Abstract
This work examined the effects of exogenously applied abscisic acid (ABA) on the content of chlorophyll, carotenoids, α-tocopherol, squalene, phytosterols, Δ9-tetrahydrocannabinol (THC) concentration, 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) and 1-deoxy-d-xylulose 5-phosphate synthase (DXS) activity in Cannabis sativa L. at flowering stage. Treatment with 1 and 10 mg l−1 ABA significantly decreased the contents of chlorophyll, carotenoids, squalene, stigmasterol, sitosterol, and HMGR activity in female cannabis plants. ABA caused an increase in α-tocopherol content and DXS activity in leaves and THC concentration in leaves and flowers of female plants. Chlorophyll content decreased with 10 mg l−1 ABA in male plants. Treatment with 1 and 10 mg l−1 ABA showed a decrease in HMGR activity, squalene, stigmasterol, and sitosterol contents in leaves but an increase in THC content of leaves and flowers in male plants. The results suggest that ABA can induce biosynthesis of 2-methyl-d-erythritol-4-phosphate (MEP) pathway secondary metabolites accumulation (α-tocopherol and THC) and down regulated biosynthesis of terpenoid primary metabolites from MEP and mevalonate (MVA) pathways (chlorophyll, carotenoids, and phytosterols) in Cannabis sativa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Terpenoids constitute the largest family of natural plant products with over 30,000 members (Dewick 2002). Members of this diverse group of natural products are found in all organisms. In higher plants, isoprenoids participate in a wide variety of biological functions such as photosynthesis, respiration, growth, cell cycle control, plant defense, and adaptation to environmental conditions (Estévez et al. 2001). Specific examples include photosynthetic pigments (chlorophylls and carotenoids), hormones (abscisic acid, gibberellins, cytokinins, and brassinosteroids), a side chain of the electron transporter (plastoquinone), structural components of membranes (phytosterols), and antimicrobial agents (phytoalexins; Estévez et al. 2001). In higher plants, the biosynthesis of terpenoids involves common building block isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP; Lichtenthaler 1999). Isoprenoids are derived from a common precursor, IPP. The two biosynthetic pathways that lead to synthesis of IPP are the cytosolic mevalonate (MVA) and the plastidic 2-methyl-d-erythritol-4-phosphate (MEP) pathways (Lichtenthaler et al. 2002; Rodriguez-Concepcion 2002; Kang et al. 2006). The cytosolic pathway, which starts from acetyl-CoA and proceeds through the intermediate MVA, provides the precursors for sterols and ubiquinone. The plastidic MEP pathway, which involves a condensation of pyruvate and glyceraldehyde-3-phosphate via 1-deoxy-d-xylulose 5-phosphate as a first intermediate, is used for the synthesis of isoprene, carotenoids, abscisic acid, and the side chains of chlorophylls and plastoquinone (Laule et al. 2003). Since terpenoids have different functions as primary metabolites (in growth and development) and secondary metabolites (in plant response to environment), therefore, it is important to understand how they interact with plant growth regulators.
ABA has been shown to regulate many aspects of plant growth and development including embryo maturation, seed dormancy, germination, cell division and elongation, and responses to environmental stresses such as drought, salinity, cold, pathogen attack, and UV radiation across membranes (Tsai et al. 1997). Although ABA has historically been thought of as a growth inhibitor, young tissues have high ABA levels, and ABA-deficient mutant plants are severely stunted because their ability to reduce transpiration and establish turgor is impaired (Tsai et al. 1997; Ahmed et al. 2006). In spite of the economic significance of the terpenoids and their many essential functions, relatively there is a little information about terpenpoid metabolism and its regulation in plants.
Cannabis is a dioecious plant that has been a source of fiber, food, oil, and medicine. Cannabinoids represent a distinctive class of compounds found only in Cannabis sativa. These C21 compounds belong to the chemical class of natural terpenophenols. Δ9-tetrahycannabinol (THC) and cannabidiol (CBD) are the most important of these compounds. The experiment with labeling patterns show that the cannabinoids are derived entirely or predominantly (>98%) from MEP pathway (Fellermeier et al. 2001). They are produced by glandular trichomes that occur on most aerial surfaces of the plant (Hilling 2004). Cannabis is used in modern medicine for the treatment of emesis in chemotherapy as anti emetics. Cannabinoids appear to have therapeutic value as antispasmodics, analgesics, anti-emetics, and appetite stimulants and may also have potential in the treatment of epilepsy, glaucoma, and asthma (Guzman 2003; Howlett et al. 2004).
To understand the role of ABA in regulation of two terpenoid biosynthesis pathways, we studied the changes of the main end products of these pathways and THC under ABA treatment on cannabis plant. The other aim of this study was the comparison of these compounds content between the male and female cannabis plants and in response to ABA treatment.
Materials and methods
Plant material
The seeds of C. sativa L. (with geographical source from Iran) were sown in pots (15 cm, i.d soil-leaf mold-perlit = 2:1:1)and cultivated in a phytotron (25°c 14 h light, 10 h dark). The plants were fertilized regularly with a Hoglanďs nutrient solution every week to the productive stage.
ABA treatment of plants
Female flowering plants were treated when glandular trichomes on bracts were globose and resinous and male flowering plants were treated when flowers bloom and pollen grains were visible. The similar size male and female plants were subjected to ABA (±cis/trans ABA; sigma) treatment by spraying the whole plants with 1 and 10 mg l−1 ABA solutions and tap water as a control, until the solution started dripping. The treatment took place with three times spraying at 24 h intervals. The plants were harvested 1 day after the treatment.
Chlorophyll and carotenoid determination
Chlorophyll and carotenoids were extracted from leaves with 95% ethanol and quantified by measuring the absorbance at 664, 648 and 470 nm as described by Lichtenthaler (1987).
Quantitative analysis of squalene and phytosterols by GC
Quantitative analysis of squalene and phytosterols (campesterol, β-sitosterol and stigmasterol) was performed by GC. Freeze-dried leaves (500 mg) were extracted with ethyl acetate at 100 rpm on a gyratory shaker (20 ml, twice, 25°C for 6 h). The acidic compounds were removed with aqueous 5% KOH (10 ml, thrice) followed by the removal of the basic compounds with aqueous 5% HCl (10 ml, twice). The organic fraction was washed with water (10 ml, twice) and then dried with anhydrous sodium sulfate. The solvent was evaporated and the residue was dissolved in hexane (2 ml) and then centrifuged for 10 min at 6,000 rpm to remove the suspended particles.
Chromatography was performed with Agilent technologies (Wilmington, DE, USA) equipment including a 7,683 automated sample-injection system, a split/split less injector, a 30 m × 320 μm I. D., 0.25 μm film thickness HP-5 fuse-silica capillary column coated with 5% phenymethyl siloxane (J&W Scientific, Folsom, CA, USA) and a flame ionization detection (FID) controlled by the Agilent Chemstation software. The oven temperature was held at 240°C for 10 min, then raised to 260°C at 2°C min−1 and then held at that temperature for 30 min; injection port 270°C; detector 300°C; split ratio 15:1, injection volume 1 μl; nitrogen carrier gas 1 ml min−1, hydrogen 30 ml min−1 and detector flow of make-up gas (nitrogen) 400 ml min−1 in the constant make-up flow mode. Stigmasterol, campesterol, β-sitosterol, and squalene standards were obtained from Sigma (USA).
Tocopherol extraction and measurement
Tocopherols were extracted basically as described for cereal seeds by Panfili et al. (2003), by grinding and homogenizing 25 mg of freeze–dried leaf material in 500 μl 100% methanol. After 20 min of incubation at 30°C, the samples were centrifuged at 14,000 rpm for 5 min, the supernatant was transferred to new tubes, and the pellet was re-extracted twice with 250 μl 100% methanol at 30°C for 30 min, pooling all supernatants.
Chromatographic conditions
Ultra-performance liquid chromatography (UPLC) analysis was performed with a Waters Acquity ultraperformance liquid chromatograph equipped with a PDA detector. An Acquity UPLC BEH C18 column (2.1 mm × 150 mm, 1.7 μm particles) was used for separation. Methanol (100%) was used as mobile phase; the flow rate was 0.48 ml min−1. PDA detector was accomplished at 290 nm for α-tocopherol. Injection volume was 10 μl. The identities of α-tocopherol in plant samples were determined by comparing with (±)-α-tocopherol standard was purchased from Sigma (Germany).
Sample collection for cannabinoids measurement
Female and male samples were collected as flowering tops and leaves separately. Flowering top samples included bracts and small leaves (small, palmately compound leaves-surrounding the flowers) in females and flowers and pollen grains in males. Mature leaves (with ~7 cm length) from male and female were used for cannabinoid measurement. All of samples were dried in room temperature in darkness.
Cannabinoids extraction and measurement
Sample material (50 mg) was placed in a test tube with 1 ml chloroform. Sonication was applied for 15 min. After filtration, the solvent was evaporated to dryness and the residue was dissolved in 0.5 ml methanol.
Chromatographic conditions
The apparatus and column conditions were the same as those of α-tocopherol analysis. The mobile phase was an acetonitrile-water gradient from 70:30 to 100:0 in 5 min, stay 100:0 in 1 min, return to 30:70 in 1 min (flow 0.4 ml min−l). Buffer (0.05% TFA resulting in a pH of 3) was added to both solvents to eliminate the tailing of phenolic compounds. PDA detector was accomplished at 230 nm for THC and CBD. Injection volume was 7 μl. Cannabinoid peaks were identified by cannabinoids standards (THC and CBD) that were a generous gift from Pr. Jun Szopa Wroclaw University, Wroclaw, Poland.
Enzyme assays
Enzymes assayed in this work were extracted from the fresh leaves with an extraction buffer of 50 mM Tris–HCl, 10 mM β-mercaptoethanol, 1% (w/v) polyvinylpyrrolidone (PVP), and pH 7.5. The leaves were ground in the extraction buffer (1 gfw ml−1) for 5 min with a pestle and mortar on ice, followed by centrifugation at 14,000 rpm and 4°C for 30 min to obtain a solid-free extract. The content of protein was determined by the Bradford (1976) using bovine serum albumin as a standard.
The activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) was determined by the method of Toroser and Huber (1998). The enzyme extract was added (ca. 50 mg protein per ml) to a 50 mM Tris–HCl assay buffer (pH 7.0) containing 0.3 mM HMG-CoA (Sigma, Cat. H6132), 0.2 mM NADPH, and 4 mM dithiothreitol. NADPH oxidation in the reaction solution was monitored at 25°C by the decreasing absorbance at 340 nm, against the solution free of HMG-CoA as a blank. One HMGR enzyme unit is equivalent to the oxidation of 1 mM of NADPH per minute.
The activity of 1-deoxy-d-xylulose 5-phosphate synthase was determined by the fluorometric method of Querol et al. (2001) which is based on the reaction of 1-deoxy-d-xylulose 5-phosphate with 3, 5-diaminobenzoic acid in an acidic medium to form a highly fluorescing quinaldine derivative. The reaction mixture contained 40 mM Tris–HCl (pH 7.5), 2.5 mM MgCl2, 5 mM β-mercaptoethanol, 1 mM thiamin diphosphate, 10 mM sodium pyruvate, 20 mM DL-glyceraldehyde 3-phosphate, and the enzyme extract (ca. 50 mg protein per ml reaction solution). The reaction solution was maintained at 37°C for 1 h, stopped by heating at 80°C for 5 min, and then spun down at 13,000 rpm for 5 min to remove the denatured proteins. The supernatant was mixed with 1 ml of 10 mM 3, 5-diaminobenzoic acid in 5 M phosphoric acid, and then heated in a boiling water bath for 15 min. The fluorescence intensity of the reaction product, which is proportional to DXS activity, was determined with a Shimadzu RF 2000 fluorescence spectrophotometer at 396 nm excitation and 510 nm emission.
Data analysis
The results presented were the mean of three replicate. Means were analyzed by one-way analysis of variance (ANOVA; SPSS 15.0). Statistical significance of difference between means was calculated using Duncan test at P < 0.05 level.
Results
Effect of ABA on levels of chlorophyll, carotenoids and α-tocopherol
Many isoprenoids are formed in plastids via the IPP produced by the MEP pathway. In order to determine the impact of ABA treatment on isoprenoid content, the quantities of plastidic isoprenoids such as chlorophylls, tocopherols, carotenoids were measured in the treated plants and compared to the control plants. These isoprenoids were chosen, in part, because they are formed from three pathways that diverge from the common plastidic IPP. The male individuals showed a slightly decrease in the contents of chlorophylls (a, b and total) at treatment of 10 mg l−1 ABA (Table 1; Fig. 1a). However, female plants submitted to ABA treatment showed a decreased in both level of ABA (1 and 10 mg l−1; Fig. 1b). Experimental evidence has clearly demonstrated that carotenoids are one of the major products derived from the MEP pathway. Treatment with ABA did not affect the contents of carotenoids in male plants (Fig. 2a). In female plants, ABA caused a significant decrease in carotenoids content in compared with the control plants (Fig. 2a).
The data analysis shows the level of α-tocopherol remained almost unchanged with ABA treatment in males (Fig. 2b), but the content of α-tocopherol increased in females. The extent of increase was higher in 1 mg l−1 ABA than 10 mg l−1.
Effect of ABA on DXS and HMGR activity
In this study, effects of ABA on key enzymes activity of terpenoid biosynthetic pathways (DXS and HMGR) were investigated. As shown in Fig. 3, some differences were obvious between the two groups of plants with different sexes. The control male plants had slightly greater DXS activity, as compared to females. No induction of DXS activity was observed in male plants when they were treated with ABA. Whereas, an increasing was detected over DXS activity in female plants which was linear with increasing ABA concentration. There was no difference in HMGR activity between the control plants in male and female sexes. HMGR activity in leaves was reduced by ABA treatment on both sexes (Fig. 3). There was not a significant difference between two levels of ABA treatment.
Effect of ABA on phytosterols
The mature leaves of male and female cannabis plant were used to determine the effect of ABA on squalene (biosynthetic precursor to all steroids), campesterol, stigmasterol and β-sitosterol (the most representative phytosterols of the MVA-pathway). Approximately, the squalene content of male plants was two times more than that of the female plants. The level of the squalene was reduced on both sexes of cannabis by ABA treatment (Fig. 4). The most decrease was observed in the high level of ABA (10 mg l−1) in male plants. As in other plants, β-sitosterol was the main phytosterol in cannabis. No significant difference was observed in campesterol content of male and female plants (Fig. 5). Treatment with ABA caused a decrease in level of stigmasterol and β-sitosterol in plants. Differences between treatments were not significant except β-sitosterol content in male plants.
Effect of ABA on THC in leaves and flowers
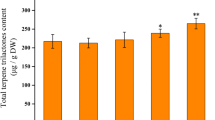
In this strain the THC concentration was dominant over CBD. CBD was found only in the leaves of the control plants in noticeable content (data not show). Therefore, this strain belongs to drug-type. A comparison between male and female plants showed that females had more amounts of THC; especially in flowers (Fig. 6). The treatment with ABA led to a significant increase in the levels of THC in leaves and flowers of both sexes. In male plants, ABA was more effective on the increasing of THC content in leaves when they were compared with flowers.
Discussion
The phytyl (C20) conjugates such as chlorophylls and tocopherols, and carotenoids (C40) are produced by MEP pathway in chloroplast. In order to investigation the ABA effects on the MEP pathway regulation, we measured the main end products of this pathway in ABA-treated plants. The results showed that ABA treatment led to a decline in chlorophyll content. A decrease in chlorophyll content has also detected in barley, wheat, and soybeans plants treated with ABA (Samet et al. 1980; Cuello et al. 1995; Xie et al. 2004).
In the present study ABA treatment caused a decrease in the carotenoid content in female plants. Similarly a significant decrease in β-carotene has been reported in Helianthus annus with ABA treatment (Keleo and Ünyayar 2004). In contrast, many researchers have shown ABA treatment caused an increased carotenoid content in plants (Zhou et al. 2005; Agarwal et al. 2005).
The treatment of ABA led to an increase in the level of α-tocopherol in the leaves of female cannabis plants. Consistent with our results a strong positive correlation between ABA and α-tocopherol level was observed in Cistus creticus and maize seedlings (Jiang and Zhang 2001; Munné-Bosch et al. 2009). Also many studies showed that drought increased tocopherol concentrations in plants, the condition that is companied with increased ABA (Munne-Bosch and Alegre 2000; Herbinger et al. 2002). As shown in Fig. 2b, the female plants treated with 10 mg l−1 ABA had lower α-tocopherol content than those of treated with 1 mg l−1 ABA. The observed decrease in the content of α-tocopherol could reflect the consumption of the antioxidant due to an increase in active oxygen species production by the high levels of ABA concentration. Because endogenous α-tocopherol levels severely affected by the extent of its degradation and recycling under stress (Simontacchi et al. 1993; Munne-Bosch 2005).
In spite of the importance of DXS activity in plastid terpenoids biosynthesis, there is not any study about the effects of phytohormones on DXS activity. The reported results support a limiting role of DXS for the production of MEP-derived isoprenoids in all systems and a regulatory role for the enzyme in controlling flux through the MEP pathway in plants (Rodriguez-Concepcion 2006). The results in this study indicated ABA treatment can induce DXS activity in female cannabis plants. However, this increase in activity was not accompanied with the increase in the entire MEP pathway end products. The results showed an increase in α-tocopherol and THC content and a decrease in chlorophyll and carotenoids contents. Furthermore, the results support that at least in female plants several enzymes can share a control over the flux of the MEP pathway, with different enzyme exhibiting different degrees of control. However, in male plants, there was a different situation and we almost found a link between DXS activity and chlorophyll, carotenoids and α-tocopherol contents. ABA treatment did not affect neither on DXS activity nor on carotenoids and α-tocopherol contents only a slightly decrease was observed in chlorophyll content with 10 mg l−1 ABA.
The enzyme HMGR is a key enzyme which controls the MVA pathway in plants (Goldstein and Brown 1990; Gondet et al. 1992). The results of this study showed that exogenous ABA decreases HMGR activity. Consistent with our results, it has reported that HMGR specific activity is inversely correlated with endogenous ABA level in maize endosperm during seed development and in avocado fruit growth (Moore and Oishi 1994; Cowan et al. 1997). Also Moore and Oishi (1993) have shown that exogenous ABA inhibits cytosolic HMGR activity in maize roots. HMGR activity is decreased in maize and cotton roots under condition of salt stress, conditions under which ABA levels have been shown to increase (Kefu et al. 1991).
There is no information about the effect of plant growth regulators on phytosterol biosynthesis. In our study male plants have more free sterol and squalene than female plants. The data suggest that exogenous ABA caused a decrease in squalene, β-sitosterol and stigmasterol in Cannabis plants. Free sterols are found predominantly in cell membranes and are tough to contribute to the proper functioning of membranes by controlling the fluidity characteristics of the membrane (Devarenne et al. 2002). It is well established that high levels ABA disrupt the membrane lipid integrity and that phytosterols inhibit this action. It is possible that ABA affects on membrane permeability by changing free sterol content (Mitchell and Cowan 2003). On the other hand, it has been shown that up-regulation of HMGR enzyme activity in tobacco dose lead to a marked increase in the accumulation of total sterols, which is consistent with the idea that HMGR activity is rate-determining for sterol synthesis (Chappell et al. 1995). Therefore ABA can change phytosterol levels by changing of HMGR enzyme activity.
This is the first report from the influence of a plant hormone on cannabinoids content in C. sativa. ABA caused an increase in THC content in leaves and flowers. The effect of ABA on increasing of THC can be indirectly by changing DXS activity. Because, cannabinoids are biosynthesized from MEP pathway (Fellermeier et al. 2001). THC usually accumulates at a quite low level in the fresh leaves of C. sativa and is considered to be derived artificially from the acidic cannabinoid tetrahydrocannabinolic acid (THCA) by non-enzymatic decarboxylation during storage or heating. THCA is biosynthesized from cannabigerolic acid (CBGA). This reaction depends on molecular oxygen and produces hydrogen peroxide and THCA at a molar ratio of 1:1 (CBGA + O2→THCA + H2O2; Sirikantaramas et al. 2004). Since ABA causes an oxidative stress in plant cells (Jiang and Zhang 2001) probably, THC biosynthesis reaction can act as a defence mechanism by using molecular oxygen. On the other hand, the high concentration of ABA can inhibit this reaction because of high H2O2 production, as we found a decrease in THC content in the high level of ABA in female plants.
Our experimental results, separately from ABA treatment, confirmed this hypothesis that physiological trade-offs between growth and defence are particularly obvious in diocious plants where males allocate more resources to the growth and females to the high concentration of secondary metabolites (Massei et al. 2006). In this study the males had more amounts of primary terpenoid metabolites such as chlorophyll, squalene, phytosterols and less amounts of secondary metabolite (THC). Also, comparing male and female plants under ABA treatment showed, in some parameters, they responded to ABA differently. It may be due to having different concentrations of phytohormones in the flowering stage whose interaction causes various responses to ABA treatment in plants.
In conclusion, the pattern of terpenoids changes in response to ABA treatment showed that apparently ABA can reduce primary terpenoids biosynthesis and induce secondary terpenoids biosynthesis in the C. sativa L at flowering stage. But in male plants, primary terpenoids from MEP pathway (carotenoids, α-tocopherol, and chlorophylls) and DXS activity did not show noticeable response to ABA treatment. However, there is still a lack of information about the secondary terpenoids metabolites induced by ABA. To understand the role of ABA in terpenoid biosynthesis regulation we need to do further investigations.
Abbreviations
- ABA:
-
Abscisic acid
- THC:
-
Δ9-Tetrahydrocannabinol
- CBD:
-
Cannabidiol
- HMGR:
-
3-Hydroxy-3-methylglutaryl coenzyme A reductase
- DXS:
-
1-Deoxy-d-xylulose 5-phosphate synthase
- MEP:
-
2-Methyl-d-erythritol-4-phosphate
- MVA:
-
Mevalonate
References
Agarwal S, Sairam RK, Srivastava GC, Meena RC (2005) Changes in antioxidant enzymes activity and oxidative stress by abscisic acid and salicylic acid in wheat genotypes. Biol Plant 49(4):541–550. doi:10.1007/s10535-005-0048-z
Ahmed S, Nawata E, Sakuratani T (2006) Changes of endogenous ABA and ACC, and their correlations to photosynthesis and water relations in mungbean (Vigna radiata L. Wilczak cv. KPS1) during waterlogging. Environ Exp Bot 57:278–284. doi:10.1016/j.envexpbot.2005.06.006
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Chem 72:248–254
Chappell J, Wolf’ F, Proulx J, Cuellar R, Saunders C (1995) Is the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase a rate-limiting step for isoprenoid biosynthesis in plans? Plant Physiol 109:1337–1343
Cowan AK, Moore-Cordon CS, Bertling L, Wolstenholme BN (1997) Metabolic control of avocado fruit growth: isoprenoid growth regulators and the reaction catalyzed by 3-hydroxy-3-methylglutaryl catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase. Plant Physiol 114:511–518
Cuello J, Quiles MJ, Rosauro J, Sabate B (1995) Effects of growth regulators and light on chloroplasts NAD(P)H dehydrogenase activities of senescent barley leaves. Plant Growth Regul 17:225–232. doi:10.1007/BF00024730
Devarenne TP, Ghosh A, Chappell J (2002) Regulation of squalene synthase, a key enzyme of sterol biosynthesis, in tobacco. Plant Physiol 129:1095–1106. doi:10.1104/pp.001438
Dewick PM (2002) The biosynthesis of C5–C25 terpenoid compounds. Nat Prod Rep 19:181–222. doi:10.1039/b002685i
Estévez JM, Cantero A, Reindl A, Reichler S, León P (2001) 1-Deoxy-d-xylulose 5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem 276:22901–22909. doi:10.1074/jbc.M100854200
Fellermeier M, Eisenreich W, Bacher A, Zenk MH (2001) Biosynthesis of cannabinoids: incorporation experiments with 13C-labeled glucoses. Eur J Biochem 268:1596–1604. doi:10.1046/j.1432-1327.2001.02030.x
Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343:425–430. doi:10.1038/343425a0
Gondet I, Weber T, Maillot-Vernier P, Benveniste P, Bach TJ (1992) Regulatory role of microsomal 3-hydroxy-3-methylglutaryl coenzyme A reductase in a tobacco mutant that overproduces sterols. Biochem Biophys Res Commun 186:888–893. doi:10.1016/0006-291X(92)90829-A
Guzman M (2003) Cannabinoids: potential anticancer agents. Natl Rev 3:745–755
Herbinger K, Tausz M, Wonisch A, Soja G, Sorger A, Grill D (2002) Complex interactive effects of drought and ozone stress on the antioxidant defence systems of two wheat cultivars. Plant Physiol Biochem 40:691–696. doi:10.1016/S0981-9428(02)01410-9
Hilling KW (2004) A chemotaxonomic analysis of terpenoid variation in cannabis. Biochem Syst Ecol 32:875–891. doi:10.1016/j.bse.2004.04.004
Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LY (2004) Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacol 47:345–358. doi:10.1016/j.neuropharm.2004.07.030
Jiang M, Zhang J (2001) Effect of Abscisic acid on active oxygen species, antioxidative defence system, and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42(11):1265–1273. doi:10.1093/pcp/pce162
Kang SM, Min JY, Kim YD, Park DJ, Jung HN, Karigar C et al (2006) Effect of supplementing terpenoid biosynthetic precursors on the accumulation of bilobalide and ginkgolides in Ginkgo biloba cell cultures. J Biotechnol 123:85–92
Kefu Z, Munns R, King RW (1991) Abscisic acid levels in NaCl treated barley, cotton, and saltbush. Aust J Plant Physiol 18:17–24
Keleo Y, Ünyayar S (2004) Responses of antioxidant defense system of Helianthus annuus to abscisic acid treatment under drought and water logging. Acta Physiol Plant 26(2):149–156. doi:10.1007/s11738-004-0004-0
Laule O, Furholz A, Chang HS, Zhu T, Wang X, Heifetz PB et al (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Plant Biol 100:6866–6871
Lichtenthaler HK (1987) Chlorophylls and caretenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. doi:10.1016/0076-6879(87)48036-1
Lichtenthaler HK (1999) The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50:47–65. doi:10.1146/annurev.arplant.50.1.47
Lichtenthaler HK, Schwender J, Disch A, Rohmer M (2002) Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. Planta 216:271–274
Massei G, Watkins R, Hartley SE (2006) Sex-related growth and secondary compounds in Juniperus oxycedrus macrocarpa. Acta Oecologica 29:135–140
Mitchell DI, Cowan AK (2003) Mevastatin-induced inhibition of cell growth in avocado suspension cultures and reversal by isoprenoid compounds. Afr J Biotechnol 2(9):264–270
Moore KB, Oishi KK (1993) Characterization of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity during maize seed development, germination, and seedling emergence. Plant Physiol 101:485–491
Moore KB, Oishi KK (1994) 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity in the endosperm of maize vivipary Mutants. Plant Physiol 105:119–125
Munné-Bosch S, Falara V, Pateraki I, López-Carbonell M, Jana CJ, Kanellis AK (2009) Physiological and molecular responses of the isoprenoid biosynthetic pathway in a drought-resistant Mediterranean shrub, Cistus creticus exposed to water deficit. J Plant Physiol 166:136–145. doi:10.1016/j.jplph.2008.02.011
Munne-Bosch S (2005) The role of α-tocopherol in plant stress tolerance. J Plant Physiol 162(7):743–748. doi:10.1016/j.jplph.2005.04.022
Munne-Bosch S, Alegre L (2000) Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta 210:925–931. doi:10.1007/s004250050699
Panfili G, Fratianni A, Irano M (2003) Normal phase high-performance liquid method for the determination of tocopherols and tocotrienols in cereals. J Agric Food Chem 51:3940–3944. doi:10.1021/jf030009v
Querol J, Besumbes O, Lois LM, Boronat A, Imperial S (2001) A fluorometric assay for the determination of 1-deoxy-d-xylulose 5-phosphate synthase activity. Anal Biochem 296:101–105. doi:10.1006/abio.2001.5234
Rodriguez-Concepcion M (2002) Elucidation of the methylerythritol phosphate pathway for isoprenoids biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol 130:1079–1089. doi:10.1104/pp.007138
Rodriguez-Concepcion M (2006) Early steps in isoprenoid biosynthesis: multilevel regulation of the supply of common precursors in plant cells. Phytochem Rev 5:1–15. doi:10.1007/s11101-005-3130-4
Samet JS, Thomas R, Sinclair TR (1980) Leaf senescence and abscisic acid in leaves of field-grown soybean. Plant Physiol 66:1164–1168. doi:10.1104/pp.66.6.1164
Simontacchi M, Caro A, Fraga CG, Puntarulo S (1993) Oxidative stress affects α-tocopherol content in soybean embryonic axes upon imbibition and following germination. Plant Physiol 103:949–953
Sirikantaramas S, Morimoto S, Shoyama Y, Ishikawa Y, Yoshiko Y, Shoyama Y et al (2004) The gene controlling marijuana psychoactivity molecular cloning and heterologous expression of tetrahydrocannabinolic acid synthase frome Cannabis sativa L. J Biol Chem 279:39767–39774. doi:10.1074/jbc.M403693200
Toroser D, Huber SC (1998) 3-Hydroxy-3-methylglutaryl-coenzyme A reductase kinase and sucrose–phosphate synthase kinase activities in cauliflower florets: Ca2+ dependence and substrate specificities. Arch Biochem Biophys 355:291–300. doi:10.1006/abbi.1998.0740
Tsai FY, Chi C, HueiKao C (1997) A comparative study of the effects of abscisic acid and methyl jasmonate on seedling growth of rice. Plant Growth Regul 21:37–42. doi:10.1023/A:1005761804191
Xie Z, Jiang D, Dai T, Jing Q, Cao W (2004) Effects of exogenous ABA and cytokinin on leaf photosynthesis and grain protein accumulation in wheat ears cultured in vitro. Plant Growth Regul 44:25–32. doi:10.1007/s10725-004-1880-4
Zhou B, Guo Z, Liu Z (2005) Effects of abscisic acid on antioxidant systems of Stylosanthes guianensis (Aublet) Sw. under chilling stress. Crop Sci 45:599–605
Acknowledgments
We would like to express our sincere gratitude to Dr. Anna Kulma for her outstanding contributions and her help with UPLC analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mansouri, H., Asrar, Z. & Szopa, J. Effects of ABA on primary terpenoids and Δ9-tetrahydrocannabinol in Cannabis sativa L. at flowering stage. Plant Growth Regul 58, 269–277 (2009). https://doi.org/10.1007/s10725-009-9375-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-009-9375-y