Abstract
Liquorice (Glycyrrhiza uralensis) is an important medicinal plant for which there is a huge market demand. It has been reported that arbuscular mycorrhizal (AM) symbiosis and drought stress can stimulate the accumulation of the active ingredients, glycyrrhizin and liquiritin, in liquorice plants, but the potential interactions of AM symbiosis and drought stress remain largely unknown. In the present work, we investigated mycorrhizal effects on plant growth and accumulation of glycyrrhizin and liquiritin in liquorice plants under different water regimes. The results indicated that AM plants generally exhibited better growth and physiological status including stomatal conductance, photosynthesis rate, and water use efficiency compared with non-AM plants. AM inoculation up-regulated the expression of an aquaporin gene PIP and decreased root abscisic acid (ABA) concentrations under drought stress. In general, AM plants displayed lower root carbon (C) and nitrogen (N) concentrations, higher phosphorus (P) concentrations, and therefore, lower C:P and N:P ratios but higher C:N ratio than non-AM plants. On the other hand, AM inoculation increased root glycyrrhizin and liquiritin concentrations, and the mycorrhizal effects were more pronounced under moderate drought stress than under well-watered condition or severe drought stress for glycyrrhizin accumulation. The accumulation of glycyrrhizin and liquiritin in AM plants was consistent with the C:N ratio changes in support of the carbon-nutrient balance hypothesis. Moreover, the glycyrrhizin accumulation was positively correlated with the expression of glycyrrhizin biosynthesis genes SQS1, β-AS, CYP88D6, and CYP72A154. By contrast, no significant interaction of AM inoculation with water treatment was observed for liquiritin accumulation, while we similarly observed a positive correlation between liquiritin accumulation and the expression of a liquiritin biosynthesis gene CHS. These results suggested that AM inoculation in combination with proper water management potentially could improve glycyrrhizin and liquiritin accumulation in liquorice roots and may be practiced to promote liquorice cultivation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Liquorice (Glycyrrhiza uralensis Fisch.) is a perennial leguminous species widely grown in the world. As a plant well adapted to low-fertility soil and arid environments, liquorice is expected to be used for ecological restoration of degraded ecosystems in arid and semi-arid areas (Liu et al. 2007; Chen et al. 2017). Glycyrrhizin, an important bioactive triterpenoid saponin, and liquiritin, the main flavonoid compound in liquorice plants, both secondary metabolites, are derived from the roots and stolons. These compounds confer several health benefits and have substantial economic and pharmacological values (Kitagawa 2002; Hayashi and Sudo 2009). As a result, liquorice also is among the most important raw materials for crude herbal drugs, and the market demand for liquorice has been increasing rapidly in recent years (Hayashi 2009; Hayashi and Sudo 2009). Currently, liquorice cultivation is developing rapidly because of gradual extinction of wild liquorice plants. Low yield and low concentrations of active ingredients (mainly glycyrrhizin and liquiritin) in cultivated liquorice plants, however, largely have limited the development of the liquorice industry (Wei et al. 2003; Hayashi and Sudo 2009). Therefore, approaches that could stimulate the accumulation of glycyrrhizin and liquiritin in liquorice plants become critically important to promote liquorice cultivation.
In liquorice plants, glycyrrhizin is mainly synthesized through the mevalonic acid (MVA) pathway and is regulated by many enzymes (Seki et al. 2008, 2011; Mochida et al. 2017; Fig. 1). Presently, most of the key genes involved in the biosynthesis of glycyrrhizin have been cloned and characterized successfully. 3-Hydroxy-3-methylglutary CoA reductase gene, HMGR, which encodes the first rate-limiting enzyme involved in the MVA pathway, largely determines the carbon flow and accumulation of glycyrrhizin (Chappell et al. 1995; Aquil et al. 2009). Several reports have shown that overexpression of the HMGR gene induced the accumulation of terpene in plants (Kim et al. 2006; Dai et al. 2011). Squalene synthase gene, SQS, which is involved in the early stage of glycyrrhizin biosynthesis and encodes the enzyme to catalyze farnesyl diphosphate (FPP) into squalene, plays an important role in the formation of triterpenoid (glycyrrhizin) skeletons (Hayashi 2009; Nasrollahi et al. 2014). β-amyrin synthase gene, β-AS, and lupeol synthase gene, LUS, are situated at a branch point to catalyze 2,3-oxidosqualene into β-amyrin and lupeol, respectively, which also play important roles in triterpenoid (glycyrrhizin) skeleton formation and accumulation (Hayashi 2009; Shen et al. 2009; Mochida et al. 2017). Liu and Liu (2012) revealed that the β-AS gene is expressed only in plant roots, not in the leaves or stems, which is consistent with the accumulation pattern of glycyrrhizin in plants. Moreover, cytochrome P450 monooxygenase 88D6 (CYP88D6) and cytochrome P450 monooxygenase 72A154 (CYP72A154), two enzymes involved in the multiple oxidation reactions that catalyze β-amyrin to glycyrrhetinic acid, the precursor of glycyrrhizin, play crucial roles in the final step of glycyrrhizin biosynthesis (Seki et al. 2008, 2011). Like the β-AS gene, both CYP88D6 and CYP72A154 only are expressed in the roots, corresponding to the accumulation pattern of glycyrrhizin (Seki et al. 2008, 2011). Similarly, as the representative flavonoid in liquorice plants, liquiritin biosynthesis also involves several enzymes, including chalcone synthase gene, CHS (Winkel-Shirley 2002; Zhang et al. 2009; Chen et al. 2017; Fig. 1). Several reports have shown that the expression level of these key genes was highly correlated with the accumulation of glycyrrhizin and liquiritin in plants (Hayashi 2009; Nasrollahi et al. 2014; Li et al. 2017).
Glycyrrhizin and liquiritin biosynthesis pathways in liquorice plants. Each arrow represents a one-step catalytic reaction, and double arrows represent multi-step catalytic reactions. Acetyl-CoA, acetyl coenzyme A; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; HMGR, 3-hydroxy-3-methylglutary coenzyme A reductase; MVA, mevalonate; IPP, isopentenyl diphosphate; DMAPP, dimethylallyl diphosphate; FPP, farnesyl pyrophosphate; SQS, squalene synthase; β-AS, beta-amyrin synthase; CYP88D6 and CYP72A154, cytochrome P450 monooxygenases; LUS, lupeol synthase; CHS, chalcone synthase
In natural environments, drought stress is considered as the most common abiotic stress affecting plant growth and development (Golldack et al. 2014; Ruiz-Lozano et al. 2016). Nevertheless, plants have evolved various mechanisms to cope with drought stress including biosynthesis of secondary metabolites (Selmar and Kleinwächter 2013). Drought stress can induce the formation of reactive oxygen species (ROS) in chloroplasts and mitochondria and cause oxidative damage (Liu et al. 2011; Selmar and Kleinwächter 2013). It has been found that triterpenoids, including glycyrrhizin, display antioxidant activity and play an important role in ROS scavenging, resulting in a significant reduction of oxidative damage (Okubo and Yoshiki 2000; Kim and Lee 2008). In addition, the production of secondary metabolites is strongly associated with plant growth conditions. Plants that are exposed to drought stress usually exhibit higher concentrations of proline and abscisic acid (ABA), higher aquaporin gene PIP expression, lower photosynthesis rate, diminished nutrient uptake, and ultimately decreased biomass (Javot and Maurel 2002; Chitarra et al. 2016; Ruiz-Lozano et al. 2016). Accordingly, plants that suffer from drought stress usually increase secondary metabolite production (Selmar and Kleinwächter 2013). For example, it has been reported that drought stress can increase phenolic compound concentrations in Hypericum brasiliense and tanshinone concentration in Salvia miltiorrhiza partially due to reduced biomass (de Abreu and Mazzafera 2005; Liu et al. 2011). Nevertheless, Nowak et al. (2010) demonstrated that the increase of monoterpenes in sage induced by drought stress was out of proportion to the corresponding decrease in biomass, indicating specific mechanisms underlying plant secondary metabolism. As reported by Nasrollahi et al. (2014), drought stress up-regulated the expression of key genes involved in the biosynthesis of triterpenoid saponins in liquorice and increased glycyrrhizin concentrations.
Arbuscular mycorrhizal (AM) fungi, as the most widespread symbiotic partners for higher plants, can improve plant mineral nutrition and growth and also affect the accumulation of secondary metabolites in host plants (Chitarra et al. 2016; Welling et al. 2016; Kapoor et al. 2017). For instance, AM inoculation significantly increased shoot biomass and artemisinin concentration in Artemisia annua (Kapoor et al. 2007) and facilitated the accumulation of phenol compounds in Medicago sativa and tomato (López-Ráez et al. 2010; Zhang et al. 2013). Liu et al. (2007) reported that inoculation with Glomus mosseae or Glomus versiforme increased the glycyrrhizin concentration in the roots of Glycyrrhiza uralensis. In general, the increases of secondary metabolites and plant dry weight of AM plants have been associated with improved photosynthesis rates, nutritional benefits, especially in phosphorus (P) nutrition, and hormonal balance, including ABA (Zeng et al. 2013; Welling et al. 2016; Kapoor et al. 2017). It has been found that AM inoculation could alter plant ABA level (Li et al. 2016a; Ruiz-Lozano et al. 2016; Chitarra et al. 2016), which could induce the biosynthesis of secondary metabolites, including terpenoids (Mansouri et al. 2009; Yang et al. 2012). The relation between ABA and terpenoid biosynthesis, however, has not been demonstrated yet. In addition, the development of AM symbiosis demands photoassimilates, and host plants therefore allocate carbon to the fungi which might disturb plant carbon-nutrient ratio and consequently carbon allocation for terpenoid synthesis (Kapoor et al. 2017). Nevertheless, the effects of AM inoculation on the accumulation of terpenoids have not been examined in relation to carbon-nutrient ratio in any studies. How AM fungi regulate the expression of genes involved in glycyrrhizin and liquiritin biosynthesis also is largely unknown. Taken together, further investigation is required to reveal the physiological mechanisms corresponding to the alteration in plant secondary metabolism by AM symbiosis.
AM inoculation could alleviate drought stress in plants through several mechanisms including alteration of ABA level and regulation of stress-responsive genes, such as aquaporin gene PIP and antioxidant gene SOD in host plants (Ruiz-Lozano et al. 2016; Chitarra et al. 2016; Li et al. 2016a). It is possible that the positive effects of drought stress on the accumulation of plant secondary metabolites might be counteracted or even disappear because of AM symbiosis. On the other hand, drought stress also could affect mycorrhiza establishment and development by influencing spore germination, hyphal growth, and arbuscule formation (Al-Karaki et al. 2004; Neumann et al. 2009; Sun et al. 2017) which could ultimately affect mycorrhiza function and plant secondary metabolism. Actually, different factors may antagonize each other, leading to negative outputs, especially for the combination of biotic and abiotic factors (Schenke et al. 2011; Babikova et al. 2014; Gorelick and Bernstein 2014). For instance, ultraviolet-B (UV-B) light induced the production of flavonols in Arabidopsis and this accumulation was attenuated by simultaneous application of the bacterial elicitor flg22 (Schenke et al. 2011). Drought stress and AM symbiosis could individually facilitate the accumulation of secondary metabolites in liquorice plants, but to the best of our knowledge, no information is available regarding the interactive effects of AM inoculation and drought stress on glycyrrhizin and liquiritin accumulations in liquorice plants.
Consequently, in this study, we hypothesize that AM inoculation could improve plant growth and facilitate the accumulation of glycyrrhizin and liquiritin in liquorice plants, and the mycorrhizal effects could be more pronounced under moderate drought stress. In a pot experiment carried out in a controlled-environment growth chamber, we examined the interactive effects of inoculation with the AM fungus Rhizophagus irregularis and drought stress on (1) plant growth, (2) plant photosynthesis, (3) plant mineral nutrition, (4) glycyrrhizin and liquiritin concentrations, and (5) the expression of genes involved in the biosynthesis of glycyrrhizin and liquiritin. We expected to unravel the underlying mechanisms for the interactions of AM inoculation and drought stress on liquorice growth and secondary metabolite accumulation, and also to check the feasibility of introducing AM fungi together with proper water management to promote liquorice cultivation.
Materials and methods
Biological materials and growth medium
Seeds of liquorice (Glycyrrhiza uralensis Fisch.) were provided by China National Traditional Chinese Medicine Corporation, Beijing, China. The seeds were immersed in 50% H2SO4 for 30 min to break the thick seed coat and then surface-sterilized for 10 min by using 10% H2O2, pregerminated on a moist sterilized filter paper at 25 °C in the dark until the emergence of radicles. After 2 days, uniform seedlings with about a 2-cm-long radicle were selected for the experiment.
The AM fungus Rhizophagus irregularis (R. irregularis) Schenck & Smith BGC AH01 was obtained from the Institute of Plant Nutrition and Resources, Beijing Academy of Agriculture and Forestry. The fungus was propagated in pot culture on Sorghum bicolor L. in a sandy soil for about 12 weeks. The inoculum consisted of sand, spores (approximately 60 spores per gram), mycelia, and colonized root fragments.
The growth medium was a 1:2 (w:w) mixture of sand (< 2 mm) and soil collected from Erdos (39° 53′ N, 110° 1′ E), Inner Mongolia, China. The soil was passed through a 2-mm sieve and then sterilized by radiation (γ rays, 20 kGy, 10-MeV electron beam); the sand was autoclaved at 121 °C for 1 h on 2 consecutive days. The soil had a pH of 8.70 (1:2.5 soil to water), organic matter content of 10.79 g/kg, and an extractable Pi (with 0.5 M NaHCO3, pH 8.5) content of 4.46 mg/kg. Before sowing, basal nutrients of 30 mg/kg Pi (KH2PO4), 120 mg/kg N (NH4NO3), and 120 mg/kg K (KH2PO4 and K2SO4) were carefully mixed into the growth medium. For convenience, this growth medium is hereinafter referred to as “soil.”
Experimental procedure
Plastic pots (15-cm diameter × 14-cm height) were first filled with 800 g soil and then 400 g soil containing 40 g of inoculum (c. 2400 spores) for AM inoculation. For the non-inoculated controls, equivalent sterilized inoculum together with 5 ml of filtrate (< 20 μm) from the AM inoculum was mixed with the soil to ensure the same microbial populations (except for AM fungi) in different inoculation treatments. Each pot was sown with three pregerminated liquorice seeds. Seedlings were thinned to two per pot at 14 days after emergence. Before water treatment, all the pots were maintained under well-watered conditions (WW, 16% water content equating to 75% field water capacity). Two months after sowing, one-third of the pots (both inoculation and non-inoculation treatments) were maintained under well-watered conditions throughout the entire experiment, while another one-third of the pots were subjected to moderate drought stress (MD, 11% water content equating to 55% field water capacity), and other pots were subjected to severe drought stress (SD, 7% water content equating to 35% field water capacity). Plants were allowed to grow for 1 more month before harvest. Water loss was daily supplemented with deionized water to maintain the desired moisture content by regular weighing. There were six treatments (full factorial combination of mycorrhizal status and water regimes) with five replicates of each treatment, giving a total of 30 pots, which were set up in a randomized block design.
The experiment was carried out in a controlled-environment growth chamber under a light intensity of 700 μmol m−2 s−1, 16:8-h light:dark photoperiod, temperature of 25 °C (light) and 20 °C (dark), and 70% relative humidity.
Photosynthesis and water use efficiency
The day before experimental harvest, net photosynthesis rate (Pn), leaf transpiration rate (Tr), and stomatal conductance (Cond) were measured on the third mature leaf from the top of the intact plants with a portable photosynthesis system (Li-6400X, Li-COR Biosciences, USA). During the measurements, a 6400-40 source provided a photosynthetic photon flux density (PPFD) of 800 μmol m−2 s−1. CO2 concentration in the sample chamber was maintained at 400 g m−3 and relative humidity was kept at about 65%. Measurements were performed in a random order during the late morning (09:00–11:00 a.m.). Four replicates per treatment were selected randomly and both plants per pot were measured. During the measurement, there was approximately 0.4–0.6% water loss (based on dry soil) for all the treatments. Water use efficiency (WUE) was calculated according to the following equation: WUE = Pn / Tr.
Plant biomass and mycorrhizal colonization
Plant shoots and roots were separately harvested and washed carefully with deionized water, and fresh shoot and root biomass were recorded. Subsamples (0.5 g fresh weight) of roots from each pot were stored at − 80 °C for RNA extraction and ABA analysis, and subsamples (0.5 g) were used for root staining and determination of mycorrhizal colonization. In brief, fresh roots were cleared in 10% KOH and stained with 0.05% trypan blue in lactic acid (v/v). Thirty pieces of randomly selected stained root fragments were observed with a light microscope to confirm the presence of fungal structures (intraradical mycelia, vesicles, and arbuscules). Intensity of mycorrhizal colonization (M%) and arbuscule abundance (A%) in the whole root system were assessed by the method described by Trouvelot et al. (1986) using MYCOCALC software (www2.dijon.inra.fr/mychintec/Mycocalc-prg/download). Shoots and the rest of the roots were dried at 60 °C for 72 h to record dry weights (DW) and used for determination of elemental concentrations and root active ingredients (glycyrrhizin and liquiritin). The mycorrhizal growth dependency (MGD) of liquorice plants under each water regime was calculated based on whole plant dry weight by using the following formula (Smith et al. 2003):
Leaf proline concentration and root ABA concentration
The extraction and determination of proline was performed according to Bates et al. (1973) with minor modifications. In brief, approximately 0.2 g leaf samples were extracted with 3% aqueous sulfosalicylic acid in a boiling water bath for 10 min. After centrifugation at 3000×g for 10 min, 1.5 ml of supernatant was added to 2 ml glacial acetic acid and 2 ml acid-ninhydrin and then incubated for 30 min at 95 °C. Five milliliters of toluene was added to extract the product after cooling. Proline concentration was measured with a spectrophotometer (UV-1700 PharmaSpec, Shimadzu, Japan) at 520 nm and calculated on the basis of a proline standard curve. Root ABA concentration was quantified following the method of Li et al. (2016a) with a plant ABA ELISA kit (Rapidbio, West Hills, USA). Briefly, frozen root samples were immersed in 2 ml distilled water and incubated for 24 h at 4 °C in the dark. After centrifugation (3000×g for 10 min), the supernatant was used for a horseradish peroxidase (HRP)-tetramethyl benzidine (TMB) chromogenic assay according to the manufacturer’s instructions. ABA concentration was measured with a Spectra Microplate Reader (SPECTRA max190, MD, USA) at 450 nm.
Root C, N, and P concentrations
Root C and N concentrations were directly determined by an Elemental Analyzer (Vario MAX, Elementar, Germany). For determination of root P concentrations, about 0.2 g dried ground root sample was digested in HNO3 (guaranteed reagent) for 12 h, followed by a microwave-accelerated reaction for 35 min in a Microwave-Accelerated Reaction System (Mars, CEM Corp., USA). The P concentrations were determined by ICP-OES (Prodigy, Teledyne Leeman, USA). Blanks and internal standards of bush leaves (GBW07603, China Standard Research Center) were used to ensure the accuracy of chemical analysis.
Glycyrrhizin and liquiritin concentrations in liquorice root
The dried root samples of liquorice plants were ground into powder with a mortar and pestle and passed through a 60-mesh sieve. Approximately 0.1 g sample was weighed accurately and extracted in 15 ml 67% methanol for 45 min in an ultrasonic bath (250 W, 40 kHz) at room temperature. The extract solution was cooled to room temperature and filtered through a 0.45-μm filter. A 10-μl aliquot of the filtered solution was injected into an injection loop and separated by high-performance liquid chromatography (HPLC, Agilent-1200, USA) through an Agilent ZORBAX-Eclipse XDB-C18 column (250 mm × 4.6 mm, 5 μm). The mobile phase comprised a gradient of deionized water:phosphoric acid (100:0.05, v/v) and acetonitrile. The separation was operated in the gradient elution mode as follows: 0–8 min, acetonitrile: 0.05% phosphoric acid at 19:81 (v/v); 8–35 min, 19–50:81–50 (v/v); 35–36 min, 50–100:50–0 (v/v); 36–40 min, 100–19:0–81 (v/v); and 40–45 min, 19:81 (v/v). The detection wavelength was 237 nm for both glycyrrhizin and liquiritin. The column temperature was 30 °C with a flow rate of 1.0 ml/min. Standard substances of glycyrrhizin and liquiritin were bought from the National Institute for the Control of Pharmaceutical and Biological Products (Batch Nos. 110731 and 11610). Stock solutions were diluted with methanol to appropriate concentrations for calibration purposes (Li et al. 2016b).
Gene expression analysis
Total RNA from roots was isolated by using CTAB (2% CTAB, 2% PVP-40, 0.1 mol/l Tris-HCl, 0.25 mol/l EDTA, 2 mol/l NaCl) following a purification procedure with a MicroElute RNA Clean-Up Kit (Omega Biotek, USA) and a DNase treatment. cDNAs were synthesized from the RNA samples using a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc., USA) following the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) was performed on the cDNAs with at least four independent biological replicates and three technical replicates for each biological replicate in a Bio-Rad iQ5 Optical system (Bio-Rad, Hercules, CA, USA), monitored by SYBR Green I fluorescence (TAKARA Biotechnology Co. Ltd). The reaction medium (25 μl) contained SYBR Green I PCR Mix, 400 nM of gene-specific primers, and the cDNA templates diluted fivefold. For plant gene expression analysis, 1.5 μl of the diluted cDNAs were added to the reaction medium. The qRT-PCR primers used are listed in supplementary Table S1. Negative control reactions (using RNA or water instead of cDNA) were included to avoid genomic DNA contamination and to exclude primer-dimers. The qRT-PCR program consisted of an initial step at 95 °C for 10 s, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 56 °C for 60 s, and extension at 72 °C for 30 s. Data collection was performed at 72 °C. To test the amplification specificity, a melting curve was produced according to the following program: 10 s at 70 °C, and heating to 100 °C at a rate of 0.5 °C/s, and data were collected continuously. The data were analyzed by the 2-ΔΔCt method (Pfaffl 2001). β-actin was used to normalize the results as the reference gene (Xu et al. 2016).
Statistical analysis
All data were checked for normality using Shapiro-Wilk test and for homoscedasticity using Levene’s test prior to statistical analysis. Percentage values (root colonization rate, arbuscule abundance, and MGD) were arcsine [square-root (X)] transformed. Other values were Box-Cox transformed to fulfill the requirement for ANOVA normality and homogeneity of variance when necessary (Box and Cox 1964). When the transformation did not improve the structure of the data, a non-parametric Kruskal-Wallis test was performed.
A two-way analysis of variance (ANOVA) was performed to examine the effects of mycorrhizal inoculation, drought stress, and their interactions on plant dry weights; photosynthetic parameters; ABA concentration; C, N, and P concentrations; C:N:P stoichiometric ratios; and gene expression, as well as glycyrrhizin and liquiritin concentrations. One-way ANOVA was performed to examine the effects of drought stress on mycorrhizal colonization and mycorrhizal growth dependency (MGD). As an exception, leaf proline concentrations of all the six treatments were compared by using a non-parametric Kruskal-Wallis test. Differences between treatments were analyzed by Duncan’s multiple range test or Kruskal-Wallis test at a 0.05 probability level. Correlations between glycyrrhizin or liquiritin concentrations and gene expressions were examined by Pearson correlation analyses. All the statistical analysis was carried out by using the software IBM SPSS Statistics v. 19.0 (IBM Corp., Armonk, NY, USA).
Results
AM root colonization
No mycorrhizal colonization was detected in non-inoculated plant roots regardless of water regime, while roots of inoculated plants were extensively colonized by R. irregularis (Table 1). There were no significant differences in M% or A% among water regimes.
Plant growth
Plant shoot and root dry weights decreased with drought stress regardless of inoculation treatment (Table 2). For AM plants, both moderate and severe drought stress significantly reduced plant shoot and root dry weights. For non-AM plants, however, the significant reduction was only observed under severe drought stress. On the other hand, AM inoculation significantly increased shoot and root dry weights under all the water regimes (Table 2). An increase in root dry weights of 14-, 8-, and 8-fold was observed in mycorrhizal plants compared to non-AM plants under well-watered conditions, moderate drought stress, and severe drought stress, respectively, corresponding to the high mycorrhizal growth dependency (MGD) of liquorice plants under our experimental conditions (MGD > 89%, Table 2). The interactions of AM inoculation and drought stress were significant for both shoot and root dry weights (Table 2). The growth differences induced by AM inoculation were weakened by drought stress, with a significant decrease of MGD values under drought stress (Table 2).
Leaf proline and root ABA concentrations
The leaf proline concentrations generally increased upon drought stress, and this increase was statistically significant under severe drought stress in AM plants (Table 3). Mycorrhizal inoculation decreased proline concentrations versus non-AM plants by 81.47 and 80.83% under well-watered conditions and moderate drought stress, respectively.
Unlike proline, drought stress significantly increased root ABA concentrations regardless of mycorrhizal status (Table 3). In contrast, AM inoculation generally decreased ABA concentrations and the decrease became significant under severe drought stress (Table 3).
Stomatal conductance and photosynthesis
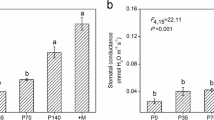
Stomatal conductance was negatively affected by drought stress in AM plants, while no significant difference among water regimes was observed for non-AM plants (Fig. 2a, Table S2). Drought stress decreased net photosynthesis rate regardless of mycorrhizal status (Fig. 2b, Table S2), while AM symbiosis significantly increased stomatal conductance and net photosynthesis rate regardless of water regime (Fig. 2a, b, Table S2). A 3.4-fold increase of stomatal conductance and 6.7-fold increase of net photosynthesis rate in AM plants compared to non-AM plants under well-watered conditions were detected (Fig. 2a, b).
Stomatal conductance (a), net photosynthesis rate (b), water use efficiency (WUE) (c), and relative expression of the aquaporin gene PIP (d) of liquorice plants as affected by mycorrhizal inoculation (I) and water regimes (W). −M and +M represent non-inoculated control and inoculation with the mycorrhizal fungus R. irregularis. WW, MD, and SD represent well-watered condition, moderate drought stress, and severe drought stress, respectively. The error bars represent the standard error (SE). Treatment effects were tested by two-way ANOVA and are shown as P values in the figure. The same letter above the error bars indicates no significant difference at P < 0.05 by Duncan’s multiple range test
WUE and expression of aquaporin gene
There were no significant changes in WUE among the water regimes for non-AM plants, while drought stress increased WUE for AM plants, this increase being significant under moderate drought stress. AM symbiosis markedly increased WUE under all the water regimes (Fig. 2c, Table S2). The expression of root aquaporin gene PIP was significantly up-regulated by severe drought stress in non-AM roots and by moderate drought stress in AM roots. AM symbiosis only induced an increase of PIP expression under moderate drought stress (Fig. 2d, Table S2); thereby, a significant interaction of AM inoculation with drought stress was observed for PIP expression (Fig. 2d, Table S2).
Root C, N, and P concentrations and stoichiometry
Drought stress steadily increased root C concentrations for both non-AM and AM plants, this increase being significant under severe drought stress in non-AM plants and under moderate drought stress in AM plants versus well-watered conditions. In contrast, AM inoculation induced a decrease in root C concentrations under all the water regimes versus non-AM plants (Table 4). In non-AM plants, root N concentrations were not changed by drought stress while root P concentrations significantly decreased under severe drought stress versus well-watered condition (Table 4). In AM plants, however, drought stress significantly increased root N but decreased root P concentrations, and the maximum N concentration and minimum P concentration were recorded under severe drought stress. In contrast with the influence of drought stress on root N and P concentrations, AM symbiosis significantly decreased root N but increased root P concentrations regardless of water regimes (Table 4). Significant interactions of AM inoculation with drought stress were observed for both root N and P concentrations (Table 4).
Root C:N, C:P, and N:P ratios all were significantly affected by drought stress and mycorrhizal inoculation (Table 5). C:N ratios were steadily decreased by drought stress in AM plants, while no changes in C:N ratios were detected in non-AM plants. AM inoculation resulted in a significant increase of C:N ratios regardless of water regimes (Table 5). Drought stress increased root C:P and N:P ratios both in non-AM and AM plants, with the maximum ratios recorded under severe drought stress (Table 5). Conversely, AM inoculation decreased root C:P and N:P ratios regardless of water regime. Significant interactions of AM inoculation with drought stress were observed for both C:N and N:P ratios (Table 5).
Root glycyrrhizin and liquiritin concentrations
Drought stress significantly increased root glycyrrhizin concentrations regardless of mycorrhizal status, this increase being significant under severe drought stress for non-AM plants (increase by 46.6%) and under moderate drought stress for AM plants (increase by 33.4%) versus well-watered conditions (Fig. 3a, Table S3). Unlike the accumulation patterns of glycyrrhizin, drought stress induced a decrease in liquiritin concentrations regardless of mycorrhizal status (Fig. 3b, Table S3). A 1.84-fold reduction of liquiritin in non-AM plants and 2.08-fold reduction in AM plants were observed under severe drought stress versus well-watered conditions (Fig. 3b).
Root glycyrrhizin (a) and liquiritin (b) concentrations of liquorice plants as affected by mycorrhizal inoculation (I) and water regimes (W). DW, dry weight. −M and +M represent non-inoculated control and inoculation with the mycorrhizal fungus R. irregularis. WW, MD, and SD represent well-watered condition, moderate drought stress, and severe drought stress, respectively. The error bars represent the standard error (SE). Treatment effects were tested by two-way ANOVA and are shown as P values in the figure. The same letter above the error bars indicates no significant difference at P < 0.05 by Duncan’s multiple range test
On the other hand, AM symbiosis increased root glycyrrhizin and liquiritin concentrations regardless of water regimes (Fig. 3, Table S3). AM symbiosis increased glycyrrhizin concentrations by 2.0-, 2.5-, and 0.9-folds under well-watered condition, moderate drought stress, and severe drought stress, respectively. The increase was about 3.8-, 4.8-, and 2.9-folds for liquiritin concentrations under the same conditions (Fig. 3). A significant interaction of AM inoculation and drought stress was observed only on glycyrrhizin concentrations (Fig. 3, Table S3). Moreover, the yields (concentrations × root dry weights) of glycyrrhizin and liquiritin of AM plants were significantly higher than those of non-AM plants, and no significant difference in glycyrrhizin yield was detected between well-watered and moderate drought stress (Table S4).
Expression of glycyrrhizin and liquiritin biosynthesis genes
HMGR expression appeared to be differentially regulated by water treatment and mycorrhizal inoculation (Fig. 4a, Table S5). In non-AM roots, the expression of HMGR was significantly up-regulated by drought stress, following a similar pattern as glycyrrhizin concentrations (r = 0.569*), but HMGR expression was unaffected by water regime in AM roots (Figs. 3a and 4a). AM inoculation significantly up-regulated HMGR expression in well-watered condition but down-regulated HMGR expression under moderate and severe drought stress (Fig. 4a). SQS1 expression was significantly influenced by drought stress, and moderate drought stress exhibited the highest gene expression regardless of mycorrhizal status. Significant increase in SQS1 expression was detected in AM plants, corresponding with the accumulation pattern of glycyrrhizin in roots (r = 0.731**, Figs. 3a and 4b, Table S5). In non-AM roots, expressions of β-AS, CYP88D6, and CYP72A154 were not regulated by water regimes, but drought stress tended to up-regulate the expressions of β-AS, CYP88D6, and CYP72A154 in AM plants (Fig. 4c–e). AM inoculation markedly up-regulated the expressions of β-AS, CYP88D6, and CYP72A154 especially under moderate drought stress, also consistent with the accumulation pattern of glycyrrhizin (β-AS: r = 0.679**; CYP88D6: r = 0.707**; CYP72A154: r = 0.502*, Figs. 3a and 4c–e, Table S5).
Relative expression of HMGR (a), SQS1 (b), β-AS (c), CYP88D6 (d), CYP72A154 (e), LUS (f), and CHS (g) in liquorice roots as affected by mycorrhizal inoculation (I) and water regimes (W). −M and +M represent non-inoculated control and inoculation with the mycorrhizal fungus R. irregularis. WW, MD, and SD represent well-watered condition, moderate drought stress, and severe drought stress, respectively. nd, not detectable. The error bars represent the standard error (SE). Treatment effects were tested by two-way ANOVA (one-way ANOVA for LUS as an exception) and are shown as P values in the figure. The same letter above the error bars indicates no significant difference at P < 0.05 by Duncan’s multiple range test
LUS gene expression appeared to be down-regulated by drought stress both in AM and in non-AM plants, although the expression of LUS became undetectable under drought stress in non-AM plants. AM inoculation significantly increased LUS expression regardless of water regimes (Fig. 4f). CHS was significantly down-regulated by drought stress regardless of mycorrhizal status. Conversely, AM inoculation up-regulated CHS expression, following the same pattern as liquiritin accumulation in roots (r = 0.679**, Figs. 3b and 4g, Table S5).
Discussion
To the best of our knowledge, this study is the first to investigate the interactive effect of AM inoculation and drought stress on plant growth and accumulations of glycyrrhizin and liquiritin in liquorice plants. We showed that AM symbiosis increased glycyrrhizin accumulation and this effect was significantly intensified by moderate drought stress. The beneficial effect of AM inoculation on liquiritin accumulation, however, was independent of water regimes. Moreover, the accumulation patterns of glycyrrhizin and liquiritin coincided with changes in the expression of genes involved in glycyrrhizin and liquiritin biosynthesis.
AM symbiosis improved plant growth under drought stress
Drought stress adversely affects plant physiology, growth, and productivity, while AM symbiosis is a key component helping plants to cope with drought stress (Ruiz-Lozano et al. 2016; Chitarra et al. 2016). In this study, drought stress decreased shoot and root dry weights of liquorice plants regardless of mycorrhizal status. Drought stress increased leaf proline and root ABA concentrations which are physiological indictors of drought stress (Szabados and Savouré 2010; Hong et al. 2013). Consequently, stomatal conductance and net photosynthesis rate decreased upon drought stress, resulting in a reduction of CO2 assimilation and an inhibition of plant growth. In contrast, mycorrhizal inoculation induced a decrease of leaf proline and root ABA concentrations under drought stress as compared with the non-inoculated treatment. Li et al. (2016a) reported that mycorrhizal symbiosis could decrease root ABA concentration to up-regulate the expression of antioxidant gene SOD and aquaporin gene PIP with the latter considered an important mechanism for plants to regulate water uptake and cope with drought stress (Javot and Maurel 2002). The enhancement of water uptake and antioxidant defense helped AM plants maintain significantly higher water use efficiency and dry biomass than non-AM plants under drought stress. In addition to those mechanisms, AM inoculation also regulated carbon flow and C:N:P balance to improve plant drought tolerance. AM plants exhibited higher root P concentrations and lower C:P and N:P ratios than non-AM plants, which were beneficial to increase root respiration and growth (Zhao et al. 2015; Kapoor et al. 2017). These results support the growth rate hypothesis that plants allocate more P to ribosomes and rRNA to support the rapid protein synthesis associated with fast growth, and fast-growing plants exhibit lower tissue C:P and N:P ratios (Elser et al. 1996; Matzek and Vitousek 2009).
Drought stress increased glycyrrhizin concentration but decreased liquiritin concentration
It has been well demonstrated that plants exposed to drought stress can accumulate elevated concentrations of secondary metabolites, including terpenes and flavonoids (Selmar and Kleinwächter 2013). In the present work, drought stress generally increased glycyrrhizin concentrations regardless of mycorrhizal status, which was consistent with previous studies (Li et al. 2011; Nasrollahi et al. 2014). Under drought stress, plants accumulated ABA which induced partial stomatal closure and the accumulation of ROS which are thought to have an adverse impact on plant physiology (Selmar and Kleinwächter 2013). Consequently, the concentration of enzymatic and non-enzymatic antioxidants in plants tended to increase to scavenge the over-accumulated ROS (Pan et al. 2006). Increasing evidence supports that glycyrrhizin, the main triterpenoid saponin in Glycyrrhiza uralensis, displays antioxidant activity and plays an important role in ROS scavenging, resulting in a significant alleviation of oxidative damages (Kim and Lee 2008). In addition, the increase of glycyrrhizin concentrations under drought stress might be attributed to the decreased root biomass. The same amount of secondary metabolites as synthesized under well-watered conditions simply might be concentrated due to the reduction in biomass under drought stress (Selmar and Kleinwächter 2013). In this study, however, we observed that drought stress steadily decreased the root biomass of AM plants and the lowest root biomass was recorded under severe drought stress, which was not consistent with the glycyrrhizin accumulation pattern, indicating a complex relationship between drought stress and secondary metabolite accumulation.
In liquorice plants, glycyrrhizin is mainly synthesized through the MVA pathway, and key genes including HMGR in this pathway have been cloned and characterized (Liu et al. 2013; Mochida et al. 2017). In non-AM plants, drought stress appeared to have no significant effect on glycyrrhizin biosynthesis genes except for a significant up-regulation of HMGR, a gene encoding the first rate-limiting enzyme in the glycyrrhizin biosynthesis pathway. That up-regulation of HMGR seemed sufficient for the accumulation of final products (Harker et al. 2003; Enfissi et al. 2005) because of a cascading effect on downstream gene expression (Mandal et al. 2015). The up-regulation of HMGR by drought stress also has been observed in non-AM roots of various other plant species (Yang et al. 2012; Zhang et al. 2016).
In addition to the reduced biomass and up-regulation of the HMGR gene leading to an increased glycyrrhizin concentration, drought stress also induced changes in the nutrient source-sink relations (C:N:P stoichiometry) of the plant and thereby affected the synthesis, translocation, and accumulation of secondary metabolites (Selmar and Kleinwächter 2013). Drought stress inhibited plant growth and decreased root biomass, simultaneously increased root C concentrations and C:P and N:P ratios in non-AM plants, and as a result, possibly stimulated the carbon-based secondary metabolism and increased total yield of glycyrrhizin and liquiritin at the cost of biomass (Elser et al. 1996; Matzek and Vitousek 2009). In AM plants, however, the highest accumulation of glycyrrhizin was detected under moderate drought stress. The highest biosynthesis gene expressions (SQS1, β-AS, CYP88D6, CYP72A154) also were induced under moderate drought stress in AM plants, suggesting that the increase of glycyrrhizin concentrations under drought stress in AM plants was due to up-regulation of the glycyrrhizin biosynthesis genes.
Drought stress steadily decreased liquiritin concentrations regardless of mycorrhizal status. The inhibitory effect by drought stress was highly correlated with the suppression of liquiritin biosynthesis gene CHS. Moreover, as liquiritin and glycyrrhizin biosynthesis both derive from acetyl coenzyme A (Acetyl-CoA), it appears that the plant potentially stimulated production of glycyrrhizin at the expense of liquiritin production under drought stress.
AM inoculation increased glycyrrhizin and liquiritin concentrations
AM inoculation has been demonstrated to affect the accumulation of secondary metabolites, including terpenes and phenolics in plants (Zeng et al. 2013; Welling et al. 2016; Kapoor et al. 2017). In this study, we found that AM inoculation markedly increased root glycyrrhizin and liquiritin concentrations of liquorice plants regardless of water regimes, suggesting a positive effect of AM inoculation on root secondary metabolism. This was consistent with previous studies showing that AM inoculation increased the glycyrrhizin and liquiritin accumulations in liquorice plants (Liu et al. 2007; Orujei et al. 2013; Chen et al. 2017). According to the carbon-nutrient balance hypothesis, the balance between carbon and nutrient availability controls carbon-based secondary metabolism in plants (Bryant et al. 1983; Hamilton et al. 2001). The present study showed that AM symbiosis altered the balance between N and P, resulting in lower root N:P ratios and N limitations for plant growth. Consequently, AM plants exhibited higher root C:N ratios than non-AM plants and could allocate more carbon to synthesize glycyrrhizin and liquiritin, the main carbon-based secondary metabolites in liquorice plants. These results suggested that AM symbiosis might cause the plant to reallocate carbon to regulate secondary metabolism. Moreover, improved plant P nutrition by AM symbiosis also has been thought to positively influence the biosynthesis of terpenoids (Kapoor et al. 2017). Phosphorus could stimulate terpenoid biosynthesis by augmenting the accumulation of pyrophosphate compounds, including isopentenyl pyrophosphate (IPP) and dimethylallylpyrophosphate (DMAPP), the most important precursors of terpenoid biosynthesis, that contain high-energy phosphate bonds (Kapoor et al. 2017). Phosphorus also is involved in the biosynthesis of other precursors, including acetyl-CoA, ATP, and NADPH in the MVA pathway and glyceraldehyde phosphate and pyruvate in the methylerythritol phosphate (MEP) pathway, that are essential for terpenoid biosynthesis. Additionally, the increased accumulation of an individual secondary metabolite could be attributed to increase of substrates involved in the biosynthesis and/or up-regulation of genes encoding metabolite synthases (Shrivastava et al. 2015). In the present study, AM inoculation markedly up-regulated the expression of glycyrrhizin biosynthesis genes SQS1, β-AS, CYP88D6, and CYP72A154, and liquiritin biosynthesis gene CHS, which were consistent with the glycyrrhizin and liquiritin accumulation patterns. It is interesting to note that despite the significantly elevated concentrations of glycyrrhizin in AM plants, the expression level of HMGR remained unaltered under all water regimes. The non-responsiveness of the HMGR gene to AM inoculation also was observed in Artemisia annua (Mandal et al. 2015). This might be because AM inoculation changed the upstream pathway of terpenoid biosynthesis from the MVA pathway to the MEP pathway and the latter became the main provider of precursors (IPP/DMAPP) to synthesize terpenoids (Mandal et al. 2015; Kapoor et al. 2017). Actually, the common isoprenoid precursors IPP/DMAPP involved in terpenoid biosynthesis could be influenced by metabolic cross-talk between the plastid MEP pathway and cytosolic MVA pathway (Laule et al. 2003; Rodríguez-Concepción 2006). In addition, the higher expression of downstream genes in the glycyrrhizin biosynthesis pathway of the AM plants might compensate for a lack of the universal precursor IPP (Kapoor et al. 2017).
The interactive effect of drought stress and AM inoculation on glycyrrhizin and liquiritin accumulation
There have been many studies focusing on drought stress or AM inoculation alone influencing plant growth and secondary metabolism (Selmar and Kleinwächter 2013; Zeng et al. 2013; Kapoor et al. 2017), but the potential interaction between drought stress and AM fungi has not been characterized. In this study, we hypothesized that the accumulation of glycyrrhizin and liquiritin in liquorice plants could be regulated by an interaction of drought stress and AM symbiosis. In agreement with our hypothesis, the highest glycyrrhizin concentration was recorded in inoculated liquorice plants under moderate drought stress. Drought stress and AM inoculation independently affected the accumulation of liquiritin, however, which failed to support our hypothesis.
AM hyphae directly link soil and plant roots and serve as the channel of water and nutrient absorption and transportation with arbuscules, a hallmark of the AM symbiosis, transferring nutrients to root cells and, in return, transferring carbohydrates to the AM fungi. The abundance of intact arbuscules could reflect the effectiveness of the AM symbiosis (Gianinazzi-Pearson 1996; Helber et al. 2011). As reported, drought stress can induce arbuscule collapse (Sun et al. 2017), which could lead to malfunction of the symbiosis. Furthermore, drought stress also could directly decrease the mobility of soil mineral nutrients and reduce P uptake both by roots and by AM hyphae (He and Dijkstra 2014; Kapoor et al. 2017). As has been demonstrated, improved P nutrition by the AM symbiosis contributed to biosynthesis of terpenoids (Kapoor et al. 2017); therefore, drought stress can potentially counteract the positive mycorrhizal effects on secondary metabolite accumulation in liquorice through negative effects on AM symbiosis development and effectiveness in improving plant P nutrition.
It has been well demonstrated that AM inoculation can enhance plant drought tolerance and regulate carbon allocation through multiple physiological mechanisms. Extraradical hyphae of AM fungi can enter fine soil pores and thus enhance uptake and transport of water to plants (Neumann et al. 2009). It has recently been demonstrated that two functional aquaporin genes (GintAQPF1 and GintAQPF2) from R. intraradices were responsible for a significant increase in the relative root water content of maize plants (Li et al. 2013), supporting the direct involvement of AM fungi in improving plant water relations under drought stress. AM symbiosis also lowered ABA concentrations in plant roots, which in turn can up-regulate the expression of stress-related genes including proline biosynthesis gene p5cs (Porcel et al. 2004) and superoxide dismutase biosynthesis gene SOD (Li et al. 2016a) to alleviate oxidative stress. In addition, AM inoculation can stimulate root respiration and augment root growth, and thereby enhance plant adaption to drought stress and nutrient limitation (Chen et al. 2010; Zhao et al. 2015). Taken together, such results suggested that the positive effect of drought stress on the accumulation of plant secondary metabolites could potentially be counteracted or even disappear in response to AM inoculation. In this study, severe drought stress exhibited no significant influence on glycyrrhizin concentrations in AM plants.
The plant hormone ABA is a chemical signal involved in plant response to both drought stress and AM inoculation, and it also is involved in the biosynthesis of secondary metabolites, including terpenoids (Mansouri et al. 2009; Yang et al. 2012). Under drought stress, the expression of ABA-biosynthesis gene NCED, encoding a 9-cis-epoxycarotenoid dioxygenase, was significantly up-regulated, resulting in an increase of ABA concentration (Aroca et al. 2008; Ruiz-Lozano et al. 2016), which would induce stomatal closure to reduce water loss and increase ROS accumulation. AM inoculation could down-regulate NCED expression and decrease ABA accumulation, however, and subsequently activate downstream stress-responsive genes and alleviate oxidative stress (Porcel et al. 2004; Chitarra et al. 2016; Li et al. 2016a). The altered ABA levels could further regulate the production of reactive oxygen species and HMGR expression, which ultimately influence the biosynthesis of secondary metabolites (Yang et al. 2012; Sivakumaran et al. 2016). Therefore, drought stress and AM fungi might interact through altering plant ABA balance in the host plant to affect secondary metabolism. Our experimental results indicated that AM inoculation had no significant influence on ABA accumulation under moderate drought stress; however, a marked decrease of ABA was observed under severe drought stress. In contrast, the highest accumulation of glycyrrhizin was achieved in AM plants under moderate drought stress. This inconsistency of ABA and glycyrrhizin accumulation patterns might suggest that glycyrrhizin accumulation is regulated by complex interactions between drought stress and AM symbiosis, but further research is needed to test this hypothesis.
In conclusion, we showed that AM symbiosis not only improved the growth of liquorice plants but also increased the accumulation of glycyrrhizin and liquiritin through several mechanisms (Fig. S1), and this beneficial effect for glycyrrhizin production was most pronounced under moderate drought stress. The experimental results confirmed an interactive effect of drought stress and AM inoculation on secondary metabolite accumulation in liquorice plants. Subsequent research should further investigate the effects of additional AM fungus species besides R. irregularis on the accumulation of secondary metabolites in liquorice plants, and also should test the effectiveness of AM fungi in combination with proper water management in promoting liquorice cultivation under field conditions.
References
Al-Karaki G, McMichael B, Zak J (2004) Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza 14:263–269
Aquil S, Husaini AM, Abdin MZ, Rather GM (2009) Overexpression of the HMG-CoA reductase gene leads to enhanced artemisinin biosynthesis in transgenic Artemisia annua plants. Planta Med 75:1453–1458
Aroca R, Vernieri P, Ruiz-Lozano JM (2008) Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J Exp Bot 59:2029–2041
Babikova Z, Gilbert L, Bruce T, Dewhirst SY, Pickett JA, Johnson D (2014) Arbuscular mycorrhizal fungi and aphids interact by changing host plant quality and volatile emission. Funct Ecol 28:375–385
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Box GEP, Cox DR (1964) An analysis of transformations. J R Stat Soc Ser B Methodol 2:211–252
Bryant JP, Chapin FS, Klein DR (1983) Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40:357–368
Chappell J, Wolf F, Proulx J, Cuellar R, Saunders C (1995) Is the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme a reductase a rate-limiting step for isoprenoid biosynthesis in plants? Plant Physiol 109:1337–1343
Chen MM, Yin HB, O’Connor P, Wang YS, Zhu YG (2010) C: N: P stoichiometry and specific growth rate of clover colonized by arbuscular mycorrhizal fungi. Plant Soil 326:21–29
Chen ML, Yang G, Sheng Y, Li PY, Qiu HY, Zhou XT, Huang LQ, Chao Z (2017) Glomus mosseae inoculation improves the root system architecture, photosynthetic efficiency and flavonoids accumulation of liquorice under nutrient stress. Front Plant Sci 8:931
Chitarra W, Pagliarani C, Maserti B, Lumini E, Siciliano I, Cascone P, Schubert A, Gambino G, Balestrini R, Guerrieri E (2016) Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol 171:1009–1023
Dai ZB, Cui GH, Zhou SF, Zhang XA, Huang LQ (2011) Cloning and characterization of a novel 3-hydroxy-3-methylglutaryl coenzyme A reductase gene from Salvia miltiorrhiza involved in diterpenoid tanshinone accumulation. J Plant Physiol 168:148–157
de Abreu IN, Mazzafera P (2005) Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol Biochem 43:241–248
Elser JJ, Dobberfuhl DR, MacKay NA, Schampel JH (1996) Organism size, life history, and N: P stoichiometry. Bioscience 46:674–684
Enfissi E, Fraser PD, Lois LM, Boronat A, Schuch W, Bramley PM (2005) Metabolic engineering of the mevalonate and non-mevalonate isopentenyl diphosphate-forming pathways for the production of health-promoting isoprenoids in tomato. Plant Biotechnol J 3:17–27
Gianinazzi-Pearson V (1996) Plant cell responses to arbuscular mycorrhizal fungi: getting to the roots of the symbiosis. Plant Cell 8:1871–1883
Golldack D, Li C, Mohan H, Probst N (2014) Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front Plant Sci 5:151
Gorelick J, Bernstein N (2014) Elicitation: an underutilized tool for the development of medicinal plants as a source for therapeutic secondary metabolites. Adv Agron 124:201–230
Hamilton JG, Zangerl AR, DeLucia EH, Berenbaum MR (2001) The carbon-nutrient balance hypothesis: its rise and fall. Ecol Lett 4:86–95
Harker M, Holmberg N, Clayton JC, Gibbard CL, Wallace AD, Rawlins S, Hellyer SA, Lanot A, Safford R (2003) Enhancement of seed phytosterol levels by expression of an N-terminal truncated Hevea brasiliensis (rubber tree) 3-hydroxy-3-methylglutaryl-CoA reductase. Plant Biotechnol J 1:113–121
Hayashi H (2009) Molecular biology of secondary metabolism: case study for Glycyrrhiza plants. In: Recent Advances in Plant Biotechnology. Springer, Boston, pp 89–103
Hayashi H, Sudo H (2009) Economic importance of licorice. Plant Biotechnol 26:101–104
He MZ, Dijkstra FA (2014) Drought effect on plant nitrogen and phosphorus: a meta-analysis. New Phytol 204:924–931
Helber N, Wippel K, Sauer N, Schaarschmidt S, Hause B, Requena N (2011) A versatile monosaccharide transporter that operates in the arbuscular mycorrhizal fungus Glomus sp is crucial for the symbiotic relationship with plants. Plant Cell 23:3812–3823
Hong JH, Seah SW, Xu J (2013) The root of ABA action in environmental stress response. Plant Cell Rep 32:971–983
Javot H, Maurel C (2002) The role of aquaporins in root water uptake. Ann Bot 90:301–313
Kapoor R, Chaudhary V, Bhatnagar AK (2007) Effects of arbuscular mycorrhiza and phosphorus application on artemisinin concentration in Artemisia annua L. Mycorrhiza 17:581–587
Kapoor R, Anand G, Gupta P, Mandal S (2017) Insight into the mechanisms of enhanced production of valuable terpenoids by arbuscular mycorrhiza. Phytochem Rev 16:677–692
Kim YJ, Lee CS (2008) Glycyrrhizin attenuates MPTP neurotoxicity in mouse and MPP+-induced cell death in PC12 cells. Korean J Physiol Pharmacol 12:65–71
Kim YS, Cho JH, Ahn J, Hwang B (2006) Upregulation of isoprenoid pathway genes during enhanced saikosaponin biosynthesis in the hairy roots of Bupleurum falcatum. Mol Cells 22:269–274
Kitagawa I (2002) Licorice root. A natural sweetener and an important ingredient in Chinese medicine. Pure Appl Chem 74:1189–1198
Laule O, Fürholz A, Chang HS, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange BM (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci 100:6866–6871
Li WD, Hou JL, Wang WQ, Tang XM, Liu CL, Xing D (2011) Effect of water deficit on biomass production and accumulation of secondary metabolites in roots of Glycyrrhiza uralensis. Russ J Plant Physiol 58:538–542
Li T, Hu YJ, Hao ZP, Li H, Wang YS, Chen BD (2013) First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol 197:617–630
Li T, Sun YQ, Ruan Y, Xu LJ, Hu YJ, Hao ZP, Zhang X, Li H, Wang YS, Yang LG, Chen BD (2016a) Potential role of D-myo-inositol-3-phosphate synthase and 14-3-3 genes in the crosstalk between Zea mays and Rhizophagus intraradices under drought stress. Mycorrhiza 26:879–893
Li YP, Yu CX, Qiao J, Zang YM, Xiang Y, Ren GX, Wang L, Zhang XY, Liu CS (2016b) Effect of exogenous phytohormones treatment on glycyrrhizic acid accumulation and preliminary exploration of the chemical control network based on glycyrrhizic acid in root of Glycyrrhiza uralensis. Rev Bras 26:490–496
Li JL, Liu SJ, Wang J, Li J, Li JX, Gao WY (2017) Gene expression of glycyrrhizin acid and accumulation of endogenous signaling molecule in Glycyrrhiza uralensis Fisch adventitious roots after Saccharomyces cerevisiae and Meyerozyma guilliermondii applications. Biotechnol Appl Biochem 64:700–711
Liu Y, Liu CS (2012) Study on the spatial and temporal expression of beta-AS gene of Glycyrriza uralensis. J Chin Med Mater 35:528–531
Liu JN, Wu LJ, Wei SL, Xiao X, Sun CX, Jiang P, Song ZB, Wang T, Yu ZL (2007) Effects of arbuscular mycorrhizal fungi on the growth, nutrient uptake and glycyrrhizin production of licorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul 52:29–39
Liu HY, Wang XD, Wang DH, Zou ZR, Liang ZS (2011) Effect of drought stress on growth and accumulation of active constituents in Salvia miltiorrhiza Bunge. Ind Crop Prod 33:84–88
Liu Y, Xu QX, Xi PY, Chen HH, Liu CS (2013) Cloning and characterization of a cDNA coding 3-hydroxy-3-methylglutary CoA reductase involved in glycyrrhizic acid biosynthesis in Glycyrrhiza uralensis. Acta Pharm Sin 48:773–779
López-Ráez JA, Flors V, García JM, Pozo MJ (2010) AM symbiosis alters phenolic acid content in tomato roots. Plant Signal Behav 5:1138–1140
Mandal S, Upadhyay S, Wajid S, Ram M, Jain DC, Singh VP, Abdin MZ, Kapoor R (2015) Arbuscular mycorrhiza increase artemisinin accumulation in Artemisia annua by higher expression of key biosynthesis genes via enhanced jasmonic acid levels. Mycorrhiza 25:345–357
Mansouri H, Asrar Z, Szopa J (2009) Effects of ABA on primary terpenoids and ∆9-tetrahydrocannabinol in Cannabis sativa L. at flowering stage. Plant Growth Regul 58:269–277
Matzek V, Vitousek PM (2009) N: P stoichiometry and protein: RNA ratios in vascular plants: an evaluation of the growth-rate hypothesis. Ecol Lett 12:765–771
Mochida K, Sakurai T, Seki H, Yoshida T, Takahagi K, Sawai S, Uchiyama H, Muranaka T, Saito K (2017) Draft genome assembly and annotation of Glycyrrhiza uralensis, a medicinal legume. Plant J 89:181–194
Nasrollahi V, Mirzaie-asl A, Piri K, Nazeri S, Mehrabi R (2014) The effect of drought stress on the expression of key genes involved in the biosynthesis of triterpenoid saponins in liquorice (Glycyrrhiza glabra). Phytochemistry 103:32–37
Neumann E, Schmid B, Römheld V, George E (2009) Extraradical development and contribution to plant performance of an arbuscular mycorrhizal symbiosis exposed to complete or partial rootzone drying. Mycorrhiza 20:13–23
Nowak M, Kleinwächter M, Manderscheid R, Weigel HJ, Selmar D (2010) Drought stress increases the accumulation of monoterpenes in sage (Salvia officinalis), an effect that is compensated by elevated carbon dioxide concentration. J Appl Bot Food Qual 83:133–136
Okubo K, Yoshiki Y (2000) The role of triterpenoid on reactive oxygen scavenging system: approach from the new chemiluminescence system (XYZ system). Biofactors 13:219–223
Orujei Y, Shabani L, Sharifi-Tehrani M (2013) Induction of glycyrrhizin and total phenolic compound production in licorice by using arbuscular mycorrhizal fungi. Russ J Plant Physiol 60:855–860
Pan Y, Wu LJ, Yu ZL (2006) Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul 49:157–165
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:45
Porcel R, Azcón R, Ruiz-Lozano JM (2004) Evaluation of the role of genes encoding for Δ1-pyrroline-5-carboxylate synthetase (P5CS) during drought stress in arbuscular mycorrhizal Glycine max and Lactuca sativa plants. Physiol Mol Plant Pathol 65:211–221
Rodríguez-Concepción M (2006) Early steps in isoprenoid biosynthesis: multilevel regulation of the supply of common precursors in plant cells. Phytochem Rev 5:1–15
Ruiz-Lozano JM, Aroca R, Zamarreño ÁM, Molina S, Andreo-Jiménez B, Porcel R, García-Mina JM, Ruyter-Spira C, López-Ráez JA (2016) Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ 39:441–452
Schenke D, Böttcher C, Scheel D (2011) Crosstalk between abiotic ultraviolet-B stress and biotic (flg22) stress signalling in Arabidopsis prevents flavonol accumulation in favor of pathogen defence compound production. Plant Cell Environ 34:1849–1864
Seki H, Ohyama K, Sawai S, Mizutani M, Ohnishi T, Sudo H, Akashi T, Aoki T, Saito K, Muranaka T (2008) Licorice β-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc Natl Acad Sci 105:14204–14209
Seki H, Sawai S, Ohyama K, Mizutani M, Ohnishi T, Sudo H, Fukushima EO, Akashi T, Aoki T, Saito K (2011) Triterpene functional genomics in licorice for identification of CYP72A154 involved in the biosynthesis of glycyrrhizin. Plant Cell 23:4112–4123
Selmar D, Kleinwächter M (2013) Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind Crop Prod 42:558–566
Shen ZY, Liu CS, Wang XY (2009) Cloning and characterization of open reading frame encoding beta-amyrin synthase in Glycyrrhiza uralensis. China J Chinese Materia Medica 34:2438–2440
Shrivastava G, Ownley BH, Augé RM, Toler H, Dee M, Vu A, Köllner TG, Chen F (2015) Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis 65:65–74
Sivakumaran A, Akinyemi A, Mandon J, Cristescu SM, Hall MA, Harren FJM, Mur LAJ (2016) ABA suppresses Botrytis cinerea elicited NO production in tomato to influence H2O2 generation and increase host susceptibility. Front Plant Sci 7:709
Smith SE, Smith FA, Jakobsen I (2003) Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol 133:16–20
Sun XG, Shi J, Ding GJ (2017) Combined effects of arbuscular mycorrhiza and drought stress on plant growth and mortality of forage sorghum. Appl Soil Ecol 119:384–391
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Trouvelot A, Kough JL, Gianinazzi-Pearson V (1986) Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de methods d’estimation ayant une signification fonctionnelle. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and genetical aspects of mycorrhizae. INRA-Press, Paris, pp 217–221
Wei SL, Wang WQ, Wang H (2003) Study on licorice resources and their sustainable utilization in center and western area of China. China J Chinese Materia Medica 28:202–206
Welling MT, Liu L, Rose TJ, Waters DLE, Benkendorff K (2016) Arbuscular mycorrhizal fungi: effects on plant terpenoid accumulation. Plant Biol 18:552–562
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5:218–223
Xu GJ, Cai W, Gao W, Liu CS (2016) A novel glucuronosyltransferase has an unprecedented ability to catalyse continuous two-step glucuronosylation of glycyrrhetinic acid to yield glycyrrhizin. New Phytol 212:123–135
Yang DF, Sheng DF, Duan QM, Liang X, Liang ZS, Liu Y (2012) PEG and ABA trigger the burst of reactive oxygen species to increase tanshinone production in Salvia miltiorrhiza hairy roots. J Plant Growth Regul 31:579–587
Zeng Y, Guo LP, Chen BD, Hao ZP, Wang JY, Huang LQ, Yang G, Cui XM, Yang L, Wu ZX (2013) Arbuscular mycorrhizal symbiosis and active ingredients of medicinal plants: current research status and prospectives. Mycorrhiza 23:253–265
Zhang HC, Liu JM, Lu HY, Gao SL (2009) Enhanced flavonoid production in hairy root cultures of Glycyrrhiza uralensis Fisch by combining the over-expression of chalcone isomerase gene with the elicitation treatment. Plant Cell Rep 28:1205–1213
Zhang RQ, Zhu HH, Zhao HQ, Yao Q (2013) Arbuscular mycorrhizal fungal inoculation increases phenolic synthesis in clover roots via hydrogen peroxide, salicylic acid and nitric oxide signaling pathways. J Plant Physiol 170:74–79
Zhang Y, Zhou ZY, Xia PG, Liang ZS, Liu SL, Liu ZL (2016) Expression of key enzyme genes and content of saikosaponin in saikosaponin biosynthesis under drought stress in Bupleurum chinense. China J Chinese Materia Medica 41:643–647
Zhao RX, Guo W, Bi N, Guo JY, Wang LX, Zhao J, Zhang J (2015) Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl Soil Ecol 88:41–49
Acknowledgements
We thank Dr. Yan Zeng from China National Traditional Chinese Medicine Corporation for kindly supplying the seeds of Glycyrrhiza uralensis.
Funding
This study was financially supported by the National Natural Science Foundation of China (project no. 41571250 and 41371264) and National Key Research and Development Program of China (project no. 2016YFC0500702).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 339 kb)
Rights and permissions
About this article
Cite this article
Xie, W., Hao, Z., Zhou, X. et al. Arbuscular mycorrhiza facilitates the accumulation of glycyrrhizin and liquiritin in Glycyrrhiza uralensis under drought stress. Mycorrhiza 28, 285–300 (2018). https://doi.org/10.1007/s00572-018-0827-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-018-0827-y