Abstract
Objectives
The aim of this study was to investigate spinal cord structure in patients with cervical spondylosis where conventional MRI fails to reveal spinal cord damage.
Methods
We performed a cross-sectional study of patients with cervical spondylosis without conventional MRI findings of spinal cord damage and healthy controls. Subjects were studied using spinal diffusion tensor imaging (DTI), precision grip and foot force-tracking tasks, and a clinical examination including assessment of neurological signs. A regional analysis of lateral and medial spinal white matter across multiple cervical levels (C1–C5) was performed.
Results
DTI revealed reduced fractional anisotropy (FA) and increased radial diffusivity (RD) in the lateral spinal cord at the level of greatest compression (lowest Pavlov ratio) in patients (p < 0.05). Patients with spondylosis had greater error and longer release duration in both grip and foot force-tracking. Similar spinal cord deficits were present in patients without neurological signs. Increased error in grip and foot tracking (low accuracy) correlated with increased RD in the lateral spinal cord at the level of greatest compression (p ≤ 0.01).
Conclusions
Spinal DTI can detect subtle spinal cord damage of functional relevance in cervical spondylosis, even in patients without signs on conventional T2-imaging and without neurological signs.
Key Points
• DTI reveals spinal cord changes in cervical spondylosis with few symptoms
• DTI changes were present despite normal spinal cord on conventional MRI
• DTI parameters correlated with force control accuracy in hand and foot
• Spinal DTI is a promising technique for patients with cervical spondylosis
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In cervical spondylotic myelopathy the spinal cord is damaged by chronic compression and altered vascular supply resulting in associated neurological symptoms and functional deficits in gait, manual dexterity and sphincter control [1, 2]. Early diagnosis and follow-up remain clinically challenging because of the poor sensitivity of neurological signs [3, 4]. Clinical signs are insufficient to confirm compression-induced spinal cord damage and MRI is routinely performed. However, clinical symptoms in cervical spondylosis are not systematically associated with spinal anatomical MRI signs [5, 6]. Diffusion tensor imaging (DTI) is a promising imaging method for quantifying spinal cord structure and some recent studies suggest that DTI could provide useful biomarkers in various spinal pathologies [7–9]. In cervical spondylosis with neurological symptoms, spinal DTI is more sensitive than conventional imaging [6, 10–13] and spinal cord damage detected with DTI can relate to severity of symptoms [14–16]. However, studies relating DTI parameters to quantitative measures of sensorimotor function in cervical spondylosis are lacking, in particular in patients with mild disability where clinical signs have poor sensitivity [4]. Studies in patients without signs of damage on conventional MRI at any cervical level are also lacking. Thus, this study investigated the structure–function relationship in cervical spondylosis patients without conventional MRI evidence of spinal cord damage, i.e. increased signal intensity on T2-weighted imaging of spinal cord. A visuomotor task was used to quantify voluntary force modulation in grip and foot force-tracking. We hypothesized that DTI would detect spinal damage in cervical spondylosis patients without signs on conventional T2-weighted imaging. We also predicted that the degree of structural (DTI assessed) spinal cord damage would correlate with reduced force control in both grip and foot tracking.
Materials and methods
Subjects
Sixteen patients (11 women, 5 men) with clinical symptoms of cervical spondylosis (neck pain with/without neurological symptoms, and absence of comorbid disorders) without MRI evidence of spinal cord damage on T2-weighted imaging were recruited from a rehabilitation and spinal disease clinic (Service de Médecine Physique et de Réadaptation, Hôpital Cochin, Paris). Twenty age-matched healthy volunteers (12 women, 8 men) without history of arthritis, neck pain or any radiating symptoms served as control group. Patients with spondylosis and controls had a mean ± SD age of 53 ± 9 and 53 ± 6 years, respectively (p = 0.88). The patients’ clinical status data are detailed in Table 1. The study received institutional review board approval and all subjects provided informed consent.
Clinical measures
All subjects were questioned regarding traumatic history, pain characteristics and sphincter control. They underwent a standardized clinical cervical spine exam including cervical spine mobility (flexion, extension, side bending and rotation), palpation of trigger points and Spurling’s manoeuvre. Pain intensity was rated on a visual analogue scale. Neurological assessment included muscle strength, surface and deep sensitivity, tendon reflexes, pyramidal reflexes, proprioception and gait patterns. We used the functional independence measure (FIM) and the European myelopathy score (EMS) to evaluate disability [17]. Three trials of maximal voluntary grip force were performed using the JAMAR® hand dynamometer and the maximum retained. A clinical measure of manual dexterity was obtained using the Moberg pick-up test (Table 1) [18]. Patients without neurological symptoms were classified as subclinical patients.
Visuomotor precision grip and toe force-tracking
Accurate control of precision grip force is necessary for dexterous manipulation. Control of toe force is important for balance and gait. Precision grip and toe control was measured using a force-tracking task [19, 20]. Subjects performed a visuomotor ramp-hold-and-release task (Fig. 1, example traces at 3 N). The task comprised six blocks each consisting of four ramp-hold-and-release target force trajectories from 0 N to 3, 6 or 9 N. Before each ramp, the target remained for 3 s at 0 N. The target force then ramped up linearly for 2 s to reach the steady-state force level, which lasted 4 s, after which the target force dropped instantaneously to 0 N (release). Between blocks, there was a 5-s pause with a black screen. Each subject performed a pseudo-randomized order of two blocks at each force level (i.e. eight trials per force level). Subjects were instructed to minimize the distance (error) between the applied (cursor) and the target force. All subjects were familiarized with the task before testing. Collection of all 24 trials took 12 min. The following force control measures were extracted for each trial, according to previous definitions:
Visuomotor force-tracking in a healthy control subject (a) and a patient with cervical spondylosis (b; same subjects as in Fig. 2). Precision grip (left) and toe (right) force-tracking examples with force recording (black line) and target force trajectory (dotted grey line). Note less accuracy relative to the target force in the patient in both grip and foot force-tracking tasks. c Mean error for the patients and controls (Newton seconds, N s; mean ± 95 % confidence intervals) in grip and foot force-tracking tasks separately for the right and left sides. d Mean release duration for the patients and controls (ms; mean ± 95 % confidence intervals) in grip and foot force-tracking tasks. Note the greater error (force modulation) and the longer release duration in the patient group. Open circles controls, filled triangles patients. *p < 0.05 group difference on each side in post hoc comparison
(i) Relative error (Ns): the total error (the absolute sum of positive error above and negative error below target) between the applied force and the target force trajectory, normalized to the target force level. (ii) Release duration (ms): the time taken to abruptly reduce the grip force (from 75 % to 25 % of the target force) at the end of the hold period. (iii) Timing of force onsets (beginning of ramp) and offsets (end of hold) [21].
DTI
Participants underwent a single imaging session in a 1.5-T Siemens Avanto scanner (Siemens, Erlangen, Germany) with collection of DTI-weighted images of the cervical spinal cord (C1–C5). The sensitivity encoding (SENSE) single-shot echo-planar imaging (EPI) sequence with SENSE factor 2 was used to reduce distortions. In the sagittal diffusion-weighted sequence 25 non-collinear gradient directions were applied with two b values (b = 0 and 900 s/mm2; TR/TE 2000/95 ms; field of view 18 cm, image matrix 128 × 128; 12 slices with slice thickness of 3 mm; slice gap = 0; voxel size 1.4 × 1.4 × 3 mm). Spatial presaturation bands were applied anterior and posterior to the vertebral column to reduce ghosting from fat outside the spinal column. The sequence was repeated four times, lasting 4 min 26 s in total. Diffusion tensor images were averaged across the four acquisitions before analysis to increase signal-to-noise ratio. Imaging parameters were similar to those used in previous spinal DTI studies [19, 22]. Sagittal and axial fast spin echo T2-weighted images were also obtained from C1 to C5 and used to evaluate T2 signal integrity and degree of spinal stenosis (by an experienced radiologist, AF).
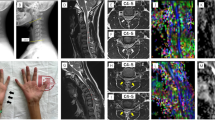
All diffusion images were examined for movement, distortion or eddy current artefacts before analysis. Visible artefacts were present in one patient and one control, subsequently excluded from DTI analysis. Fractional anisotropy (FA), apparent diffusion coefficient (ADC), axial diffusivity (AD) and radial diffusivity (RD) values were calculated voxel-by-voxel using MedINRIA (1.9.0) software (http://www-sop.inria.fr/asclepios/software/MedINRIA). AD describes the principal eigenvector (λ1) and RD the mean of the remaining two eigenvectors perpendicular to λ1 (λ2 + λ3/2) [23]. Regions of interest (ROIs) covering the entire spinal white and grey matter were drawn on b0 images (axial views). ROIs included (1) the lateral part and (2) the medial part of the spinal cord on both sides (Fig. 2c). The lateral ROI was defined so as to include the lateral corticospinal tract (CST) and the medial ROI was defined as the remaining part of the spinal cord (e.g. Fig. 2c–e) [19].
Diffusion tensor imaging and spinal stenosis in cervical spondylosis patients without conventional MRI findings of spinal cord damage. a, b Left: Sagittal T2-weighted images showing cervical spinal cord anatomy and fibre tracking in a control subject (top) and a cervical spondylotic myelopathy (CSM) patient (bottom; patient 7 in Table 1). Note the presence of spondylotic stenosis at C4–C5 and C5–C6 (arrow) levels in the patient. Right: fibre tracking shows ascending and descending spinal cord fibres (green craniocaudal FA direction) in both subjects. c Axial b0 image illustrating positioning of medial (M) and lateral (L) ROIs. d, e Axial view at C5 level of FA map with lateral and medial spinal cord ROIs in a healthy control (top) and a patient (bottom; same subjects as in a, b). f Pavlov’s ratio (mean ± 95 % confidence intervals) was lower in patients than controls at all levels (C2–C5) indicating reduced spinal canal space in CSM patients. *Significant post hoc comparisons. Open circles controls, black triangles cervical spondylosis patients
To limit the partial volume effect (i.e. inclusion of voxels containing cerebrospinal fluid, CSF) we verified that ROI placement on the b0 image did not contain voxels extending into CSF (voxels covering both were removed). Only voxels with diffusion predominantly in the craniocaudal direction on FA colour maps were included. Two raters (PL and FO) separately performed ROI measurements, and reliability of DTI parameters within these ROIs were evaluated using intraclass correlation coefficients (ICC). ICC > 0.7 was considered to reflect good reliability. Deterministic tractography using DTItrack (MedINRIA software, with FA > 0.2) with seed regions covering whole spinal cord at C2 and C5 levels was used to ensure anatomical correspondence between tracked fibres and T2-weighted images.
Pavlov’s ratio
The degree of spinal stenosis was measured on sagittal T2-weighted images according to Pavlov’s ratio, i.e. the ratio of the diameter of the spinal canal to the diameter of the vertebral body [24]. The lowest Pavlov ratio for each subject was used to define the cervical level with greatest spinal compression (greatest stenosis).
Statistics
Group differences in clinical variables were evaluated using the Chi-square and Mann–Whitney U tests. Group differences in DTI parameters (FA, ADC, AD and RD) were evaluated using general linear model ANOVAs with two within-group factors: LEVEL (C1–C5) and ROI (medial/lateral), and one between-group factor GROUP. DTI parameters across sides (left/right) correlated (r > 0.79) and values were pooled for ANOVA. A second separate ANOVA of DTI parameters was confined to the level of greatest stenosis (according to Pavlov’s ratio) and performed with one within-group (ROI) and one between-group (GROUP) factor. Group differences in Pavlov’s ratio were also analysed using ANOVA (with factors LEVEL and GROUP). Force data were analysed using Matlab v7 (The MathWorks, Inc., Natick, MA, USA). Differences in force-tracking parameters (error, release duration, onsets and offsets) were analysed separately using ANOVA with two within-subject factors (HAND/FOOT, and SIDE: right, left) and a between-subject factor (GROUP). Post hoc tests were performed using Fisher LSD test. The level of significance was set to p ≤ 0.05 for ANOVAs and post hoc tests. Relations between force-tracking and DTI parameters were investigated using Pearson’s correlation tests. Force-tracking performance was similar on both sides in controls and patients and data were pooled across hands for correlation analyses. Correlation tests were limited to variables showing significant group differences and corrected for multiple comparisons (Bonferroni, p < 0.0125). Statistical analysis was performed using Statistica 10 (StatSoft, Inc., Tulsa, OK, USA).
Results
Clinical characteristics and anatomical MRI
All 16 patients reported cervical neck pain and most had a combination of radicular and neuropathic pain (Table 1). Seven patients (44 %) complained of night awakening secondary to pain and 11 patients (69 %) reported previous cervical trauma. Patients had significantly reduced neck extension by about 2 cm. In terms of neurological signs, sensory deficits, sphincter and gait trouble were significantly more frequent in the patient group (Table 1). This was not the case for motor deficits and reflexes. However, five (31 %) patients had no neurological signs (subclinical patients) and three (19 %) patients had only one sign. Eight patients (50 %) had small reduction in independence according to FIM (patients: 124 ± 4, controls: 126 ± 0; P = 0.01) and ten patients (63 %) were classified as ‘normal functioning’ according to EMS, confirming mild disability. Patients did not differ in age, gender, functional dexterity (pick-up task) or maximal grip force (Table 1).
Anatomical MRI showed signs of cervical spinal canal stenosis in 13 (81 %) patients and in four (20 %) controls. None of the patients had signs of spinal cord compression. Patients also showed more signs of herniated discs than age-matched controls (Table 2). In patients, stenosis was most frequent at C5–C6 (81 %) followed by C4–C5 (44 %, example Fig. 2b). Quantification of stenosis according to Pavlov’s ratio showed the same pattern with the majority of patients showing lowest ratios (least canal space) at C5 (69 %). Three patients (19 %) had lowest ratio at C4 and two patients (12 %) at C3. ANOVA comparison of groups showed lower Pavlov ratios in patients than controls (GROUP: F = 13.4, p < 0.001; Fig. 2f). Post hoc tests showed diminished spinal canal space at all cervical levels in patients (p < 0.05).
Diffusion parameters
All subjects showed good quality FA, FAdir maps and fibre tracking from C1 to C5 (examples Fig. 2). Inter-rater reliability was good for FA, ADC, AD and RD (ICC 0.93, 0.94, 0.98 and 0.75, respectively), without systematic bias between raters (paired t test, p > 0.1). Whole spinal cord ROIs included a similar number of voxels in patients (40 ± 8) and controls (41 ± 8, P = 0.64), as did lateral spinal cord ROIs (8 ± 1 voxels in patients and 9 ± 2 voxels in controls, P = 0.84).
ANOVA across all spinal levels (C1–C5) showed an effect of ROI and LEVEL on all parameters, consistent with previous studies [19, 25, 26]. FA was greater in the lateral than in the medial spinal cord (ROI: F = 62.9, p < 0.001) and higher at C1–C3 compared to C4–C5 (LEVEL: F = 13.4, p < 0.001). ADC was reduced in the lateral ROI (ROI: F = 68.9, p < 0.001) and lower at higher cervical levels (LEVEL: F = 9.3, p < 0.001). Patients and controls both showed this pattern and groups were similar in all parameters when analysed across all spinal levels. However, when confining the ANOVA to the level of greatest stenosis significant group differences were found. FA was reduced in patients (GROUP: F = 4.1, p = 0.05) and differed between lateral and medial ROIs (GROUP*ROI, F = 9.9, p = 0.004). Post hoc comparison showed a significant reduction of about 9 % in the lateral ROI only (p = 0.003, Fig. 3a). RD showed an inverse pattern tending to be increased in patients and regionally different (GROUP: F = 3.9, p = 0.06; GROUP*ROI: F = 3.7, p = 0.06). Post hoc tests showed a 16 % increase in RD confined again to the lateral ROI (p = 0.02, Fig. 3d). ADC and AD showed no GROUP differences (Fig. 3b, c). The five subclinical patients showed the exact same group differences (p < 0.05).
Regional spinal DTI parameters of lateral and medial spinal cord across C1–C5 levels and at the level of greatest stenosis. a FA (mean ± 95 % confidence intervals) was lower in patients in the lateral spinal cord, only at the level of greatest stenosis (p = 0.003). No significant difference in FA was found in the medial spinal cord. b Patients had similar spinal ADC values as controls in both whole spinal cord and regionally at the level of greatest stenosis. c Axial diffusivity (AD) was also comparable across groups. d RD was higher in the lateral spinal cord in patients at the level of greatest stenosis only (p = 0.02). Open circles controls, black triangles cervical spondylosis patients, *significant group differences
Force control
Visuomotor precision grip and toe force-tracking was more variable in patients (Fig. 1a, b). The ANOVA showed a significant effect of GROUP (F = 5.5, P = 0.03) with patients producing 38 % more error in grip and 27 % more error in foot tracking (Fig. 1c). Post hoc tests showed significantly higher error in both grip and foot tracking in the patients (mean across right and left sides, p < 0.05). The average error was greater in foot compared to grip tracking (HAND/FOOT: F = 44.8, p < 0.001). In patients, error in grip tracking correlated with error in foot tracking (R = 0.82, p < 0.001).
Patients also took significantly longer to release force (57 % longer in grip and 42 % longer in foot tracking) compared to controls (GROUP: F = 16.4, p < 0.001; Fig. 1d). Post hoc tests showed longer release duration across both hands and feet in patients (p < 0.05). The effect of HAND/FOOT was not significant (F = 0.21, p = 0.66), although mean grip and foot release duration was 30 and 31 ms longer than in controls, respectively. In patients, grip release duration did not correlate with foot release duration (R = 0.25, p = 0.35). Timing of force onsets and offsets was similar in patients and controls (p > 0.5).
Subclinical patients also showed increased error (p = 0.05) and prolonged release duration (p < 0.001) compared to controls.
Relation between force control and DTI parameters
In the patients, RD in the lateral spinal cord at the level of greatest stenosis correlated positively with grip and foot tracking error (Fig. 4). FA (in this ROI) showed a tendency for negative correlation (R = −0.49, p = 0.06 and R = −0.56, p = 0.03, respectively). Thus, patients with more accurate foot tracking (less error) had higher FA and lower RD values than less accurate patients. No relations were found between release duration and diffusion parameters.
Relation between spinal cord structure and error during force-tracking in cervical spondylosis patients. RD in the lateral spinal cord at level of greatest stenosis correlated with error in precision grip (top) and foot tracking (bottom; error log transformed). Higher error was present in patients with higher RD values
Discussion
Our findings extend previous work on the sensitivity of spinal DTI by providing quantitative evidence of impaired spinal cord structure–function in cervical spondylosis patients without conventional MRI evidence of spinal cord damage with few or lacking neurological signs. The degree of (DTI-assessed) spinal damage correlated with the accuracy in voluntary grip and foot force control: patients with greatest cervical damage were least accurate.
Although the patient group showed more signs of spinal stenosis and cervical arthritis than the age-matched control group (Table 2), no signs of spinal cord damage were present on conventional T2-weighted imaging at any cervical level. Despite normal-appearing T2-weighted imaging, DTI showed reduced FA in the lateral spinal cord at the cervical level of greatest compression (stenosis) in the spondylosis patients. This was also the case in subclinical patients without neurological signs. Spinal damage confined to the area of greatest compression agrees with previous animal studies [27] and with recent DTI studies in cervical spondylotic myelopathy [6, 14, 15]. In this study, the reduced FA in the lateral spinal cord was primarily due to increased RD. RD reflects diffusion perpendicular to the axon and informs on myelin content, whereas AD, which was not affected, is more related to axonal changes [23]. The reduced RD suggests detectable changes in myelin content in the lateral spinal cord confined to the level of maximal stenosis.
The CST is the major descending tract in the lateral spinal cord and our findings suggest that damage was most pronounced in the lateral white matter [28]. We thus hypothesize that the lateral CST is affected in early cervical spondylotic myelopathy. This is coherent with human postmortem findings in showing white matter changes occurring mainly in the lateral CST [29]. It is also consistent with abnormal motor, but normal sensory-evoked potentials in cervical spondylosis with neurological symptoms [30]. Similarly, a recent animal model of cervical myelopathy showed early occurring corticospinal degeneration and compromised spinal cord microvasculature [27]. This pattern of initial damage to CST axons may be due to an increased vulnerability of large myelinated fibres as suggested by animal and human studies [31, 32].
Accuracy in grip and foot force control correlated with degree of spinal cord damage, i.e. with RD in the lateral spinal cord as assessed by DTI (Fig. 4). This confirmed our hypothesis of a cervical spinal structure–function relationship in patients with cervical spondylosis. This hypothesis was grounded on the assumption that the CST is essential for adequate force control in both the upper and lower limbs. Functionally, the CST provides major excitatory input to hand and foot motorneuron pools and force control deficits in chronic spinal cord injury are likely due to impaired motor unit modulation (accuracy), including impaired ability to stop motor unit activity (release duration) [33, 34]. In this study, the patients were less accurate in force control (greater error) and showed prolonged release duration in both feet and hands. This extends previous findings showing that force control impairments may be present even in cervical spondylosis patients without neurological signs [35, 36]. Anatomically, the CST represents the predominant tract in the lateral spinal cord and we have previously shown a correlation between FA in the lateral cervical spinal cord and accuracy of force control in the precision grip in a larger sample of healthy subjects (same task as in this study) [19]. The present findings show the presence of such a spinal cord structure–function relationship in spondylosis patients. This is in line with recent findings showing a correlation of severity of clinical symptoms with reduced FA at the level of greatest spinal canal stenosis [15] and extends these findings by showing that the spinal DTI changes relate to specific deficits in force control, a key function of the corticospinal tract. In addition we show that this correlation is present in patients without conventional MRI signs of spinal cord damage, which has not been specifically tested before.
In a more general perspective, our findings add to growing evidence that spinal DTI can be used to probe the structural integrity of spinal white matter and that altered integrity (assessed by DTI parameters) may have functional consequences. For example, sensory function has been found to correlate with DTI integrity of posterior tracts, and fine finger movements with CST integrity [6, 37]. Furthermore, DTI measures also correlate with symptom severity and functional deficits in other disorders affecting the spinal cord such as amyotrophic lateral sclerosis [7], syringomyelia [38], multiple sclerosis [8] and spinal cord injury [25, 37, 39].
These results should be considered within the limitations of the current study. Limitations concern the sample size and DTI methods: although a relatively small sample of patients was included (all, however, without signs of spinal cord damage on T2-weighted imaging), power was sufficient to detect group DTI and behavioural differences. Relatively poor spatial DTI resolution is not uncommon: we employed a previously used sequence [19, 22] with similar resolution to previous studies [40, 41]. Spinal imaging remains difficult because of the small size and physiological motion of the spinal cord and optimizing the imaging methods remains a major challenge [9, 16, 42]. ROIs were defined manually; nonetheless, results showed good reliability across two separate raters [19, 22]. Furthermore, our findings agree with previous studies in other pathologies showing a correlation between spinal CST integrity and motor function [7, 8, 37].
In conclusion, this study shows that DTI is sensitive for detection of spinal cord damage in cervical spondylosis without conventional MRI evidence of spinal cord damage. The correlation of quantitative force control measures with the regional changes in spinal DTI parameters suggests that these structural changes are functionally relevant and can impact sensorimotor control in both the hand and foot. Spinal DTI may be more sensitive for the detection of spinal damage than the use of neurological signs and could be used early in the course of cervical spondylosis to improve diagnosis and medical management.
Abbreviations
- AD:
-
Axial diffusivity
- ADC:
-
Apparent diffusion coefficient
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- N:
-
Newton
- RD:
-
Radial diffusivity
- ROI:
-
Region of interest
- SD:
-
Standard deviation
References
Brain WR, Northfield D, Wilkinson M (1952) The neurological manifestations of cervical spondylosis. Brain 75:187–225
Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG (2015) Degenerative cervical myelopathy: epidemiology, genetics and pathogenesis. Spine (Phila Pa 1976). doi:10.1097/BRS.0000000000000913
Holly LT (2009) Management of cervical spondylotic myelopathy with insights from metabolic imaging of the spinal cord and brain. Curr Opin Neurol 22:575–581
Chikuda H, Seichi A, Takeshita K et al (2010) Correlation between pyramidal signs and the severity of cervical myelopathy. Eur Spine J 19:1684–1689
Morio Y, Teshima R, Nagashima H, Nawata K, Yamasaki D, Nanjo Y (2001) Correlation between operative outcomes of cervical compression myelopathy and MRI of the spinal cord. Spine (Phila Pa 1976) 26:1238–1245
Kerkovský M, Bednarík J, Dušek L et al (2012) Magnetic resonance diffusion tensor imaging in patients with cervical spondylotic spinal cord compression: correlations between clinical and electrophysiological findings. Spine (Phila Pa 1976) 37:48–56
Nair G, Carew JD, Usher S, Lu D, Hu XP, Benatar M (2010) Diffusion tensor imaging reveals regional differences in the cervical spinal cord in amyotrophic lateral sclerosis. Neuroimage 53:576–583
Naismith RT, Xu J, Klawiter EC et al (2013) Spinal cord tract diffusion tensor imaging reveals disability substrate in demyelinating disease. Neurology 80:2201–2209
Stroman PW, Wheeler-Kingshott C, Bacon M et al (2014) The current state-of-the-art of spinal cord imaging: methods. Neuroimage 84:1070–1081
Facon D, Ozanne A, Fillard P, Lepeintre JF, Tournoux-Facon C, Ducreux D (2005) MR diffusion tensor imaging and fiber tracking in spinal cord compression. AJNR Am J Neuroradiol 26:1587–1594
Mamata H, Jolesz FA, Maier SE (2005) Apparent diffusion coefficient and fractional anisotropy in spinal cord: age and cervical spondylosis-related changes. J Magn Reson Imaging 22:38–43
Demir A, Ries M, Moonen CT et al (2003) Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology 229:37–43
Banaszek A, Bladowska J, Szewczyk P, Podgórski P, Sąsiadek M (2014) Usefulness of diffusion tensor MR imaging in the assessment of intramedullary changes of the cervical spinal cord in different stages of degenerative spine disease. Eur Spine J 23:1523–1530
Jones JG, Cen SY, Lebel RM, Hsieh PC, Law M (2013) Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. AJNR Am J Neuroradiol 34:471–478
Ellingson BM, Salamon N, Grinstead JW, Holly LT (2014) Diffusion tensor imaging predicts functional impairment in mild-to-moderate cervical spondylotic myelopathy. Spine J 14:2589–2597
Ellingson BM, Salamon N, Woodworth DC, Holly LT (2015) Correlation between degree of subvoxel spinal cord compression measured with super-resolution tract density imaging and neurological impairment in cervical spondylotic myelopathy. J Neurosurg Spine 6:1–8
Vitzthum HE, Dalitz K (2007) Analysis of five specific scores for cervical spondylogenic myelopathy. Eur Spine J 16:2096–2103
Amirjani N, Ashworth NL, Gordon T, Edwards DC, Chan KM (2007) Normative values and the effects of age, gender, and handedness on the Moberg pick-up test. Muscle Nerve 35:788–792
Lindberg PG, Feydy A, Maier MA (2010) White matter organization in cervical spinal cord relates differently to age and control of grip force in healthy subjects. J Neurosci 30:4102–4109
Roche N, Bussel B, Maier MA, Katz R, Lindberg P (2011) Impact of precision grip tasks on cervical spinal network excitability in humans. J Physiol 589:3545–3558
Lindberg P, Ody C, Feydy A, Maier MA (2009) Precision in isometric precision grip force is reduced in middle-aged adults. Exp Brain Res 193:213–224
Lindberg PG, Bensmail D, Bussel B, Maier MA, Feydy A (2011) Wallerian degeneration in lateral cervical spinal cord detected with diffusion tensor imaging in four chronic stroke patients. J Neuroimaging 21:44–48
Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17:1429–1436
Pavlov H, Torg JS, Robie B, Jahre C (1987) Cervical spinal stenosis: determination with vertebral body ratio method. Radiology 164:771–775
Ciccarelli O, Wheeler-Kingshott CA, McLean MA et al (2007) Spinal cord spectroscopy and diffusion-based tractography to assess acute disability in multiple sclerosis. Brain 130:2220–2231
Van Hecke W, Leemans A, Sijbers J, Vandervliet E, Van Goethem J, Parizel PM (2008) A tracking-based diffusion tensor imaging segmentation method for the detection of diffusion-related changes of the cervical spinal cord with aging. J Magn Reson Imaging 27:978–991
Karadimas SK, Moon ES, Yu WR et al (2013) A novel experimental model of cervical spondylotic myelopathy (CSM) to facilitate translational research. Neurobiol Dis 54:43–58
Nathan PW, Smith MC, Deacon P (1990) The corticospinal tracts in man. Course and location of fibres at different segmental levels. Brain 113:303–324
Ogino H, Tada K, Okada K et al (1983) Canal diameter, anteroposterior compression ratio, and spondylotic myelopathy of the cervical spine. Spine (Phila Pa 1976) 8:1–15
Simó M, Szirmai I, Arányi Z (2004) Superior sensitivity of motor over somatosensory evoked potentials in the diagnosis of cervical spondylotic myelopathy. Eur J Neurol 11:621–626
Blight AR (1991) Morphometric analysis of a model of spinal cord injury in guinea pigs, with behavioral evidence of delayed secondary pathology. J Neurol Sci 103:156–171
Quencer RM, Bunge RP, Egnor M et al (1992) Acute traumatic central cord syndrome: MRI-pathological correlations. Neuroradiology 34:85–94
Lemon RN (2008) Descending pathways in motor control. Annu Rev Neurosci 31:195–218
Zijdewind I, Thomas CK (2003) Motor unit firing during and after voluntary contractions of human thenar muscles weakened by spinal cord injury. J Neurophysiol 89:2065–2071
Yoshikawa M, Doita M, Okamoto K, Manabe M, Sha N, Kurosaka M (2008) Impaired postural stability in patients with cervical myelopathy: evaluation by computerized static stabilometry. Spine (Phila Pa 1976) 33:E460–E464
Olindo S, Signate A, Richech A et al (2008) Quantitative assessment of hand disability by the Nine-Hole-Peg test (9-HPT) in cervical spondylotic myelopathy. J Neurol Neurosurg Psychiatry 79:965–967
Freund P, Schneider T, Nagy Z et al (2012) Degeneration of the injured cervical cord is associated with remote changes in corticospinal tract integrity and upper limb impairment. PLoS One 7, e51729
Hatem SM, Attal N, Ducreux D et al (2010) Clinical, functional and structural determinants of central pain in syringomyelia. Brain 133:3409–3422
Koskinen E, Brander A, Hakulinen U et al (2013) Assessing the state of chronic spinal cord injury using diffusion tensor imaging. J Neurotrauma 30:1587–1595
Ducreux D, Lepeintre JF, Fillard P, Loureiro C, Tadié M, Lasjaunias P (2006) MR diffusion tensor imaging and fiber tracking in 5 spinal cord astrocytomas. AJNR Am J Neuroradiol 27:214–216
Agosta F, Lagana M, Valsasina P et al (2007) Evidence for cervical cord tissue disorganisation with aging by diffusion tensor MRI. Neuroimage 36:728–735
Cui JL, Wen CY, Hu Y, Mak KC, Mak KH, Luk KD (2011) Orientation entropy analysis of diffusion tensor in healthy and myelopathic spinal cord. Neuroimage 58:1028–1033
Acknowledgments
This study was in part supported by grants to Påvel Lindberg of Hjärnfonden (the Swedish Brain Foundation) and of the Institut pour la Recherche sur la Moelle Epinière et l'Encéphale (IRME, Paris). The authors thank URC-CIC Paris Centre for implementation and monitoring of the study. The scientific guarantor of this publication is Marc A. Maier. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: cross-sectional study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindberg, P.G., Sanchez, K., Ozcan, F. et al. Correlation of force control with regional spinal DTI in patients with cervical spondylosis without signs of spinal cord injury on conventional MRI. Eur Radiol 26, 733–742 (2016). https://doi.org/10.1007/s00330-015-3876-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3876-z