postoperative adjuvant chemotherapy was based on the Japanese gastric cancer treatment guidelines 2014 (ver. 4) [16]. Briefly, S-1 adjuvant chemotherapy was indicated for one year among patients with pathological stage II or III (except T3N0M0). The decision on chemotherapy was based on the patient’s age, general condition, and consent.
Measurement and definition of skeletal muscle mass
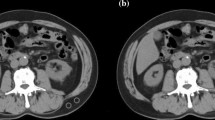
A preoperative CT image of the L3 cross-sectional area was selected for measurements. To minimize measurement bias, one investigator (Y.T.) who was blinded to the patients’ outcomes at that time measured all evaluation items using Volume Analyzer SYNAPSE VINCENT (Fujifilm, Tokyo, Japan). Fat tissue was identified and quantified using Hounsfield units (HU) with thresholds of −50 to −200. Tissue boundaries were manually corrected.
The skeletal muscle index (SMI) was calculated by dividing the area of all skeletal muscles at the L3 level by the squared value of the height. The muscles of the L3 level contain the psoas, erector spine, quadratus lumborum, multifidus, transversus abdominis, external and internal obliques, and rectus abdominis. TPA was obtained by dividing the area of the psoas muscle of the L3 level by the square of the height. The IMAC was calculated by dividing the region of interest (ROI) of the multifidus muscle by the ROI of subcutaneous fat (HU) [10]. The MPM score was defined as the mean of the short axis-to-long axis ratios of the right and left psoas muscles. The MPM grade was defined by the MPM score (range, 0 to 4) as follows: grade 0, score >2/3; grade 1, score >1/2 to ≤2/3; grade 2, score >1/3 to ≤1/2; grade 3, score >1/4 to ≤1/3; and grade 4, score ≤1/4 [11]. The VSR was the ratio of the visceral fat area to the subcutaneous fat area.
Outcomes
Overall survival (OS) was defined as the time from gastrectomy to the date of death from any cause. Relapse-free survival (RFS) was defined as the time from gastrectomy to the first recurrence (local, regional, or distant) or death from any cause (whichever occurred first).
Statistical analysis
The continuous and categorical variables were summarized, respectively, by the median (min–max) and frequency (percent). We dichotomized the continuous quantities (SMI, TPA, IMAC, MPM, and VSR) at all possible thresholds. We compared these variables to OS in a two-group comparison using the log–rank test in a gender-specific dataset. In each of these variable- and sex-specific comparisons, the threshold with the maximum log–rank statistic (the minimum p value) was adopted as the sex-specific cutoff value for SMI, TPA, IMAC, MPM, and VSR. The Cox proportional hazard model was used for univariate and multivariate analyses to investigate prognostic factors for OS. Age, Eastern cooperative oncology group performance status (PS), clinical stage, and type of resection [17] are known prognostic factors. Univariate analysis of OS was performed with these known prognostic factors and CT measurements. Multivariate analyses were conducted using the significant factors determined by the univariate analysis. Non-parametric values of more than two groups were compared using the Kruskal–Wallis test. The correlation of each item was checked using the absolute Pearson correlation coefficient. A prognostic score for each patient was defined by the frequency of positive variables selected by the multivariate analyses.
The OS and RFS were calculated using the Kaplan–Meier method, and the curves among groups were compared using the log–rank test. We considered it significant when p < 0.05 from two-tailed tests. Statistical analyses were performed using SAS (SAS Institute Inc., Cary, NC, USA).
Results
Patient demographics and characteristics
Between January 2007 and December 2015, a total of 1290 gastric cancer patients underwent gastrectomy at Shizuoka General Hospital. Among 366 patients who had clinical stage I, II, or III and were 75 years or older, patients with urgent conditions (n = 42), double primary cancer (n = 64), or no CT before treatment at our institution (n = 3) were excluded. After exclusion, 257 patients were included in this study.
Patient characteristics are shown in Table 1. Only 16 patients (6.2%) had a performance status score of 2 or worse. One hundred seventy-one patients (66.5%) had clinical stage I. The modified Charlson scores of 47 patients (18.3%) were one or higher. Postoperative complications occurred in 52 patients (20.2%).
Outcomes
The median follow-up period was 5.0 years (range, 0.16–11.0 years). A total of 48 patients (18.7%) were diagnosed with gastric cancer recurrence. Ninety-two patients (35.8%) died during the observation period (from any cause). Five-year OS and RFS rates of all patients were 64.7% (Fig. 1a) and 80.7% (Fig. 1b), respectively.
Optimal measurements of CT images and cutoff values
Sex-specific cutoff levels are shown in Table 2. The cutoff levels of IMAC could not be determined because the OS rates of patients with extremely high IMAC and low IMAC were lower than those of patients with medium IMAC.
Evaluation of CT image measurements in terms of OS
Univariate and multivariate Cox regression analyses of clinical findings and CT image measurements to determine OS are shown in Table 3. Univariate analyses indicated that, in addition to the four known prognostic factors (age, PS, clinical stage, and types of resection), SMI and VSR were significant prognostic factors that can be obtained by measuring CT images. A multivariate analysis with these six variables revealed that, in addition to the four known prognostic factors, SMI and VSR were independent prognostic factors (SMI: hazard ratio (HR), 1.78; 95% confidence interval (CI), 1.11–2.83; p = 0.016; VSR: HR, 1.59; 95% CI, 1.01–2.49; p = 0.046).
Correlation between clinical stage, SMI, and VSR
According to the Kruskal–Wallis test, no correlation can be observed between clinical stage and SMI or VSR (Fig. 2a and b). Similarly, according to the analysis using Pearson’s correlation coefficient, there is no correlation between SMI and VSR (r = 0.055).
Association of postoperative adjuvant chemotherapy with SMI and VSR
A total of 60 patients (23.3%) were eligible for postoperative adjuvant chemotherapy. Among the 60 patients, 41 (68.3%) had low SMI and 43 (71.6%) had low VSR, both of which tended to be slightly higher than the overall percentage. Nineteen of the 60 patients received adjuvant chemotherapy, and 10 received more than 50% of dose intensity. Among these 10 patients, 7 had low SMI (17.1%) and 7 had low VSR (16.3%). Moreover, no association was observed between postoperative adjuvant chemotherapy with dose intensity ≥ 50% and SMI or VSR (p = 1.00, for both); however, dose intensity ≥ 50% was a significant prognostic factor for OS. (p = 0.027)
Prognostic scores according to SMI and VSR and long-term outcomes
Patients with both high SMI and high VSR were assigned a prognostic score of 0. Patients with either low SMI or low VSR were assigned a prognostic score of 1. Patients with both low SMI and low VSR were assigned a prognostic score of 2. Five-year OS rates of patients with prognostic scores of 0, 1, and 2 were 90.9%, 62.3%, and 52%, respectively (p < 0.001) (Fig. 3a). Five-year RFS rates of patients with prognostic scores of 0, 1, and 2 were 96.2%, 80.2%, and 71.3%, respectively (p = 0.004) (Fig. 3b). The clinical stage-specific OS and RFS rates are shown in Fig. 4. The prognostic score significantly discriminates OS for clinical stage I and clinical stage II (p = 0.021 and 0.012, respectively).
Discussion
Clinical stage, PS, and sarcopenia are known prognostic factors. However, the definition of sarcopenia has not been clearly described, and there are many CT image measurements used to determine sarcopenia. In this study, we showed that preoperative SMI and VSR were significant prognostic factors for OS after gastrectomy. To the best of our knowledge, this is the first report to compare CT findings and show the optimal CT measurement items for predicting long-term outcomes. Among muscle measurements, SMI was superior to TPA, MPM, and IMAC for determining the prognosis. These results suggest that total muscle volume is more important than partial muscle volume, muscle shape, and muscle density.
Shachar et al. performed a meta-analysis of 7843 patients with solid tumors from 38 studies and revealed that low SMI was associated with poor OS (HR, 1.44; 95% CI, 1.32–1.56; p < 0.001) [18]. The SMI has been reported as a prognostic factor for many types of cancer, such as gastric cancer [19], hepatocellular cancer [20], colon cancer [21], and esophageal cancer [22]. However, some studies have shown no significant differences in OS [23,24,25]. Because SMI alone is not a strong prognostic factor, using an additional factor is expected to improve the prognostication.
Moon et al. reported that high VSR was a poor prognostic factor for colorectal cancer patients [12]. However, our data showed that patients with high VSR had more prolonged survival. We estimated that this discrepancy was due to the differences in the body mass index (BMI) of the patients involved in these studies. The mean BMI of the patients in the study by Moon et al. [12] was 23.9, but that of our patients was 21.7. Visceral adipose tissue loss is preferential to subcutaneous abdominal adipose tissue loss with modest weight loss [26]. Visceral fat tissue has a physiological role in providing energy at times of acute negative energy balance [26]. Gonzalez et al. reported that obesity can be used to predict a high survival rate when sarcopenia is absent [27]. Our data also showed that high VSR was a better prognostic factor when SMI was high (log–rank: p = 0.013), but not when SMI was low (log–rank: p = 0.296).
A combination of SMI and VSR discriminates the Kaplan–Meier curves for OS and RFS. This result indicated that both muscle volume and energy storage are important for the prolonged survival of gastric cancer patients. In previous reports, several factors such as body weight loss, muscle attenuation [28], comorbidity [29], age [30], and sex [30] were combined with SMI to explain prolonged survival. However, regarding muscle attenuation, it is difficult to discriminate between fat in muscle and fat between muscle, especially around the intercostal muscle and multifidus muscle. The combination of SMI and VSR is logically simple, and both could be measured on CT images without much bias between measurers. The prognostic scores of SMI and VSR stratified the OS. This result supported the fact that SMI and VSR are both independent prognostic factors.
The SMI and VSR discriminate OS for patients with clinical stage I and for those with clinical stage II, but not for those with clinical stage III. The impact of SMI on OS at each stage varies between reports. Sakurai et al. reported that SMI was a poor prognostic factor for stage I but not for stages II and III [7], whereas Zhuang et al. reported that SMI was a poor prognostic factor for stages II and III but not for stage I [6]. In our study, the prognostic score in stage III was not statistically significant because the number of patients with clinical stage III was insufficient (n = 33), especially patients with prognostic score 0 (n = 1). Furthermore, there were not enough events to evaluate RFS (n = 48). Therefore, the impact of the prognostic score among stage III patients is unknown in our study. There were 74 deaths and 34 recurrences for patients with clinical stages I and II. Therefore, more than half of the patients died from causes other than gastric cancer. Using Kaplan–Meier curves for OS and RFS, we estimated that SMI and VSR predicted death from both gastric cancer and other causes.
The cutoff value of SMI differs between studies [6, 20, 28]. Nishigori et al. compared five previously reported cutoff values for advanced gastric cancer [19]. However, the best cutoff value for the prognosis depends on race, disease, and disease progression, and it differs between populations. Therefore, we did not use the previously reported cutoff values. We calculated the most distinguishable cutoff value using our dataset because the purpose of this study was to identify the optimal CT measurements for diagnosing sarcopenia and predicting the long-term prognosis. Therefore, our cutoff value was not ubiquitous, and a retrospective study to define cutoff value for each population is required to apply our results.
Our results are useful for assessing patients’ long-term risk and personalizing nutritional interventions. For older people, in addition to an oncologic assessment, evaluations of comorbidity, activities of daily living, and nutrition are important. Our prognostic score enables physicians to extract patients at high risk for malnutrition so they can be offered the nutritional intervention. Imamura et al. reported that nutritional support after gastrectomy with an oral elemental diet of amino acids prevented body weight loss [31]. However, treatment compliance was not high (mean, 68.7% ± 30.4%), probably because of its bad taste. Using our prognostic score, physicians and patients can recognize the necessity for nutritional intervention. This prognostic score might also be used for nutritional follow-up in addition to body weight and hematological examinations because gastric cancer patients usually undergo CT regularly after gastrectomy.
Nevertheless, there were several limitations in our study. First, our analysis was limited to patients 75 years or more. Because sarcopenia is an age-related disorder, and because older people are easily affected by their nutritional status, we decided that this population is suitable for the study of sarcopenia. Nakajima et al. reported that elderly patients with esophageal cancer and sarcopenia had worse OS than those without sarcopenia; however, this was not observed for younger patients with esophageal cancer [32]. Therefore, the concept of sarcopenia might not apply to young people. Second, this study was retrospective and was conducted at a single institution. More extensive prospective research should be performed to validate our results. Finally, the BMI of our study participants was relatively low. Therefore, the meaning of VSR might also be different for people with different BMI.
Conclusions
Preoperative SMI, VSR, and CT measurement scores were prognostic for OS of gastric cancer patients aged 75 years or older after gastrectomy.
References
Rosenberg IH (1989) Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutr. 50:1231
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 48:16–31
Bray F, Ferlay J, Soerjomataram I, Siegel RL et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424
Japanese Gastric Cancer Association (2020) Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer
Simonsen C, de Heer P, Bjerre ED, Suetta C et al (2018) Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta-analysis. Ann Surg. 268:58–69
Zhuang CL, Huang DD, Pang WY, Zhou CJ et al (2016) Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine (Baltimore). 95:e3164
Sakurai K, Kubo N, Tamura T, Toyokawa T et al (2017) Adverse effects of low preoperative skeletal muscle mass in patients undergoing gastrectomy for gastric cancer. Ann Surg Oncol. 24:2712–2719
Fukuda Y, Yamamoto K, Hirao M, Nishikawa K et al (2016) Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer. 19:986–993
Peng PD, van Vledder MG, Tsai S, de Jong MC et al (2011) Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford). 13:439–446
Kitajima Y, Hyogo H, Sumida Y, Eguchi Y et al (2013) Severity of non-alcoholic steatohepatitis is associated with substitution of adipose tissue in skeletal muscle. J Gastroenterol Hepatol. 28:1507–1514
Hanaoka M, Yasuno M, Ishiguro M, Yamauchi S et al (2017) Morphologic change of the psoas muscle as a surrogate marker of sarcopenia and predictor of complications after colorectal cancer surgery. Int J Colorectal Dis. 32:847–856
Moon HG, Ju YT, Jeong CY, Jung EJ et al (2008) Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol. 15:1918–1922
Quan H, Li B, Couris CM, Fushimi K et al (2011) Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 173:676–682
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 240:205–213
Sobin LH, Gospodarowicz MK (2009) Wittekind C. TNM classification of malignant tumours, 7th edn. Wiley-Blackwell, New York
Japanese Gastric Cancer Association (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 20:1–19
Liu Z, Feng F, Guo M, Liu S et al (2017) Distal gastrectomy versus total gastrectomy for distal gastric cancer. Medicine (Baltimore). 96:e6003
Shachar SS, Williams GR, Muss HB, Nishijima TF (2016) Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 57:58–67
Nishigori T, Tsunoda S, Obama K, Hisamori S et al (2018) Optimal cutoff values of skeletal muscle index to define sarcopenia for prediction of survival in patients with advanced gastric cancer. Ann Surg Oncol. 25:3596–3603
Voron T, Tselikas L, Pietrasz D, Pigneur F et al (2015) Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. 261:1173–1183
Malietzis G, Currie AC, Athanasiou T, Johns N et al (2016) Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. 103:572–580
Tamandl D, Paireder M, Asari R, Baltzer PA et al (2016) Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol. 26:1359–1367
Cooper AB, Slack R, Fogelman D, Holmes HM et al (2015) Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol. 22:2416–2423
Harada K, Ida S, Baba Y, Ishimoto T et al (2016) Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma. Dis Esophagus. 29:627–633
Rollins KE, Tewari N, Ackner A, Awwad A et al (2016) The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr. 35:1103–1109
Chaston TB, Dixon JB (2008) Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes (Lond). 32:619–628
Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB (2014) Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr. 99:999–1005
Martin L, Birdsell L, Macdonald N, Reiman T et al (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 31:1539–1547
Kuwada K, Kuroda S, Kikuchi S, Yoshida R et al (2018) Sarcopenia and comorbidity in gastric cancer surgery as a useful combined factor to predict eventual death from other causes. Ann Surg Oncol. 25:1160–1166
Hu CL, Jin XH, Yuan ZD, Xiong SW et al (2019) Prognostic significance of preoperative skeletal muscle status in patients with gastric cancer after radical gastrectomy. Asia Pac J Clin Nutr. 28:442–449
Imamura H, Nishikawa K, Kishi K, Inoue K et al (2016) Effects of an oral elemental nutritional supplement on post-gastrectomy body weight loss in gastric cancer patients: a randomized controlled clinical trial. Ann Surg Oncol. 23:2928–2935
Nakashima Y, Saeki H, Nakanishi R, Sugiyama M et al (2018) Assessment of sarcopenia as a predictor of poor outcomes after esophagectomy in elderly patients with esophageal cancer. Ann Surg. 267:1100–1104
Funding
This study was supported by the Medical Research Scholarship of the Shizuoka Prefectural Hospital Organization.
Author information
Authors and Affiliations
Contributions
Study conception and design: Yusuke Taki and Shinsuke Sato. Acquisition of data: Yusuke Taki. Analysis and interpretation of data: Yusuke Taki, Shinsuke Sato, and Eiji Nakatani. Drafting of manuscript: Yusuke Taki. Critical revision of manuscript: Shinsuke Sato, Eiji Nakatani, Kazuya Higashizono, Erina Nagai, Masato Nishida, Masaya Watanabe, Ko Ohata, Hideyuki Kanemoto, and Noriyuki Oba.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The content of this research was disclosed on the hospital’s website, and patients had the opportunity to reject it. The Institutional Review Board of Shizuoka General Hospital approved this study and has determined that individual patient consent is not required due to its retrospective nature. Patients’ data were anonymized and de-identified before analysis.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taki, Y., Sato, S., Nakatani, E. et al. Preoperative skeletal muscle index and visceral-to-subcutaneous fat area ratio are associated with long-term outcomes of elderly gastric cancer patients after gastrectomy. Langenbecks Arch Surg 406, 463–471 (2021). https://doi.org/10.1007/s00423-021-02092-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02092-1