Abstract
Introduction
The radiological diagnosis of cervical spondylotic myelopathy (CSM) has to be made as soon as possible, since surgery performed in earlier stages during the course of CSM was reported to be more successful when compared with later stages. We hypothesized that diffusion tensor imaging (DTI) may detect CSM in earlier stages, before the appearance of signal increase in T2-weighted sequences.
Methods
A total of 16 patients with neurological signs and symptoms of CSM but without hyperintensity in spinal cord on T2-weighted sequences enrolled in the study. The magnetic resonance (MR) examinations were performed on a 3-T MR imaging system. Apparent diffusion coefficient (ADC) and fractional anisotropy (FA) maps were generated on axial plane. The ADC and FA measurements in each individual were made at the level of most severe cervical canal stenosis and at a nonstenotic level. Student’s t test was used to compare FA and ADC values of the spinal cord in stenotic and nonstenotic segments. We also investigated if there was a correlation between DTI parametrics and duration of clinical symptoms by using Pearson correlation analysis.
Results
All patients showed changes in DTI parametrics at stenotic segments. While FA values of the spinal cord at the stenotic level showed a statistically significant reduction, there was a statistically significant increase in the measured ADC values (p < 0.001). There was no statistical correlation between the duration of symptoms and DTI parametrics.
Conclusion
Our preliminary findings indicate that DTI may show abnormalities in the spinal cord before the development of T2 hyperintensity on conventional sequences in patients with CSM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cervical spondylotic myelopathy (CSM) is the most serious complication of cervical spondylosis and the most common cause of spinal dysfunction in the elderly [1–4]. Its diagnosis is based primarily on clinical manifestations. Magnetic resonance imaging (MRI) is a useful tool for evaluating the spinal cord, where increased signal intensity on T2-weighted images may be observed at the level of the spinal canal stenosis. However, this finding is not seen in every patient with clinical signs of myelopathy, and its sensitivity is reported to be low (between 15% and 65%) [4–7]. Furthermore, increased signal intensity on T2-weighted sequences is generally observed only in later stages of the disease [8]. However, the diagnosis of CSM must be made as early as possible, since the results of surgical treatments are better in mildly affected individuals than in those severely affected [4, 9–14]. Therefore, newer neuroimaging strategies are needed for the demonstration of structural changes in the spinal cord before the development of T2 hyperintensity. Preliminary reports have shown that spinal applications of advanced MRI techniques such as diffusion weighted imaging (DWI) and diffusion tensor imaging (DTI) may be useful in understanding pathophysiology and early detection of spinal cord involvement by various pathologic processes [15–17]. DTI is a novel sequence that evaluates the translation of extracellular water molecules within the white matter fibers providing quantitative measurements and three-dimensional reconstruction of the white matter tracts. From the diffusion tensor matrix, several scalar metrics may be calculated. Fractional anisotropy (FA) is one of these parameters, which indicates the degree of anisotropy [18]. It is reported that FA has higher sensitivity to detect abnormal areas inside the cord, compared with conventional T2-weighted imaging [8, 19–21]. There are also studies that investigated the DTI findings of patients with CSM in an early clinical stage using 0.2- and 1.5-T systems [20, 22]. In light of preliminary studies, we think that DTI may provide further information about early detection of CSM. To achieve this, we evaluated the FA and apparent diffusion coefficient (ADC) values of spinal cord in patients with clinical manifestations of CSM, whose conventional MRI examinations showed no abnormal signal in T2-weighted sequences.
Materials and methods
Patient selection
This prospective study was performed between May 2009 and May 2010 with close cooperation of departments of orthopedic surgery, neurosurgery, neurology, and radiology. Only patients who met both of the following criteria were enrolled in the study: (a) patients with symptoms and signs of purely cervical myelopathy examined by a neurosurgeon or neurologist and (b) MRI findings consistent with cervical canal stenosis, but without signal changes in spinal cord on T2-weighted images. The exclusion criteria were as follows: (a) patients who had increased signals on T2-weighted images of the cervical spinal cord, (b) previous spine surgery, (c) history of cervical trauma, (d) history of stroke and other neurological disease, (e) cervical radiculopathy with no clinical evidence of cervical myelopathy, (f) patients without radiological evidence of cervical canal stenosis, and (e) contraindication to MRI (including severe claustrophobia). The institutional review board approved our study, and informed consent was obtained from all of the patients.
Imaging protocols
The MR examinations were performed on a 3-T MRI system (GE Healthcare, Milwaukee, WI, USA). The MRI protocol routinely included sagittal T2-, sagittal T1-, and axial T2-weighted FSE sequences and a 25-direction single-shot echo-planar imaging (EPI)-based DTI sequence in an axial plane. The parameters of the DTI sequence were repetition time/echo time = 5,000/85 ms, \( {\hbox{matrix size}} = {128} \times {128} \), FOV = 200 mm, b value of 500 s/mm2, slice thickness/spacing = 4.00/0.5 mm, and 16 slices. To reduce the susceptibility artifacts, volume shim over 140 mm has been used in the DTI protocol during the slice prescription.
Data processing and measurements
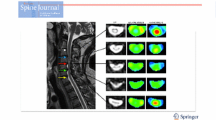
Acquired DTI images were processed using commercially available software (Functool DTI processing, GE Healthcare, Milwaukee, WI, USA) to produce ADC and FA maps of acquired axial slices. The EPI artifacts reduction option was used on all data sets in order to reduce typical EPI related artifacts such as eddy currents and geometric distortion. Furthermore, to reduce the measurement errors, the ADC and FA measurements were made on the axial plane at four sites in each individual. The first two regions of interest (ROIs) were placed at nonstenotic levels of the spinal cord, typically the C2–C3 level (Figs. 1 and 2). The last two sites were at the level of most severe cervical canal stenosis (Figs. 1 and 3). Instead of healthy individuals, we preferred to make measurements from upper nonstenotic segments (e.g., C2–C3 level) as a control group, since the study of Facon et al. showed that the comparison of FA and ADC values of healthy volunteers and on healthy areas of patients with spinal cord compression did not show a statistically significant difference [21]. The measurements were made by using a small ROI, which included both gray and white matter. The morphology and the cross-sectional area of the spinal cord were different between stenotic and nonstenotic segments. Therefore, the sizes of ROIs were not the same always in stenotic and nonstenotic segments and between patients and ranged from 40 to 60 mm2.
Statistical analysis
Student’s t test was used to compare FA and ADC values of the spinal cord in stenotic and nonstenotic segments. We also investigated if there was a correlation between DTI parametrics and duration of clinical symptoms by using Pearson correlation analysis. p values smaller than 0.05 was considered as “statistically significant.”
Results
A total of 16 patients (ten females and six males) met the inclusion criteria and enrolled in the study. Patients’ age varied between 27 and 86 years (mean 59.9 years). The mean duration of symptoms was 42.1 months (ranged from 8 to 120 months). Patients’ neurological findings were summarized in Table 1. All of the patients showed DTI abnormalities in stenotic segments when compared with nonstenotic segments. The FA and ADC values of spinal cord of each individual measured at nonstenotic segments are given in Table 2. The FA and ADC measurements obtained from stenotic segments are shown in Table 3. At the nonstenotic segment, the mean FA and ADC values were 0.65 ± 0.04 and 1.01 ± 0.19 (in 1 × 10−3 mm2/s), respectively. At the stenotic segment, the mean FA and ADC values were 0.47 ± 0.07 and 1.40 ± 0.37 (in 1 × 10−3 mm2/s), respectively (Table 4). While FA values of the spinal cord at the stenotic level showed a statistically significant reduction, there was a statistically significant increase in the measured ADC values (p < 0.001). We also observed that FA values were pretty consistent from one patient to another and resulted in a smaller standard deviation compared to ADC measurements. There was no statistical correlation between the duration of symptoms and DTI parametrics.
The symptoms were resolved in all of the surgically treated patients (10 of 16). However, we were not able to perform follow-up DTI in these patients due to artifacts created by metallic instrumentations implanted for cervical stabilization.
Discussion
The clinical success of decompression surgery in CSM depends on the timing of the surgical intervention. Surgery performed in earlier stages during the course of CSM was reported to be more successful when compared with later stages [9–14]. Therefore, the radiological diagnosis of CSM has to be made as soon as possible. CSM appears as high signal intensity on conventional T2-weighted sequences, but it occurs only at a late stage of the disease. This condition led the investigators to use novel MRI techniques to depict lesions in the spinal cord before the appearance of increased signal intensity on T2-weighted images. Studies in late 1990s showed that the DWI is more sensitive than T2-weighted imaging in patients with CSM [23] (Dousset et al., presented at the 1997 annual meeting of the American Society of Neuroradiology). The study by Demir et al. is one of the first reports on the use of the diffusion tensor concept in CSM [8]. In their study, a multishot echo-planar imaging sequence with calculation of ADC and ADT was applied in 36 patients with symptomatic cervical spondylosis. They found that patients with myelopathy had abnormal ADC and ADT maps with increased ADC and ADT values and decreased anisotropy. They also found that ADT maps have a sensitivity of 78%, whereas T2-weighted images have 57%. Mamata et al. evaluated 72 cervical spondylosis patients [20]. Among them, 39 cases (54%) without any abnormal signal on T2-weighted images showed ADC and FA abnormality at the narrowed spinal canal level. A total of 15 out of 39 patients demonstrated clinical myelopathy, while the other 24 cases were neurologically intact. The authors assumed that those cases that did not show clinical signs of myelopathy could present early stages of the disease. Hori et al. investigated the line scan DTI findings in patients with CSM using a 0.2-T MR imager [22]. They found that line scan DTI was able to detect changes in the spinal cord while T2-weighted scans were unable to reveal any abnormality. Our findings support the results of the studies mentioned above. We also found that the mean FA value in stenotic segments was 0.47, which was statistically lower than the nonstenotic segments (mean 0.65). The ADC values of stenotic segments were also increased compared with nonstenotic segments (the mean ADC values of the spinal cord in stenotic and nonstenotic segments were 1.40 × 10−3 and 1.01 × 10−3 mm2/s, respectively).
We found that there was no statistical correlation between the duration of symptoms and DTI parametrics. The reason for this lack of correlation is unclear. One possible explanation for this may be that the degree of the cervical canal stenosis and the degree of affection of the spinal cord at the stenotic segment differ in each individual. Additionally, some of the patients had multiple levels of canal stenosis. We preferred to obtain measurements only from the most stenotic level, and the symptoms might be related to a different stenotic segment in some patients. The reported FA values of the cervical spinal cord ranged from 0.52 to 0.83 [24–26]. In our study, we found that the mean FA value at the nonstenotic segments was 0.65 ± 0.04, similar to those reported previously.
We think that our study has two advantages. Firstly, our study cohort was quite homogenous clinically because we focused only on patients who had degenerative canal stenosis accompanied by pure myelopathic manifestations but no increased signal intensity on T2-weighted images. We did not enroll the patients who had neurological signs and symptoms related to coexistence of other spinal disorders. Therefore, we think that the DTI abnormalities that we found are related to the effects of cervical canal narrowing on the spinal cord. Secondly, the 3-T MRI system enabled us to have precise diffusion tensor images and the opportunity to make the measurements on the axial plane. Former studies mentioned above used the sagittal plane for DTI acquisitions and measurements. Acquisitions on the axial plane enabled us to generate FA maps in the axial plane. We believe that this is a more reliable method for measuring the FA values than using the sagittal plane, as the ROI can be placed precisely on spinal cord without cerebrospinal fluid contamination.
DTI enabled us to evaluate the degree of anisotropy of diffusion, which reflects the integrity and organization of the white matter tracts. From brain studies, it is known that anisotropy of diffusion in white matter mainly results from oriented membranes, such as axonal structures and myelin. Their disintegration results in a loss of anisotropy, which is detected by a decrease of FA [27]. DTI metrics in the various spinal cord tracts have also been shown to correlate with specific histological measures including axon counts and myelin volume [28]. Other experimental studies on spinal cord compression in rats found decreased FA values secondary to mechanical disruption, tearing of fibers and myelin sheaths, extracellular edema, Wallerian degeneration, and demyelination [21]. Recent studies demonstrated that apoptosis of the oligodendrocyte cell population is an early feature of CSM and may play a central role in the development of neurological deterioration seen with this order. Oligodendrocyte apoptosis causes demyelination of axons, which was observed both in human and animal studies of chronic spinal cord compression [29, 30]. Recently, Cheung et al. investigated the nature of chronic spinal cord compression in a rat model by using in vivo DTI (Cheung MM et al., presented at the 2009 annual international conference of the IEEE Engineering in Medicine and Biology Society). For simulation of chronic spinal cord compression, they inserted a urethane-containing polymer into the lateral column of the spinal cord of Sprague-Dawley rats at the C5–C6 level. FA and radial diffusivity values were measured by using regions of interest of four white matter regions (ventral, dorsal, ipsilesional, contralesional) in eight slices covering 4.4 mm caudal and 4.4 mm rostral to the epicenter of injury. They found that the ipsilesional region has a lower FA and higher radial diffusivity than other regions near the lesion site. The authors suggested that these DTI changes were related to demyelination in spinal cord, which caused by prolonged compression. In light of the data mentioned above, we think that in the course of CSM, prior to development of increased signal intensity on T2-weighted images, decreased FA values may reflect the axonal demyelination. Unfortunately, we do not have histopathological correlation for this argument.
When compared to brain studies, spinal applications of DTI are more challenging due to technical limitations. First of all, the small size of the spinal cord requires the use of small voxel sizes for better spatial resolution, which decreases the signal-to-noise ratio (SNR). Others factors, which make obtaining of DTI of the spinal cord difficult, are pulsation artifacts arising from the cerebrospinal fluid, cardiac and respiratory motion, and magnetic susceptibility artifacts caused by adjacent bones [19, 31]. Even though most of these difficulties are present in 1.5- or 3-T MR systems, due to its inherently higher SNR, 3 T poses some advantages. Since the 3-T system is more sensitive to motion and susceptibility artifacts, the DTI sequence has to be adopted for the spinal cord to get the main benefits of the high magnetic field imaging. In this study, we optimized the 3-T DTI protocol to obtain the shortest echo time possible, using a small, localized, shim volume to reduce susceptibility artifacts. To strike a good balance between scan time and SNR, we used 25 diffusion directions in all studies. In our experience, 55 directions created more gross and physiological motion artifacts due to extended scan time and exposure to the loud noise for longer time. Therefore, we think that a very high number of directions for routine DTI of spinal cord are not necessary when using a 3-T system.
There are several limitations in the present study. Firstly, the patient population is relatively small. Secondly, we do not have a long-term follow-up of the patients. Our hypothesis that the reduced FA values may indicate an early stage of CSM would only be proven if a majority of the patients went on to develop hyperintensity on T2-weighted images during the follow-up. However, the majority of the patients (10 of 16) underwent surgery, and the instrumentations implanted for cervical stability do not allow DTI examination of the cervical spinal cord in these patients. Therefore, we do not know how many of our patients would develop T2 hyperintensity if they were left untreated. We think that our data could be valuable for the establishment of a cutoff value of DTI parameters if we had a long-term follow-up of the patients, especially those treated conservatively. Lastly, one can argue that the use of DTI measurements of the spinal cord at the patients’ nonstenotic segments as the control group instead of measurements obtained from healthy individuals is a drawback in the study. From previous studies, it is known that the FA values of the spinal cord may differ from person to person and the upper and lower limits of normal FA values in a cervical spinal cord are not yet established. In addition, the study of Facon et al. showed that the comparison of FA and ADC values of healthy volunteers and on healthy areas of patients with spinal cord compression did not show a statistically significant difference [21]. We think that the DTI abnormalities in the affected segment of the spinal cord should be compared with a nonaffected segment if there is enough distance between them, as there was in our patients (the stenotic segments of our patients were located between C5 and C7). Therefore, when we designed the study, we thought that it would be more appropriate to take the nonstenotic upper cervical segments (usually C2–C3 level) as a reference level instead of using a cohort of healthy subjects.
In conclusion, our preliminary findings indicate that DTI may show abnormalities in the spinal cord before the development of T2 hyperintensity on conventional sequences in patients with CSM. The results of the present study also support the findings of previous studies, which concluded that DTI is more sensitive than conventional T2-weighted sequences in the early detection of CSM [8, 20, 22]. Our study showed that a 3-T system enables a technically feasible and precise data acquisition for quantitative measurements. However, further studies with larger patient populations are needed to determine the clinical utility of this novel MRI technique as an additional sequence in the routine examination of patients with CSM.
References
Shedid D, Benzel EC (2007) Cervical spondylosis anatomy: pathophysiology and biomechanics. Neurosurgery 60:7–13
Baptiste DC, Fehlings MG (2006) Pathophysiology of cervical myelopathy. Spine J 6:190S–197S
Lyu RK, Tang LM, Chen CJ, Chen CM, Chang HS, Wu YR (2004) The use of evoked potentials for clinical correlation and surgical outcome in cervical spondylotic myelopathy with intramedullary high signal intensity on MRI. J Neurol Neurosurg Psychiatry 75:256–261
Baron EM, Young WF (2007) Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery 60:S35–S41
Matsuda Y, Miyazaki K, Tada K, Yasuda A, Nakayama T, Murakami H, Matsuo M (1991) Increased MR signal intensity due to cervical myelopathy. J Neurosurg 74:887–892
Takahashi M, Yamashita Y, Sakamoto Y, Kojima R (1989) Chronic cervical cord compression: clinical significance of increased signal intensity on MR images. Radiology 173:219–224
Matsumoto M, Toyoma Y, Ishikawa M, Chiba K, Suzuki N, Fujimura Y (2000) Increased signal intensity of the spinal cord on magnetic resonance images in cervical compressive myelopathy: does it predict the outcome of conservative treatment? Spine 25:677–682
Demir A, Ries M, Moonen CT, Vital JM, Dehais J, Arne P, Caillé JM, Dousset V (2003) Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology 229:37–43
McCormick WE, Steinmetz MP, Benzel EC (2003) Cervical spondylotic myelopathy: make the difficult diagnosis, then refer for surgery. Cleve Clin J Med 70:899–904
Montgomery DM, Brower RS (1992) Cervical spondylotic myelopathy. Clinical syndrome and natural history. Orthop Clin North Am 23:487–493
Moore AP, Blumhardt LD (1997) A prospective survey of the causes of non-traumatic spastic paraparesis and tetraparesis in 585 patients. Spinal Cord 35:361–367
Yagi M, Ninomiya K, Kihara M, Horiuchi Y (2010) Long-term surgical outcome and risk factors in patients with cervical myelopathy and a change in signal intensity of intramedullary spinal cord on magnetic resonance imaging. J Neurosurg Spine 12:59–65
Chatley A, Kumar R, Jain VK, Behari S, Sahu RN (2009) Effect of spinal cord signal intensity changes on clinical outcome after surgery for cervical spondylotic myelopathy. J Neurosurg Spine 11:562–567
Matz PG, Anderson PA, Holly LT et al (2009) The natural history of cervical spondylotic myelopathy. J Neurosurg Spine 11:104–111
Rutgers DR, Toulgoat F, Cazejust J, Fillard P, Lasjaunias P, Ducreux D (2008) White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. AJNR Am J Neuroradiol 29:514–519
Huang J, Friedland RP, Auchus AP (2007) Diffusion tensor imaging of normal-appearing white matter in mild cognitive impairment and early Alzheimer disease: preliminary evidence of axonal degeneration in the temporal lobe. AJNR Am J Neuroradiol 28:1943–1948
Yu CS, Lin FC, Li KC, Jiang TZ, Zhu CZ, Qin W, Sun H, Chan P (2006) Diffusion tensor imaging in the assessment of normal-appearing brain tissue damage in relapsing neuromyelitis optica. AJNR Am J Neuroradiol 27:1009–1015
Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL (2004) Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol 25:356–369
Shanmuganathan K, Gullapalli RP, Zhuo J, Mirvis SE (2008) Diffusion tensor MR imaging in cervical spine trauma. AJNR Am J Neuroradiol 29:655–659
Mamata H, Jolesz FA, Maier SE (2005) Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis-related changes. J Magn Reson Imaging 22:38–43
Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D (2005) MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol 26:1587–1594
Hori M, Okubo T, Aoki S, Kumagai H, Araki T (2006) Line scan diffusion tensor MRI at low magnetic field strength: feasibility study of cervical spondylotic myelopathy in an early clinical stage. J Magn Reson Imaging 23:183–188
Castillo M, Arbelaez A, Fisher LL, Smith JK, Mukherji SK (1999) Diffusion weighted imaging in patients with cervical spondylosis. Int J Neuroradiol 5:79–85
Ries M, Jones RA, Dousset V, Moonen CT (2000) Diffusion tensor MRI of the spinal cord. Magn Reson Med 44:884–892
Cercignani M, Horsfield MA, Agosta F, Filippi M (2003) Sensitivity-encoded diffusion tensor MR imaging of the cervical cord. AJNR Am J Neuroradiol 24:1254–1256
Xiangshui M, Xiangjun C, Xiaoming Z, Qingshi Z, Yi C, Chuanqiang Q, Xiangxing M, Chuanfu L, Jinwen H (2010) 3 T magnetic resonance diffusion tensor imaging and fibre tracking in cervical myelopathy. Clin Radiol 65:465–473
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 15:435–455
Hesseltine SM, Law M, Babb J, Rad M, Lopez S, Ge Y, Johnson G, Grossman RI (2006) Diffusion tensor imaging in multiple sclerosis: assessment of regional differences in the axial plane within normal-appearing cervical spinal cord. AJNR Am J Neuroradiol 27:1189–1193
Kim DH, Vaccaro AR, Henderson FC, Benzel EC (2003) Molecular biology of cervical myelopathy and spinal cord injury: role of oligodendrocyte apoptosis. Spine J 3:510–519
Yu WR, Baptiste DC, Liu T, Odrobina E, Stanisz GJ, Fehlings MG (2009) Molecular mechanisms of spinal cord dysfunction and cell death in the spinal hyperostotic mouse: implications for the pathophysiology of human cervical spondylotic myelopathy. Neurobiol Dis 33:149–163
Thurnher MM, Law M (2009) Diffusion-weighted imaging, diffusion-tensor imaging, and fiber tractography of the spinal cord. Magn Reson Imaging Clin N Am 17:225–244
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kara, B., Celik, A., Karadereler, S. et al. The role of DTI in early detection of cervical spondylotic myelopathy: a preliminary study with 3-T MRI. Neuroradiology 53, 609–616 (2011). https://doi.org/10.1007/s00234-011-0844-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-011-0844-4