Abstract
Purpose
To compare diffusion tensor imaging (DTI) parameters of the spinal cord between patients with cervical spondylotic myelopathy (CSM) and normal subjects, and investigate their significance in the clinical diagnosis, surgical planning and post-operative evaluation of CSM.
Methods
Routine sequence magnetic resonance imaging (MRI) and DTI scans were performed in 50 normal subjects and 60 cases of CSM with 3.0-T MR. DTI images, apparent diffusion coefficient (ADC) and fractional anisotropy (FA) colormaps corresponding to spinal cord cross-sections were obtained. The spinal cord function of CSM patients was measured using modified Japanese Orthopaedic Association (mJOA) scoring and Nurick grade at different times. The changes in DTI parameters and their correlation with spinal cord function scores were analysed by SPSS 19.
Results
There were significant differences in DTI parameters of the spinal cord between normal subjects and patients with CSM (ADC: 1.119 ± 0.087 vs. 1.395 ± 0.091, P < 0.01; FA: 0.661 ± 0.057 vs. 0.420 ± 0.080, P < 0.01). The FA values at the maximal compression level of the spinal cord in the patients with CSM were significantly associated with the mJOA score pre-operatively, 1 week, and 1, 3 and 6 months post-operatively, with Pearson’s correlation coefficients of 0.58 (P < 0.01), 0.53 (P < 0.05), and 0.51 (P < 0.05), 0.54 (P < 0.05) and 0.55 (P < 0.05), respectively. However, the FA values were significantly negatively associated with the Nurick grade, with Pearson’s correlation coefficients of − 0.40 (P < 0.05), − 0.39 (P < 0.05), and -0.41 (P < 0.05), − 0.45 (P < 0.05) and − 0.44 (P < 0.05), respectively.
Conclusions
DTI may play a significant role in diagnosing and predicting the development of CSM.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cervical spondylotic myelopathy (CSM) is the most common cause of cervical spinal cord dysfunction [1]. It is a progressive, degenerative spine abnormality, causing cervical spinal cord damage [2]. Among people above 40 years old, approximately 60% suffer from this disease [3]. Its pathologic mechanisms include mechanical compression and secondary biological damage [4].
Imaging technology is an integral part of CSM patient management. The earliest imaging diagnosis of CSM began with the X-ray, CT and computed tomography myelography (CTM) on the morphological description of the cervical spinal canal. Some scholars have tried to determine cervical spinal cord compression by measuring the spinal canal parameters, thus providing imaging evidence for the clinical diagnosis of CSM [5,6,7,8]. Magnetic resonance imaging (MRI), which has changed the condition in which radiology solely relied on morphological changes to predict the diagnosis of the disease, became the most important imaging diagnostic methods for CSM [9]. However, traditional MRI has its limits in the prediction of neurological function, often underestimating the extent and severity of spinal cord injury [10]. Therefore, a new imaging technique that can clearly describe the function of the spinal cord is called for improving the promptness and sensitivity of diagnosis.
Diffusion tensor imaging (DTI) is a new magnetic resonance technique that emerged in recent years. Studies have shown that DTI can display the damage of the spinal cord before MR T2-weighted imaging (T2 WI) [11,12,13]. Therefore, DTI of the spinal cord marked a significant advancement in the field of neurological imaging, and its application will greatly expand the diagnosis and treatment evaluation of CSM.
In this study, we measured the DTI parameter of cervical spinal cord in normal subjects and CSM patients, and explore its correlation with clinical function scores.
Subjects and methods
Subjects
Fifty normal subjects were randomly sampled from healthy volunteers in whom a physical examination, X-ray and MRI were performed. Those with cervical diseases, such as cervical spine trauma, deformity and other cervical spine diseases were excluded. Normal subjects included 32 males and 18 females, with a mean age of 45 years (22–74 years). Sixty patients with CSM were randomly sampled from those admitted to the hospital during a period from October 2013 to October 2016. All patients underwent physical examinations, X-ray and MRI scan, and patients with ossification of posterior longitudinal ligament (OPLL), amyotrophic lateral sclerosis (ALS) and spinal tumour were excluded. Of 60 patients, 39 were male and 21 were female, with a mean age of 55 years (35–82 years). Most patients were in a chronic process, and showed a progressive exacerbation. The duration of the disease ranged from 2 months to 16 years, with a mean of 4.6 years. After the onset of the disease, all subjects experienced neck and shoulder discomfort, numbness of limbs, unsteady gait and zonesthesia (a feeling or sensation of constriction in the body) in the chest or abdomen. Physical examination showed that compression was mainly on the lower cervical spinal cord, and different degrees of incomplete paralysis were detected in the limbs. In addition, there were obvious sensor and activity disorders in the limbs, and hyperreflexia was observed and the pathological tendon reflex was positive.
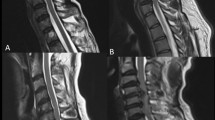
All patients with CSM underwent surgical treatment. According to the location and segment of spinal cord compression, different approaches (anterior, posterior or anterior–posterior) and techniques were chosen. The anterior approach in the study included anterior cervical discectomy and fusion (ACDF) and anterior cervical corpectomy and fusion (ACCF). The posterior approach was an open-door extensive laminoplasty. Pre-operative and post-operative MRI of patients with CSM is shown in Fig. 1. Cervical spinal cord function of patients with CSM was measured using the modified Japanese Orthopaedic Association (mJOA) scoring [14] and Nurick grade [15] method pre-operatively, 1 week, and 1, 3 and 6 months post-operatively (Table 1). The study was approved by the Ethics Committee of University and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from volunteers and patients.
Measurement methods
All MRI conventional sequence scans and DTI scans were performed on a GE Signa HDxt 3.0-T magnetic resonance machine (General Electric Company, USA). Subjects were in supine position, wore earplugs, and calmly breathed to avoid swallowing. Their cervical spines were in neutral position. Their heads and necks were in the middle of the HDNV Array head and neck coil, and their hands were on both sides of the body. Scanning range was from C1 to C7 level. The scanning parameters were as follows: field strength 40 mT/m, switching rate 150 mT/m/s. FSE sequence sagittal T1 WI (TE26.1 ms, TR2927 ms), T2 WI (TE117.6 ms, TR3200 ms), axial T2 WI (TE120.2 ms, TR2500 ms), and DTI (TE87.2 ms, TR8000 ms) scans were performed on all subjects, with slice thickness of 3 mm, sagittal field of view (FOV) 240 mm × 240 mm, and axial FOV 180 mm × 180 mm. DTI used a single stimulated echo planar imaging (EPI) sequence, with a b value (Dispersion Sensitive Gradient, DSG) of 1000 s/mm2, FOV 24 mm × 24 mm, slice thickness 4 mm, slice spacing 0.4 mm, and scanning matrix 130 × 128.
All scanning images were conveyed to the Advantage Workstation Volume Share 5 (GE Medical Systems S.C.S, France). DTI data image processing is performed using GE Functool 9.4.05 software. The correct programme was used to correct the scanned images. Then DTI images, apparent diffusion coefficient (ADC) colormaps and fractional anisotropy (FA) colormaps of the corresponding cross-sections of the cervical spinal cord were obtained. Normal subjects were analysed according to the regions of interest (ROI) (C2–C3, C3–C4, C4–C5, C5–C6 and C6–C7 level corresponding to spinal cord), which was determined by the FSE T2 WI sequence. The patients with CSM were examined by MRI pre-operatively, 1 week, and 1, 3 and 6 months post-operatively. The ROI was selected according to the FSE T2 WI sequence, including C2–C3 (white arrow), C3–C4 (blue arrow), C4–C5 (red arrow), C5–C6 (green arrow) and C6–C7 (yellow arrow) intervertebral disc level corresponding to the cervical spinal cord. Additionally, the level of T2 hyperintensity or maximum compression level (MCL) on T2 WI was recorded. The DTI parameters of all ROIs were measured and recorded: ADC values and FA values. Corresponding DTI images, ADC and FA colormaps of the spinal cord in patients with CSM are shown in Fig. 2. Scales of ADC colormaps and FA colormaps are shown in Fig. 3. To reduce subjective errors, all measurements were done by a spine surgeon and a radiologist, respectively. The final results were determined as the average of the two.
Statistical analysis
All data were described as the mean ± standard deviation (SD), and all statistical analyses were performed using the statistical software SPSS 19 (IBM SPSS Statistics, USA). Differences of DTI parameters between maximum compression level of spinal cord in CSM patients and the same level in normal subjects were tested for statistical significance using two-sample t tests. Pearson’s correlation analysis was used to investigate relationships between the FA value at the maximal compression level of the spinal cord in CSM patients and the mJOA score and Nurick grade. P < 0.05 was considered statistically significant.
Results
-
1.
There were significant differences in DTI parameters of the spinal cord between normal subjects and patients with CSM (ADC: 1.119 ± 0.087 vs. 1.395 ± 0.091, P < 0.01; FA: 0.661 ± 0.057 vs. 0.420 ± 0.080, P < 0.01). The ADC value of the spinal cord increased, but the FA value decreased at the maximal compression level of the spinal cord in patients with CSM (Table 2).
Table 2 ADC and FA value between the maximal compression level of patients with CSM and the same level of normal subjects (mean ± SD) -
2.
DTI images, ADC colormaps and FA colormaps corresponding to the C2–C3 (white arrow), C3–C4 (blue arrow), C4–C5 (red arrow), C5–C6 (green arrow) and C6–C7 (yellow arrow) intervertebral level of the cervical spinal cord are shown in Fig. 2. The DTI images, ADC colormaps and FA colormaps of the cervical spinal cord in patients with CSM not only reflected compression of the spinal cord from the form but also reflected the functional status of the spinal cord from the visual colour changes. In ADC colormaps, the ADC value increased as the spinal cord compression increased, which was manifested as a colour change from blue to → green. However, in the FA colormaps, the FA value gradually reduced as the spinal cord compression increased, which was manifested as a colour change from red to → yellow to → green (Fig. 2).
-
3.
The FA values at the maximal compression level of the spinal cord in patients with CSM were significantly associated with the mJOA score pre-operatively, 1 week, and 1, 3 and 6 months post-operatively, with Pearson’s coefficient correlations of 0.58 (P < 0.01), 0.53 (P < 0.05), and 0.51 (P < 0.05), 0.54 (P < 0.05) and 0.55 (P < 0.05), respectively (Table 3). However, they were negatively and significantly associated with the Nurick grade, with Pearson’s coefficient correlations of − 0.40 (P < 0.05), − 0.39 (P < 0.05), and − 0.41 (P < 0.05), − 0.45 (P < 0.05) and − 0.44 (P < 0.05), respectively (Table 4).
Table 3 Correlations between the FA value of the maximal compression level and mJOA score in patients with CSM at different times Table 4 Correlations between the FA value of the maximal compression level and Nurick grade in patients with CSM at different times
Discussion
With the ageing of the world population, the incidence of CSM has increased each year, especially in young patients. This seriously jeopardises people’s health. Thus, early diagnosis and treatment is urgently necessary. In the existing CSM imaging diagnostic technology, MRI undoubtedly plays a crucial role.
The shortcomings of traditional MRI
MRI provides excellent anatomical images of the macroscopic structure of the spinal cord, clearly showing compression and signal changes of the spinal cord, and even showing some pathological changes. Histopathological changes in CSM include myelomalacia, oedema gliosis and ischaemic changes in white matter, which are the pathological basis of the high signal in the spinal cord on MR T2 WI [1, 16]. Some scholars believe that these changes indicate irreversible damage of the spinal cord [3]. Therefore, if a patient’s traditional MR T2 WI shows a high signal, this indicates the function of the spinal cord has been severely damaged; thus, surgery is often slightly too late.
Many of the current radiological anatomical images are often inconsistent with the functional status of the patient, placing clinical treatment in a dilemma [17]. For example, the same MR images may have different clinical manifestations in different people. This makes clinical diagnosis and timely treatment of CSM challenging, urging for a new MRI technique.
DTI in CSM
DTI can quantify the size and direction of water molecules diffusion in tissues, which reflects the integrity of axon fibres in the spinal cord and small pathological changes of the spinal cord [18, 19]. The FA value is the most commonly used quantitative description of the dispersion of the diffusion tensor index [20]. ADC is the mean of the diffusion rate, and its increase or decrease depends on the process of histopathological damage of the tissue. The 3.0-T MR can provide higher image quality in DTI of the cervical spinal cord and better accordance with the expected anatomy compared to 1.5-T. In general, DTI parameters are affected by microstructural changes in tissues that affect the diffusion of water molecules, which is difficult to detect by traditional MRI. This is the theoretical basis of DTI in the diagnosis and evaluation of post-operative spinal cord function in patients with CSM.
It is interesting that in our study, in patients with CSM, the ADC value is elevated and FA value decreased, which is in contrast to acute compression of spinal cord [21, 22]. The changes in patients with CSM are very similar to those observed in chronic spinal cord injury [23, 24] and are also confirmed by animal models of chronic spinal compression. We conclude that this may be due to chronic, recurrent ischaemic damage of the spinal cord leading to downstream nerve fibre histopathology changes, including gliosis, neuron function loss, angioedema and eventual necrosis and cavitation. These pathological changes are intertwined, leading to an increase in the ADC value at the site of the spinal cord compression (increase of extracellular water) and decrease of the FA value (reduced anisotropy tissue). In the ADC colormaps and FA colormaps of patients with CSM, we can clearly visualise the above changes in DTI parameters (Fig. 2).
A study completed by Jones et al. [25] showed that patients with a high pre-operative FA value were likely to achieve better functional recovery after surgery. We analysed the FA values of spinal cord in 60 patients with CSM, compared it with the mJOA score and Nurick grade at different times, and found the FA value was positively correlated with the mJOA score but negatively correlated with the Nurick grade. Therefore, we believe that the FA value at the site of the spinal cord compression can be used as a potential imaging marker in selecting appropriate surgical cases, or that it can serve as a useful predictor of surgical outcomes.
Studies have found that a high pre-operative MR T2 WI signal indicates poorer neurological outcomes following surgery, and it may continue to exist even if patients’ mJOA score and Nurick grade are constantly improving [26]. Compared with an all-or-none high signal on T2 WI, the FA value is a continuous variable, which can provide a more accurate description of the spinal function in patients with CSM. Moreover, under the clinical suspicion of cervical myelopathy, DTI can find the abnormal microstructure changes of the spinal cord before a high signal emerges on traditional MR T2 WI. Although DTI was able to identify and quantify the severity of the spinal cord injury, we suggest that the establishment of the clinical FA value for the prognosis of spinal cord recovery still requires a larger sample size and multicentre, randomized controlled prospective studies.
In conclusion, DTI colormaps of spinal cord in patients with CSM can reflect compression and functional status of spinal cord. The FA value of spinal cord was associated with post-operative recovery of spinal cord function. These suggest DTI may play a significant role in diagnosing and predicting the development of CSM.
References
Baptiste DC, Fehlings MG (2006) Pathophysiology of cervical myelopathy. Spine J 6(6 Suppl):190S–197S
Matsunaga S, Komiya S, Toyama Y (2015) Risk factors for development of myelopathy in patients with cervical spondylotic cord compression. Eur Spine J 24(Suppl 2):142–149
Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG (2015) Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976) 40(12):E675–E693
Shedid D, Benzel EC (2007) Cervical spondylosis anatomy: pathophysiology and biomechanics. Neurosurgery 60(1):S7–S13
Morishita Y, Naito M, Hymanson H, Miyazaki M, Wu G, Wang JC (2009) The relationship between the cervical spinal canal diameter and the pathological changes in the cervical spine. Eur Spine J 18(6):877–883
Suk KS, Kim KT, Lee JH, Lee SH, Kim JS, Kim JY (2009) Reevaluation of the Pavlov ratio in patients with cervical myelopathy. Clin Orthop Surg 1(1):6–10
Naganawa T, Miyamoto K, Ogura H, Suzuki N, Shimizu K (2011) Comparison of magnetic resonance imaging and computed tomogram-myelography for evaluation of cross sections of cervical spinal morphology. Spine (Phila Pa 1976) 36(1):50–56
Dong F, Shen C, Jiang S, Zhang R, Song P, Yu Y, Wang S, Li X, Zhao G, Ding C (2013) Measurement of volume-occupying rate of cervical spinal canal and its role in cervical spondylotic myelopathy. Eur Spine J 22(5):1152–1157
Tetreault LA, Dettori JR, Wilson JR, Singh A, Nouri A, Fehlings MG, Brodt ED, Jacobs WB (2013) Systematic review of magnetic resonance imaging characteristics that affect treatment decision making and predict clinical outcome in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976) 38(22 Suppl 1):S89–110
Mohanty C, Massicotte EM, Fehlings MG, Shamji MF (2015) Association of preoperative cervical spine alignment with spinal cord magnetic resonance imaging hyperintensity and myelopathy severity: analysis of a series of 124 cases. Spine (Phila Pa 1976) 40(1):11–16
Liu Y, Kong C, Cui L, Yuan X, Zhao P, Zhang Y, Guan Y, Chen X (2017) Correlation between diffusion tensor imaging parameters and clinical assessments in patients with cervical spondylotic myelopathy with and without high signal intensity. Spinal Cord 55(9):1079–1083
Lindberg PG, Sanchez K, Ozcan F, Rannou F, Poiraudeau S, Feydy A, Maier MA (2016) Correlation of force control with regional spinal DTI in patients with cervical spondylosis without signs of spinal cord injury on conventional MRI. Eur Radiol 26(3):733–742
Kara B, Celik A, Karadereler S, Ulusoy L, Ganiyusufoglu K, Onat L, Mutlu A, Ornek I, Sirvanci M, Hamzaoglu A (2011) The role of DTI in early detection of cervical spondylotic myelopathy: a preliminary study with 3-T MRI. Neuroradiology 53(8):609–616
Bartels RH, Verbeek AL, Benzel EC, Fehlings MG, Guiot BH (2010) Validation of a translated version of the modified Japanese orthopaedic association score to assess outcomes in cervical spondylotic myelopathy: an approach to globalize outcomes assessment tools. Neurosurgery 66(5):1013–1016
Revanappa KK, Rajshekhar V (2011) Comparison of Nurick grading system and modified Japanese Orthopaedic Association scoring system in evaluation of patients with cervical spondylotic myelopathy. Eur Spine J 20(9):1545–1551
Yukawa Y, Kato F, Yoshihara H, Yanase M, Ito K (2007) MR T2 image classification in cervical compression myelopathy: predictor of surgical outcomes. Spine (Phila Pa 1976) 32(15):1675–1678
Fernández de Rota JJ, Meschian S, Fernández de Rota A, Urbano V, Baron M (2007) Cervical spondylotic myelopathy due to chronic compression: the role of signal intensity changes in magnetic resonance images. J Neurosurg Spine 6(1):17–22
Vedantam A, Jirjis MB, Schmit BD, Wang MC, Ulmer JL, Kurpad SN (2014) Diffusion tensor imaging of the spinal cord: insights from animal and human studies. Neurosurgery 74(1):1–8
Xu J, Shimony JS, Klawiter EC, Snyder AZ, Trinkaus K, Naismith RT, Benzinger TL, Cross AH, Song SK (2013) Improved in vivo diffusion tensor imaging of human cervical spinal cord. Neuroimage 67:64–76
Budzik JF, Balbi V, Le Thuc V, Duhamel A, Assaker R, Cotten A (2011) Diffusion tensor imaging and fibre tracking in cervical spondylotic myelopathy. Eur Radiol 21(2):426–433
Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D (2005) MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol 26(6):1587–1594
Kelley BJ, Harel NY, Kim CY, Papademetris X, Coman D, Wang X, Hasan O, Kaufman A, Globinsky R, Staib LH, Cafferty WB, Hyder F, Strittmatter SM (2014) Diffusion tensor imaging as a predictor of locomotor function after experimental spinal cord injury and recovery. J Neurotrauma 31(15):1362–1373
Song T, Chen WJ, Yang B, Zhao HP, Huang JW, Cai MJ, Dong TF, Li TS (2011) Diffusion tensor imaging in the cervical spinal cord. Eur Spine J 20(3):422–428
Ellingson BM, Ulmer JL, Kurpad SN, Schmit BD (2008) Diffusion tensor MR imaging in chronic spinal cord injury. AJNR Am J Neuroradiol 29(10):1976–1982
Jones JG, Cen SY, Lebel RM, Hsieh PC, Law M (2013) Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. AJNR Am J Neuroradiol 34(2):471–478
Shin JJ, Jin BH, Kim KS, Cho YE, Cho WH (2010) Intramedullary high signal intensity and neurological status as prognostic factors in cervical spondylotic myelopathy. Acta Neurochir 152(10):1687–1694
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, F., Wu, Y., Song, P. et al. A preliminary study of 3.0-T magnetic resonance diffusion tensor imaging in cervical spondylotic myelopathy. Eur Spine J 27, 1839–1845 (2018). https://doi.org/10.1007/s00586-018-5579-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-018-5579-z