Abstract
Purpose
To compare volume-occupying rate of cervical spinal canal between patients with cervical spondylotic myelopathy (CSM) and normal subjects, and to investigate its significance in cervical spine disease.
Methods
Spiral computed tomography (CT) scan (C4–C6 cervical spine unit) was performed in 20 normal subjects and 36 cases of CSM at a neutral position, and data were transferred to the Advantage Workstation Version 4.2 for assessment. Bony canal area and fibrous canal area in each cross section, and sagittal diameters of cervical spinal canal and cervical spinal body were measured. Volume-occupying rate of cervical spinal canal was calculated using MATLAB. Cervical spinal canal ratio and effective cervical spinal canal ratio were calculated, and Japanese Orthopaedic Association score was used to assess cervical spinal cord function.
Results
Volume-occupying rate of cervical spinal canal at a neutral position was significantly higher in CSM patients as compared to normal subjects (P < 0.01). There was no correlation between cervical spinal canal ratio and JOA score in CSM patients, with a Pearson’s correlation coefficient of 0.171 (P > 0.05). However, sagittal diameter of secondary cervical spinal canal, effective cervical spinal canal ratio and volume-occupying rate of cervical spinal canal were significantly associated to JOA score, with Pearson’s coefficient correlations of 0.439 (P < 0.05), 0.491 (P < 0.05) and −0.613 (P < 0.01), respectively.
Conclusions
Volume-occupying rate of cervical spinal canal is an objective reflection of compression on cervical spine and spinal cord, and it is associated with cervical spinal cord function. These suggest that it may play a significant role in predicting the development of CSM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cervical canal stenosis is an anatomic cause and a risk factor for cervical spondylotic myelopathy (CSM) [1, 2]. Currently, most morphological measurements of cervical canal stenosis focused on radial line length of cervical spinal canal and cervical spinal body [3–8] or cross-sectional area of cervical spinal canal [9–14], and thus compression on the cervical spinal cord cannot be objectively and accurately examined. Some data regarding the measurement of spinal canal volume originated from cadaver specimens [15], which had reference value but could not be applied in clinical practices. The purpose of the present study was to investigate change of volume-occupying rate of cervical spinal canal and explore its clinical significance through measurement of cervical canal volume in normal subjects and patients with CSM at a neutral position using spiral computed tomography (CT) scan and MATLAB language.
Subjects and methods
Subjects
Twenty normal subjects were randomly sampled from healthy volunteers in whom physical examination, X-ray and magnetic resonance imaging (MRI) were performed. Those with cervical diseases such as cervical spine deformity, trauma and cervical spine disease were excluded. Normal subjects included 14 males and 6 females, with mean age of 39 years (24–55 years). Thirty-six patients with CSM were randomly sampled from those admitted to hospital during period from January 2008 to June 2010. All patients had physical examinations, X-ray and MRI scan, and patients with ossification of posterior longitudinal ligament, amyotrophic lateral sclerosis and spinal tumor were excluded. Of 36 patients, 26 were male and 10 were female, with mean age of 47 years (35–57 years). Most patients were in a chronic process, and showed a progressive exacerbation. The duration of the disease ranged from 5 months to 9 years, with a mean of 4.3 years. After the onset of the disease, all subjects experienced neck and shoulder discomfort, numbness of limbs, unsteady gait and zonesthesia (a feeling or sensation of constriction in the body) in chest or abdomen. Physical examination showed that compression was mainly on lower cervical spinal cord, and different degrees of incomplete paralysis were detected in limbs. In addition, there were obvious sensor and activity disorders in limbs, and hyper-reflexia was observed and the pathological tendon reflex was positive. Cervical spinal cord function of all patients with CSM was measured using Japanese Orthopaedic Association (JOA) scoring method [16]. JOA score of all CSM patients ranged from 7 to 15, with a mean score of 11.14.
The study was approved by the Ethics Committee of Anhui Medical University and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from volunteers and patients.
Measurement methods
All scanning was performed on a 64-Slice LightSpeed CT Scanner (GE Healthcare). The CT was acquired in a supine and neutral position (cervical spine was maintained at an angle of 12°) [17]. Lateral position of cervical spine was scanned and photographed. The scanning was performed between upper cervical spine C4 and lower cervical spine C6. The scanning parameters were: voltage of 120 kV, current 600 mA, field-of-view (FOV) 150 mm, slice thickness of 2.5 mm, pitch 1.375:1, slice interval 0.625 mm, scan width of 300 mm, and scan length of 35 mm.
All scanning images were conveyed to Advantage Workstation 4.2 (GE Healthcare), and then post-treatment imaging evaluations including volume rendering (VR), multiplanar reformation (MPR) and maximum intensity projection (MIP) were performed. Bony canal area, fibrous canal area of each section and sagittal diameter of spinal canal were measured in the workstation. To reduce subjective error of the measurement, measurements of cross-sectional area and sagittal diameter of spinal canal were performed by an orthopaedic surgeon and a radiographer, and the final results were the means of both measurements.
Cross-sectional area of spinal canal
Border of spinal canal was defined according to effective lacuna of spinal canal. For bony canal, the front was bordered on posterior margin of vertebral body, and the back was bordered on interior margin of vertebral laminae. For fibrous canal, the border was defined as the disco-ligamentous space: the front was bordered on posterior margin of intervertebral disk, and the back was bordered on interior margin of ligamentum flavum. Two sides were bordered on interior margin of pedicle of vertebral arch. If images of spinal canal appeared as opening shape on both sides, posterior margin of nerve root was considered as the border. The area measured was input into MATLAB 7.0.1, and canal volume of cervical vertebra was calculated using the trapezoidal rule integration method, and then volume-occupying rate of spinal canal was estimated using the following formula:
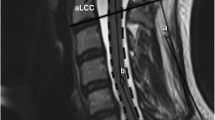
The measurement is shown in Fig. 1.
Radial line of cervical spinal canal and cervical spinal body
Sagittal diameter of secondary spinal canal in the most narrowing segment of spinal canal C4–C6 was measured in soft tissue window. Sagittal diameter of secondary spinal canal, also called sagittal diameter of effective spinal canal, was sagittal diameter of spinal canal excluding pathological volume-occupying lesions such as herniated intervertebral disk and osteophyte. Mid-sagittal diameters of vertebral body and sagittal diameters of developmental spinal canal in the same fragment of the spinal canal C4–C6 were measured in the bony window. For measuring the mid-sagittal diameters of the vertebral body, proliferative osteophytes were excluded. Cervical spinal canal ratio, namely Pavlov’s ratio, and effective cervical spinal canal ratio were calculated: Sagittal diameter of vertebra, a; sagittal diameter of developmental spinal canal, b; sagittal diameter of secondary spinal canal, c; cervical spinal canal ratio, b/a; and effective cervical spinal canal ratio, c/a. The measurement is shown in Fig. 2.
Statistical analysis
All data were described as mean ± standard deviation (SD), and all statistical analyses were performed using the statistical software Statistical Package for the Social Sciences V. 13.0 (SPSS 13.0, SPSS Inc., Chicago, IL, USA). Differences of volume-occupying rate of cervical spinal canal between normal subjects and CSM patients were tested for statistical significance using two-sample t tests. Pearson’s correlation analysis was used to investigate relationships between JOA score and cervical spinal canal ratio, sagittal diameter of secondary cervical spinal canal, effective cervical spinal canal ratio and volume-occupying rate of cervical spinal canal. P value of <0.05 was considered statistically significant.
Results
Volume-occupying rate of cervical spinal canal was significantly higher in CSM patients compared with normal subjects (P < 0.01) (Table 1). There was no correlation between cervical spinal canal ratio and JOA score, with a Pearson’s correlation coefficient of 0.171 (P > 0.05). However, sagittal diameter of secondary cervical spinal canal, effective cervical spinal canal ratio and volume-occupying rate of cervical spinal canal were significantly associated with JOA score, with Pearson’s correlation coefficients of 0.439 (P < 0.05), 0.491 (P < 0.05) and −0.613 (P < 0.01), respectively (Tables 2, 3).
Discussion
We measured volume-occupying rate of cervical spinal canal at a neutral position, and found that volume-occupying rate of cervical spinal canal was significantly higher in CSM patients as compared to normal subjects, and was associated with cervical spinal cord function. Volume-occupying rate of cervical spinal canal had a higher correlation with JOA score than sagittal diameter of secondary cervical spinal canal and effective cervical spinal canal ratio.
Anatomical features of spinal canal
The present study selected C4–C6 vertebrae to measure volume-occupying rate of spinal canal, because changes in the volume of cervical spinal canal mainly occurred at C3–C7 vertebrae [18]. Cervical vertebra bending and extension was one of the most basic activities. Bending and extension of cervical vertebra led to changes in anatomical structures in spinal canal, including sagittal diameter, cross-sectional area, as well as volume [15]. Both flexion and extension worsened spinal canal stenosis [19]. Therefore, in the present study, CT scanning of cervical vertebra was performed at a neutral position.
Measurement of cervical spinal canal
Measurement of cervical spinal canal using sagittal diameter of spinal canal, cervical spinal canal ratio (i.e., Torg ratio or Pavlov ratio) [3, 4], ratio of effective cervical spinal canal, the sagittal diameter of secondary spinal canal, the ratio of effective cervical spinal canal (calculated using sagittal diameter of secondary spinal canal), and axial cross-sectional area of cervical spinal cord [11] have been proposed; however, various ratio methods could not reflect the size of cervical spinal canal and could not comprehensively reflect the spinal canal stenosis and compression on cervical spinal cord, thus are limited for clinical use [5–7].
The most ideal assessment was to measure spinal canal volume directly. Many methods like perfusion method [15] and finite element model method [20, 21] have been used, which have some reference values but could not be applied in clinical diagnosis. Currently, there is no precise method for direct measurement of spinal canal volume. Previously, B-type ultrasound was used to measure spinal canal in the newborn and infants. Due to incomplete ossification of vertebra and vertebral arch, the entire and clear two-dimensional images of spinal canal and spinal cord were obtained using ultrasonic examination [22]. However, ossification of adult vertebra and vertebral arch, together with many gas-containing channels in pre-lateral side, imbricate shape in posterior spinous process, presence of Luschka joint at lateral side of the neighboring vertebra, led to lack of acoustic window, resulting in large measurement bias.
With rapid advances in imaging and computer technology, the development of spiral CT thin layer scanning and three-dimensional reconstruction technology remarkably enhanced the ability of medical imaging for differentiating microscopic anatomy [23]. Thin layer scanning data were used for 3-D reconstruction, and volume was extracted automatically according to variation in density in the workstation. As cervical spinal canal was an irregular semi-sealed cavity, accuracy and repeatability of this method were both low, and this method could not identify fibrous spinal canal. Based on CT scan, the present study used the MATLAB language (MathWorks Company) for numerical integration. Basic principle was to divide integral space (cervical spinal canal) [a, b] into several sub-spaces (of 0.625 mm thick in each section). x i , x i+1, i = 1, 2,…, N, where x 1 = a, x N+1 = b. The division of the integral space is shown in the following formula:
Spinal canal volume in each sub-space was calculated approximately. MATLAB language proved that accuracy of integration gradually increased with reduction in interval h [24]. Therefore, in the present study, we selected 64-slice spiral LightSpeed CT scan with rapid scanning and a slice interval of 0.625 mm (MRI with slice thickness of more than 2 mm was insensitive to bony structures and the imaging took long period, therefore, MRI scan was not used in the present study), which insured the accuracy of measurement of spinal canal volume. The present study showed that CT scan was more accurate and reproducible than the perfusion method, and it could be used for measurement of in vivo vertebra. The present study was the first to solve the problem for the measurement of spinal canal volume, which was of great clinical significance.
Volume-occupying rate of cervical spinal canal
Currently, it is assumed that bony spinal canal stenosis is the basis of cervical canal stenosis, but fibrous spinal canal stenosis can directly cause most cervical canal stenosis [25]. Fibrous spinal canal composed of intervertebral disk and ligamenta flava, which were at anterior and posterior positions of the dural sac, respectively. The fibrous spinal canal measured in the present study was the functional canal [11].
Based on volume measurement, we proposed volume-occupying rate of spinal canal. The volume-occupying rate of spinal canal reflected the sizes of storage cavity and compensation gap of spinal canal. The storage cavity emphasized on the concept of space and as an assessment of volume, it reflected compression on spinal cord more accurately. The increased ratio resulted from two changes: one was a decrease in volume of bony spinal canal, and the other was an increase in volume of fibrous spinal canal. The increase in volume-occupying rate of spinal canal implied an increase in soft tissues (intervertebral disk and ligamenta flava) occupation and a reduction in storage space of spinal canal, which led to frequent compression on spinal cord. The compression from the intervertebral space would result in occurrence of CSM; therefore, storage space of spinal canal is more important than absolute volumes of bony spinal canal and fibrous spinal canal in the pathogenesis of CSM.
Significance of volume-occupying rate of spinal canal
The present study showed that volume-occupying rate of spinal canal in a neutral position was higher in patients with CSM as compared to that in normal subjects. Spinal cord in patients with CSM had smaller space than spinal canal, which was the important anatomical reason for the onset of CSM. In addition, the present study revealed no relationship between spinal canal ratio and JOA score, which supported the views proposed by Blackley et al. [5] and Prasad et al. [11]. Sagittal diameter of secondary spinal canal correlated with JOA score, but it did not reflect compression on spinal cord accurately. Effective spinal canal ratio eliminated the impact of individual difference in anteroposterior diameter of cervical spinal cord to a certain degree, and it had a significant correlation with JOA score. Volume-occupying rate of spinal canal reflected actual occupying space of cervical spinal cord in cervical spinal canal, and more precisely reflected compression on cervical spinal cord; therefore, it had a stronger correlation with JOA score. These results suggest measurement of volume-occupying rate of spinal canal would help analyze the effects of spinal canal stenosis on cervical spinal cord, and may have clinical utility for diagnosis of CSM and selection of therapeutic strategy.
The compression that caused CSM was mainly derived from anterior part of the cervical spinal canal and spinal cord. In critical patients, due to compression from both anterior and posterior parts of spinal cord, a “clamping” state was observed [26]. The main purpose of surgery was to remove bony and fibrous compression on spinal cord. However, the complexity of compression caused difficulties in selection of the surgery [27]. Anterior–posterior decompression was the most ideal surgery. Schultz et al. [28] proposed single-stage anterior–posterior decompression for the treatment of CSM with anterior and posterior compression. Such a surgery was supposed to enhance the surgical efficacy and provide an ideal environment for recovery of nerve functions. Currently, the success of anterior–posterior decompression depends on imaging manifestations and operators’ clinical experience. In patients with high pre-surgical volume-occupying rate of spinal canal, because of decreased storage space for spinal canal and reduced buffering space, there was increased risk of spinal cord injury during anterior compression. In such cases, posterior laminoplasty (including open-door type or double-door type) for enlarging accommodating space of spinal cord [29] followed by stage I or II anterior decompression, was considered a better choice for treatment of CSM. Judicious choice of anterior or posterior approach should be made after individualizing each case [27]. Thus, estimation of volume-occupying rate of spinal canal prior to operation, assessment of compression on cervical spinal cord, were of great significance to formulate an optimized individual operation scheme and improve efficacy of surgical treatment for cervical spine diseases.
In conclusion, volume-occupying rate of cervical spinal canal is an objective reflection of compression on cervical spine and spinal cord, and it is associated with cervical spinal cord function. These suggest that it may play a significant role in predicting the development of CSM.
References
Bernhardt M, Hynes RA, Blume HW, White AA 3rd (1993) Cervical spondylotic myelopathy. J Bone Joint Surg Am 75(1):119–128
Morishita Y, Naito M, Hymanson H, Miyazaki M, Wu G, Wang JC (2009) The relationship between the cervical spinal canal diameter and the pathological changes in the cervical spine. Eur Spine J 18(6):877–883
Torg JS, Pavlov H, Genuario SE, Sennett B, Wisneski RJ, Robie BH, Jahre C (1986) Neurapraxia of the cervical spinal cord with transient quadriplegia. J Bone Joint Surg Am 68(9):1354–1370
Pavlov H, Torg JS, Robie B, Jahre C (1987) Cervical spinal stenosis: determination with vertebral body ratio method. Radiology 164(3):771–775
Blackley HR, Plank LD, Robertson PA (1999) Determining the sagittal dimensions of the canal of the cervical spine. The reliability of ratios of anatomical measurements. J Bone Joint Surg Br 81(1):110–112
Lim JK, Wong HK (2004) Variation of the cervical spinal Torg ratio with gender and ethnicity. Spine J 4(4):396–401
Keny SM, Suh SW, Song HR, Vaidya SV, Machavarapu MM (2006) Morphometric determinants of the sagittal dimensions of the cervical spinal canal in achondroplasia: an analysis of the reliability of the Torg ratio. J Spinal Disord Tech 19(7):523–527
Suk KS, Kim KT, Lee JH, Lee SH, Kim JS, Kim JY (2009) Reevaluation of the Pavlov ratio in patients with cervical myelopathy. Clin Orthop Surg 1(1):6–10
Okada Y, Ikata T, Katoh S, Yamada H (1994) Morphologic analysis of the cervical spinal cord, dural tube, and spinal canal by magnetic resonance imaging in normal adults and patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976) 19(20):2331–2335
Golash A, Birchall D, Laitt RD, Jackson A (2001) Significance of CSF area measurements in cervical spondylitic myelopathy. Br J Neurosurg 15(1):17–21
Prasad SS, O’Malley M, Caplan M, Shackleford IM, Pydisetty RK (2003) MRI measurements of the cervical spine and their correlation to Pavlov’s ratio. Spine (Phila Pa 1976) 28(12):1263–1268
Tench CR, Morgan PS, Constantinescu CS (2005) Measurement of cervical spinal cord cross-sectional area by MRI using edge detection and partial volume correction. J Magn Reson Imaging 21(3):197–203
Kadanka Z, Kerkovsky M, Bednarik J, Jarkovsky J (2007) Cross-sectional transverse area and hyperintensities on magnetic resonance imaging in relation to the clinical picture in cervical spondylotic myelopathy. Spine (Phila Pa 1976) 32(23):2573–2577
Naganawa T, Miyamoto K, Ogura H, Suzuki N, Shimizu K (2011) Comparison of magnetic resonance imaging and computed tomogram-myelography for evaluation of cross sections of cervical spinal morphology. Spine (Phila Pa 1976) 36(1):50–56
Holmes A, Han ZH, Dang GT, Chen ZQ, Wang ZG, Fang J (1996) Changes in cervical canal spinal volume during in vitro flexion–extension. Spine (Phila Pa 1976) 21(11):1313–1319
Wada E, Suzuki S, Kanazawa A, Matsuoka T, Miyamoto S, Yonenobu K (2001) Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: a long-term follow-up study over 10 years. Spine (Phila Pa 1976) 26:1443–1447 discussion 48
Schriger DL, Larmon B, LeGassick T, Blinman T (1991) Spinal immobilization on a flat backboard: does it result in neutral position of the cervical spine? Ann Emerg Med 20(8):878–881
Yanase M, Matsuyama Y, Hirose K, Takagi H, Yamada M, Iwata H, Ishiguro N (2006) Measurement of the cervical spinal cord volume on MRI. J Spinal Disord Tech 19(2):125–129
Chen CJ, Hsu HL, Niu CC, Chen TY, Chen MC, Tseng YC, Wong YC, Wang LJ (2003) Cervical degenerative disease at flexion-extension MR imaging: prediction criteria. Radiology 227(1):136–142
Wheeldon J, Khouphongsy P, Kumaresan S, Yoganandan N, Pintar FA (2000) Finite element model of human cervical spinal column. Biomed Sci Instrum 36:337–342
Panzer MB, Cronin DS (2009) C4–C5 segment finite element model development, validation, and load-sharing investigation. J Biomech 42(4):480–490
Raghavendra BN, Epstein FJ (1985) Sonography of the spine and spinal cord. Radiol Clin North Am 23(1):91–105
Kaiser JA, Holland BA (1998) Imaging of the cervical spine. Spine (Phila Pa 1976) 23(24):2701–2712
Moor H (2008) MATLAB for Engineers, 2/E. Prentice Hall, Englewood Cliffs
Stafira JS, Sonnad JR, Yuh WT, Huard DR, Acker RE, Nguyen DL, Maley JE, Ramji FG, Li WB, Loftus CM (2003) Qualitative assessment of cervical spinal stenosis: observer variability on CT and MR images. AJNR Am J Neuroradiol 24(4):766–769
Nuckley DJ, Konodi MA, Raynak GC, Ching RP, Mirza SK (2002) Neural space integrity of the lower cervical spine: effect of normal range of motion. Spine (Phila Pa 1976) 27(6):587–595
Bapat Mihir R, Chaudhary Kshitij, Sharma Amit, Laheri Vinod (2008) Surgical approach to cervical spondylotic myelopathy on the basis of radiological patterns of compression: prospective analysis of 129 cases. Eur Spine J 17(12):1651–1663
Schultz KD Jr, McLaughlin MR, Haid RW Jr, Comey CH, Rodts GE Jr, Alexander J (2000) Single-stage anterior–posterior decompression and stabilization for complex cervical spine disorders. J Neurosurg 93(2 Suppl):214–221
Hirabayashi Shigeru, Yamada Hironobu, Motosuneya Takao, Watanabe Yoshinobu, Miura Makoto, Sakai Hiroya, Matsushita Takashi (2010) Comparison of enlargement of the spinal canal after cervical laminoplasty: open-door type and double-door type. Eur Spine J 19(10):1690–1694
Conflict of interest
There is no actual or potential conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dong, F., Shen, C., Jiang, S. et al. Measurement of volume-occupying rate of cervical spinal canal and its role in cervical spondylotic myelopathy. Eur Spine J 22, 1152–1157 (2013). https://doi.org/10.1007/s00586-012-2622-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-012-2622-3