Abstract
Salvia miltiorrhiza is one of the most popular traditional Chinese medicinal plants because of its excellent performance in treating coronary heart disease. Tanshinones, a group of active compounds in S. miltiorrhiza, are derived from two biosynthetic pathways: the mevalonate (MVA) pathway in the cytosol and the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway in the plastids. Water stress is well known to stimulate the accumulation of secondary metabolites in plants. Reactive oxygen species (ROS) serve as important secondary messengers in water stress-induced signal transduction pathways. In this study, the effects of polyethylene glycol (PEG) and abscisic acid (ABA) on tanshinone production in S. miltiorrhiza hairy roots were investigated and the roles of ROS in PEG- and ABA-induced tanshinone production were further elucidated. The results showed that contents and yields of four tanshinones in S. miltiorrhiza hairy roots were significantly enhanced by 2 % PEG and 200 μM ABA. Simultaneously, the mRNA levels and activities of two key enzymes (3-hydroxy-3-methylglutaryl coenzyme A reductase and 1-deoxy-D-xylulose 5-phosphate synthase) involved in tanshinone biosynthesis were upregulated. Both PEG and ABA were able to trigger the burst of H2O2 and O2 −. The PEG- and ABA-induced increases of tanshinone production, gene expression, and enzyme activity were all dramatically suppressed by two ROS scavengers, catalase and superoxide dismutase. In addition, ROS treatments resulted in a significant increase in tanshinone production. These results demonstrated that the MVA and MEP pathways were activated by PEG and ABA to stimulate tanshinone biosynthesis, and the increase of tanshinone production was probably via ROS signaling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
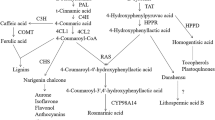
Salvia miltiorrhiza (Danshen in Chinese) is one of the most popular traditional Chinese medicinal plants and has been widely used in the prevention and treatment of coronary heart disease (Sugiyama and others 2002). Tanshinones, a group of diterpenoids, are the main biologically active compounds in S. miltiorrhiza (Yang and others 2009a, 2009b). The elucidation of tanshinone biosynthesis is very important due to their excellent performance in treating human disease. Terpenoids are biosynthesized via at least two pathways in plants: the mevalonate (MVA) pathway in the cytosol (Lichtenthaler 2000) and the 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway in the plastids (Rohmer 1999). 3-Hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) is the rate-limiting enzyme in the MVA pathway (Lichtenthaler 2000) and 1-deoxy-D-xylulose 5-phosphate synthase (DXS) is the rate-limiting enzyme in the MEP pathway (Rohmer 1999). The involvement of the two pathways in tanshinone biosynthesis has been suggested (Dai and others 2011; Ge and Wu 2005; Kai and others 2011; Wu and others 2009). Overexpression of HMGR and DXS in transgenic hairy root lines could significantly enhance the production of tanshinones (Kai and others 2011). Great attention has been paid to the development of a high-speed tanshinone production system using plant tissue culture technology. Various elicitors to stimulate tanshinone production in S. miltiorrhiza hairy roots, including biotic elicitors such as yeast extracts and bacteria, and abiotic elicitors such as heavy metal ions and plant response-signaling compounds (Chen and Chen 1999; Ge and Wu 2005; Zhao and others 2010), have been investigated. However, tanshinone production in S. miltiorrhiza hairy roots is generally low.
Water deficit is one of the most important manifestations of abiotic stresses and is well known to increase the amount of secondary metabolites in a wide variety of plant species (Blanch and others 2009; Liu and others 2011). One of the inevitable consequences of water stress is the enhancement of reactive oxygen species (ROS) production in the different cellular compartments. ROS, including hydrogen peroxide (H2O2), superoxide anion (O2 −), hydroxyl radical (HO•), and singlet oxygen (1O2), are the result of the partial reduction of atmospheric O2, and they can cause cellular toxicity and damage. Plants have developed a complex mechanism to scavenge ROS from cells (Apel and Hirt 2004). The antioxidant enzyme systems, including superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione peroxidase (GPX), play a very important role in scavenging ROS. SOD is the frontline enzyme in ROS attack and can convert O2 − to oxygen and H2O2. The major enzymatic cellular scavengers of H2O2 are CAT and APX. In fact, SOD and CAT have been widely used as ROS scavengers in cell culture (Preston and others 2001; Xu and Dong 2005). The involvement of ROS in elicitor-induced accumulation of secondary metabolites has been suggested. For example, O2 − from the fungus-induced oxidative burst is necessary for triggering phenylalanine ammonia-lyase activation and catharanthine synthesis in Catharanthus roseus cell cultures (Xu and Dong 2005), and H2O2 mediates oligogalacturonic acid-induced saponin synthesis in suspension-cultured cells of Panax ginseng (Hu and others 2003). It is also well known that ROS are involved in the plant response to water stress (Miller and others 2010). However, the role of ROS in water stress-induced accumulation of secondary metabolites in medicinal plants is still largely unknown.
The aim of this study was to reveal the role of ROS in water stress-induced tanshinone production in S. miltiorrhiza hairy roots. For this purpose, polyethylene glycol 6000 (PEG) and abscisic acid (ABA) were employed to induce water stress responses, and CAT and SOD were used as the scavengers of H2O2 and O2 − (Preston and others 2001; Xu and Dong 2005). The growth, tanshinone production, ROS generation, and gene expression and enzyme activity of HMGR and DXS in S. miltiorrhiza hairy roots under different treatments were measured.
Materials and Methods
Hairy Root Culture and Treatment
S. miltiorrhiza hairy roots were derived after the infection of plantlets with Agrobacterium rhizogenes bacterium (ATCC 15834). Experiments in this study were carried out in a 250 ml shake flask on an orbital shaker running at 110–120 rpm and at 25 °C in darkness (Hu and Alfermann 1993). Each flask was filled with 50 ml liquid medium and inoculated with 0.3 g fresh hairy roots from a 3-week-old shake-flask culture (Ge and Wu 2005). The liquid medium was made of hormone-free MS medium with 30 g l−1 sucrose but without ammonium nitrate as previously described (Hu and Alfermann 1993).
Stock solutions of ±-cis, trans-ABA (Wolsen, China) (Gagné and others 2011; Lacampagne and others 2010), CAT (Sigma, USA), and SOD (Sigma, USA) were prepared in distilled water. Then they were sterilized by filtration (0.22 μm membrane). PEG 6000 (Merck, Germany) was sterilized by autoclaving at 121 °C for 30 min.
ABA in the culture medium was stable during the period of treatment in our experiments (Gagné and others 2011). A final concentration of 2 % PEG (w/v) and 200 μM ABA in the culture medium was chosen to study their effects on tanshinone production on the basis of preliminary experiments (data not shown). To scavenge ROS in S. miltiorrhiza hairy roots, the final concentrations of 1,000 U ml−1 CAT and SOD were added separately to the culture together with PEG or ABA treatments. The concentrations of CAT and SOD were chosen on the basis of a previous study (Xu and Dong 2005). Pyrogallol was a donor of O2 −. To elucidate further the effects of ROS on tanshinone production, pyrogallol at 50 μM and H2O2 at 200 μM were applied to the hairy root culture. An equal volume of distilled water was added to the hairy root culture as the control (CK). Treatments were conducted on day 18 post inoculation. All treatments were performed in four replicates.
Hairy roots were harvested from the culture medium on day 6 after treatments, blotted dry with paper towels (yielding fresh weight, FW), and then dried at 45 °C in an oven until constant weight was reached (yielding dry weight, DW).
ROS Determination
Hairy roots (0.5 g) were homogenized with 8 ml of 50 mM sodium phosphate buffer (pH 7.8), including 1 % PVP, and centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatant was used for ROS determination. O2 − production was measured as previously described (Sun and Hu 2006; Zhu and others 2009) by monitoring the absorption of benzenesulfonic acid-azo-p-α-naphthylamine at 530 nm produced from the reaction of O2 −, hydroxylamine hydrochloride, benzenesulfonic acid, and α-naphthylamine. The reaction mixture containing 65 mM sodium phosphate buffer (pH 7.8) and 10 mM hydroxylamine hydrochloride was incubated at 25 °C for 20 min, followed by the addition of 17 mM benzenesulfonic acid and 7 mM α-naphthylamine and then incubated at 30 °C for 30 min. The absorbance was measured at 530 nm. The amount of H2O2 was measured by the H2O2 Determination Kit (Nanjing, Jiancheng, China) according to the manufacturer’s instructions.
Tanshinone Extraction and HPLC Analysis
The dried hairy roots were ground with a mortar and pestle and sieved through a 0.45-mm screen. The sample (0.1 g) was extracted ultrasonically with 2 ml of methanol–water solution (7:3 v/v) for 45 min. The extract was centrifuged at 10,000 rpm for 15 min and filtered through a 0.45 μm filter.
Stock solutions of tanshinone ΠA, cryptotanshinone, dihydrotanshinone I, and tanshinone I were prepared in methanol and diluted to the desired concentrations. A series of the standard solutions was used to determine the linear range of the analytes. The detector response was linearly correlated with the concentration of the tested compound, with a range of 0.6875–137.5 μg ml−1 for tanshinone ΠA, 0.3125–62.5 μg ml−1 for cryptotanshinone, 0.51–102.0 μg ml−1 for dihydrotanshinone I, and 0.55–55.0 μg ml−1 for tanshinone I.
HPLC analysis was performed using a Waters (Milford, MA, USA) system with a binary pump and photodiode array detector (DAD). A SunFire C18 column (250 × 4.6-mm, 5 μm) was used at a column temperature of 30 °C. The flow rate was 1 ml min−1 and the injection volume was 20 μl. The DAD detection wavelength was 270 nm. Data were acquired and processed using Empower 2 software. Separation was achieved by elution with a linear gradient with solvent A (acetonitrile) and solvent B (water). The gradient was t = 0 min 40 % A; t = 5 min, 60 % A; t = 20 min, 60 % A; t = 23 min, 80 % A; and t = 25 min, 100 % A. Quantitative analysis was repeated for four replicates of each treatment, and the results were represented by their means ± standard deviation (SD).
RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR
Hairy roots 24 h after treatment were homogenized to a fine powder in liquid nitrogen. Total RNA was extracted by RNAiso™ Plus (Takara, Tokyo, Japan). The first-strand cDNA was synthesized from 500 ng total RNA using the reverse-transcription PCR system according to the manufacturer’s protocol for the PrimeScript® RT Reagent Kit (Takara). The primer used for the first-strand cDNA synthesis was the oligo (dT) primer supplied with the kit. The quantitative RT-PCR reactions were performed using a Bio-Rad CFX96 system (Bio-Rad Labs, Hercules, CA, USA) with Brilliant SYBR® Green qPCR Master Mix (Agilent, Santa Clara, CA, USA). A total reaction volume of 25 μl included 12.5 μl Brilliant SYBR® Green QPCR Master Mix, 1.0 μl of 10 μM forward primer, 1.0 μl of 10 μM reverse primer, 1.0 μl cDNA template, and 9.5 μl nuclease-free PCR-grade water. The conditions were as follows: predenaturation at 95 °C for 10 min, 40 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 60 s, and collection fluorescence at 72 °C for 30 s. Experiments were performed in four replicates and the results were represented by their means ± SD. β-actin was used as the reference gene. The relative expression levels of HMGR and DXS were detected. The primers were designed by the software Primer-Premier 5.0 (Palo Alto, CA, USA) (Table 1).
Enzyme Assays
Hairy roots (1.0 g) were ground with 1 ml of extraction buffer [50 mM Tris–HCl, 10 mM β-mercaptoethanol, 1 % (w/v) polyvinylpyrrolidone (pH 7.5)] for 5 min on ice, and then centrifuged at 14,000 rpm for 30 min. The activities of HMGR and DXS were determined by the method previously described (Ge and Wu 2005). The reaction mixture (50 mM Tris–HCl (pH 7.0), 0.3 mM HMG-CoA, 0.2 mM NADPH, 4 mM dithiothreitol, and the enzyme extract) was monitored at 340 nm to determine HMGR activity. One HMGR enzyme unit (U/mg protein) is equivalent to the oxidation of 1 μmol of NADPH per minute. To determine DXS activity, the reaction mixture (40 mM Tris–HCl (pH 7.5), 2.5 mM MgCl2, 5 mM β-mercaptoethanol, 1 mM thiamine diphosphate, 10 mM sodium pyruvate, 20 mM d,l-glyceraldehyde 3-phosphate, and the enzyme extract) was incubated at 37 °C for 1 h and at 80 °C for 5 min, followed by centrifugation at 13,000 rpm for 5 min. Then, after the supernatant was mixed with 1 ml of 10 mM of 3,5-diaminobenzoic acid in 5 M phosphoric acid and incubated at 100 °C for 15 min, the fluorescence intensity of the reaction product was measured at 396 nm excitation and 510 nm emission with a fluorescence spectrophotometer. The activities of HMGR and DXS in PEG- and ABA-treated S. miltiorrhiza hairy roots were calculated against those in the control, and the results were shown as folds of the control.
Statistical Analysis
All data in this work were obtained from three independent experiments with four replicates each. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Duncan’s multiple-range test using Data Processing System (DPS) for Windows. The difference between treatments was considered to be statistically significant when p ≤ 0.05.
Results
Effects of ROS Scavengers on the Growth of PEG- and ABA-treated S. miltiorrhiza Hairy Roots
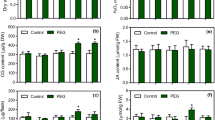
We initially measured the growth of S. miltiorrhiza hairy roots under PEG and ABA treatments. Concentrations of 2 % PEG and 200 μM ABA were chosen according to the preliminary experiments. As shown in Table 2, applications of PEG and ABA significantly reduced the growth of S. miltiorrhiza hairy roots to 0.43 and 0.41 g DW, respectively, compared to that of the control (0.54 g DW). No significant effect on hairy root growth by treatments with CAT and SOD alone was observed; however, SOD was able to increase the growth of PEG- and ABA-treated S. miltiorrhiza hairy roots. With PEG + CAT and ABA + CAT treatments, root growth was almost unchanged when compared to that with PEG and ABA treatments. With PEG + SOD and ABA + SOD treatments, root growth increased to 0.49 and 0.51 g DW, respectively. Treatments with pyrogallol and H2O2 resulted in a significant decrease of hairy root growth, which was 0.48 and 0.40 g DW, respectively.
Effects of ROS Scavengers on PEG- and ABA-induced Tanshinone Production
With an appropriate HPLC method, the accumulation of four tanshinones in S. miltiorrhiza hairy roots was determined. Application of either PEG or ABA led to a significant increase of tanshinone contents, as shown in Fig. 1. With PEG treatment, the contents of tanshinone I, cryptotanshinone, dihydrotanshinone I, and tanshinone ΠA in S. miltiorrhiza hairy roots were increased by 70, 100, 60, and 70 %, respectively, when compared to the control. With ABA treatment, they were increased by 110, 170, 60, and 90 %, respectively, when compared to the control. To confirm further roles of PEG and ABA in tanshinone production, yields of four tanshinones in the hairy root culture were investigated. Figure 2 shows that yields of tanshinone I, cryptotanshinone, dihydrotanshinone I, and tanshinone ΠA in the hairy roots were all improved by PEG and ABA. When compared to the control levels of yields of the four tanshinones (0.7, 1.3, 3.2, and 1.5 mg l−1, respectively), they were increased to 0.9, 2.1, 3.9, and 2.0 mg l−1 by PEG treatment and to 1.1, 1.6, 3.9, and 2.2 mg l−1 by ABA treatment.
Contents of four tanshinones in S. miltiorrhiza hairy roots under different treatments. CK control, PEG polyethylene glycol at 2 %, ABA abscisic acid at 200 μM, CAT catalase at 1,000 U ml−1, SOD superoxide dismutase at 1,000 U ml−1, pyrogallol, 50 μM, H2O2, 200 μM. Different letters indicate significant difference at p ≤ 0.05 using Duncan’s multiple-range test. Means ± standard deviation (SD) (n = 4) are shown
Yields of four tanshinones in S. miltiorrhiza hairy roots under different treatments. CK control, PEG polyethylene glycol at 2 %, ABA abscisic acid at 200 μM, CAT catalase at 1,000 U ml−1, SOD superoxide dismutase at 1,000 U ml−1, pyrogallol, 50 μM, H2O2, 200 μM. Different letters indicate significant difference at p ≤ 0.05 using Duncan’s multiple-range test. Means ± standard deviation (SD) (n = 4) are shown
By using two ROS scavengers (CAT and SOD), the PEG- and ABA-induced increase of tanshinone contents was suppressed (Fig. 1). With CAT treatment, the contents of the four tanshinones in PEG-treated hairy roots were reduced by 43, 53, 39, and 48 %, and those in ABA-treated hairy roots were reduced by 47, 49, 39, and 53 %. With SOD treatment, the contents of the four tanshinones in PEG-treated hairy roots were reduced by 45, 64, 52, and 43 %, and those in ABA-treated hairy roots were reduced by 68, 68, 59, and 69 %. Correspondingly, the PEG- and ABA-induced increase of tanshinone yields in S. miltiorrhiza hairy roots was also suppressed by the two ROS scavengers (Fig. 2). However, when CAT and SOD were applied to the culture alone, contents and yields of four tanshinones remained constant. These results indicated that the PEG- and ABA-induced increase of tanshinone production was probably via ROS signaling.
To confirm further the roles of ROS in tanshinone production, the effects of H2O2 and pyrogallol on the contents and yields of the four tanshinones were investigated. With H2O2 treatment, the contents of the four tanshinones were increased by 35, 62, 114, and 125 %, and their yields were increased by 1, 21, 60, and 68 %. With pyrogallol treatment, their contents were 3.6-, 1.9-, 1.6-, and 1.5-fold of the control, and their yields were 3.2-, 1.7-, 1.4-, and 1.3-fold of the control. This demonstrated that ROS were effective in inducing tanshinone production, which confirmed further the roles of ROS in tanshinone production.
Gene Expression and Enzyme Activity of HMGR and DXS in PEG- and ABA-treated S. miltiorrhiza Hairy Roots
To gain further insight into the regulation mechanism of tanshinone biosynthesis by PEG and ABA, the mRNA levels of HMGR and DXS and the enzyme activities of HMGR and DXS were detected. Figure 3 shows that the mRNA levels of HMGR and DXS and the enzyme activities of HMGR and DXS were significantly upregulated by PEG and ABA treatments, whereas they were almost not affected by treatments with CAT and SOD alone. The mRNA levels of HMGR and DXS were increased by 17- and 14-fold, respectively, by PEG treatment and increased by 10- and 13-fold, respectively, by ABA treatment compared to the control. The PEG- and ABA-induced increase of HMGR and DXS expression was significantly inhibited by CAT and SOD. Correspondingly, enzyme activities of HMGR and DXS were upregulated by more than twofold over the control by PEG and ABA, and the upregulation was also suppressed by CAT and SOD. These results revealed that both the MVA and the MEP pathways were activated by applications of PEG and ABA. The PEG- and ABA-induced upregulation of gene expression and enzyme activity probably depended on the ROS burst.
Gene expression and enzyme activity of HMGR and DXS in S. miltiorrhiza hairy roots under different treatments. HMGR, 3-hydroxy-3-methylglutaryl coenzyme A reductase; DXS, 1-deoxy-D-xylulose 5-phosphate synthase; CK control, PEG polyethylene glycol at 2 %, ABA abscisic acid at 200 μM, CAT catalase at 1,000 U ml−1, SOD superoxide dismutase at 1,000 U ml−1. Different letters indicate significant difference at p ≤ 0.05 using Duncan’s multiple-range test. Means ± standard deviation (SD) (n = 4) are shown
Generation of ROS in PEG- and ABA-treated S. miltiorrhiza Hairy Roots
To verify further the roles of ROS in PEG- and ABA-induced responses of S. miltiorrhiza hairy roots, ROS generation was determined. As shown in Fig. 4, both PEG and ABA triggered a ROS burst in S. miltiorrhiza hairy roots. The amounts of H2O2 and O2 − were increased to 50.1 μmol g−1 FW and 19.5 μg g−1 FW by PEG treatment and to 46.2 μmol g−1 FW and 17.9 μg g−1 FW by ABA treatment from the control levels (32.2 μmol g−1 FW and 10.5 μg g−1 FW, respectively). Treatment with CAT or SOD alone was unable to affect ROS generation. However, the increase of PEG- and ABA-induced H2O2 generation was completely reversed by CAT, but it was just partially reversed by SOD. SOD totally arrested O2 − generation induced by PEG and ABA, but CAT just partially arrested the generation.
Generation of H2O2 and O2 − in S. miltiorrhiza hairy roots under different treatments. CK control, PEG polyethylene glycol at 2 %, ABA abscisic acid at 200 μM, CAT catalase at 1,000 U ml−1, SOD superoxide dismutase at 1,000 U ml−1. Different letters indicate significant difference at p ≤ 0.05 using Duncan’s multiple-range test. Means ± standard deviation (SD) (n = 4) are shown
Discussion
It is well known that water stress not only reduces plant growth but also changes the metabolite flux, including primary and secondary metabolites. Plant secondary metabolites play an important role in the interaction between plants and their environment, and they are involved in water stress tolerance (Hartmann 2007; Oh and others 2009). In medicinal plants, our previous studies indicated that the accumulation of secondary metabolites was significantly enhanced by water stress, including saikosaponin in Bupleurum chinense DC (Zhu and others 2009), iridoid glycosides in Scrophularia ningpoensis (Wang and others 2010), and tanshinone in S. miltiorrhiza (Liu and others 2011). ABA was defined as a stress plant hormone because of its rapid accumulation in response to stresses and its important role in the regulation of plant growth, development, and tolerance under stress (Zhang and others 2006). Earlier reports indicated that ABA could induce the biosynthesis of secondary metabolites, including terpenoids and anthocyanin (Gagné and others 2011; Mansouri and others 2009). In the present study, we observed that although the growth of S. miltiorrhiza hairy roots was inhibited by PEG and ABA treatments, both contents and yields of four tanshinones were significantly enhanced by these treatments. These results confirmed further our earlier observations (Liu and others 2011). It is well known that terpenoids are biosynthesized via the MVA and MEP pathways in plants. Although the contribution of each pathway to tanshinone production is still unclear, it is believed that the MVA and MEP pathways are involved in tanshinone biosynthesis (Dai and others 2011; Ge and Wu 2005; Kai and others 2011; Wu and others 2009). In yeast- and Ag+-treated S. miltiorrhiza hairy roots, tanshinones were predominantly derived from the MEP pathway but could depend on crosstalk between the two pathways (Ge and Wu 2005). Our results showed that the mRNA levels and enzyme activities of HMGR and DXS were significantly stimulated by PEG and ABA. It was suggested that the MVA and MEP pathways were activated by PEG and ABA to increase tanshinone production, which confirmed further the involvement of the two pathways in tanshinone biosynthesis.
Understanding the physiological mechanism of tanshinone accumulation in response to stress is critical to ramping up tanshinone production. A significant physiological response of plant cells to water stress is a ROS burst. The production of ROS is an early event in the plant response to water stress, and ROS act as secondary messengers in a stress-response signal transduction pathway (Cruz de Carvalho 2008). Increasing evidence indicates that ROS generation is triggered by PEG and ABA to improve stress tolerance (Boo and Jung 1999; Hu and others 2010; Leshem and others 2010). Similar results were obtained in this study. The amounts of H2O2 and O2 − in S. miltiorrhiza hairy roots were both enhanced by PEG and ABA treatments. ROS play an important signaling role in plants controlling processes such as growth, development, and response to biotic and abiotic environmental stimuli (Bailey-Serres and Mittler 2006). A signaling role of ROS in water stress-induced responses of plants has been suggested (Cruz de Carvalho 2008). Our previous work indicated that water stress-induced secondary metabolite accumulation in Bupleurum chinense roots was probably stimulated via ROS signaling (Zhu and others 2009). This hypothesis was supported by the present study. The PEG- and ABA-induced increase in tanshinone production was significantly arrested by ROS scavengers (CAT and SOD). Simultaneously, the upregulation of mRNA levels of HMGR and DXS and of enzyme activities of HMGR and DXS induced by PEG and ABA was significantly inhibited by CAT and SOD. It demonstrated that PEG- and ABA-induced tanshinone production was ROS-dependent. The activation of the MVA and MEP pathways by PEG and ABA was probably via ROS signaling. In addition, ROS treatments also resulted in a significant increase in tanshinone production, confirming further the roles of ROS in tanshinone biosynthesis.
In conclusion, the data from this study suggests that both PEG and ABA are able to enhance tanshinone production, upregulate mRNA levels of HMGR and DXS and enzyme activities of HMGR and DXS, and trigger a ROS burst in S. miltiorrhiza hairy roots. These results also indicated that the PEG- and ABA-induced increase of tanshinone production was probably via ROS signaling.
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bailey-Serres J, Mittler R (2006) The roles of reactive oxygen species in plant cells. Plant Physiol 141:311
Blanch JS, Peñuelas J, Sardans J, Llusià J (2009) Drought, warming and soil fertilization effects on leaf volatile terpene concentrations in Pinus halepensis and Quercus ilex. Acta Physiol Plant 31:207–218
Boo YC, Jung J (1999) Water deficit-induced oxidative stress and antioxidative defenses in rice plants. J Plant Physiol 155:255–261
Chen H, Chen F (1999) Effects of methyl jasmonate and salicylic acid on cell growth and cryptotanshinone formation in Ti transformed Salvia miltiorrhiza cell suspension cultures. Biotechnol Lett 21:803–807
Cruz de Carvalho MH (2008) Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav 3:156–165
Dai Z, Cui G, Zhou SF, Zhang X, Huang L (2011) Cloning and characterization of a novel 3-hydroxy-3-methylglutaryl coenzyme A reductase gene from Salvia miltiorrhiza involved in diterpenoid tanshinone accumulation. J Plant Physiol 168:148–157
Gagné S, Cluzet S, Mérillon J-M, Gény L (2011) ABA initiates anthocyanin production in grape cell cultures. J Plant Growth Regul 30:1–10
Ge X, Wu J (2005) Tanshinone production and isoprenoid pathways in Salvia miltiorrhiza hairy roots induced by Ag+ and yeast elicitor. Plant Sci 168:487–491
Hartmann T (2007) From waste products to ecochemicals: fifty years of research of plant secondary metabolism. Phytochemistry 68:2831–2846
Hu ZB, Alfermann AW (1993) Diterpenoid production in hairy root cultures of Salvia miltiorrhiza. Phytochemistry 32:699–703
Hu XY, Neill S, Cai WM, Tang ZC (2003) Hydrogen peroxide and jasmonic acid mediate oligogalacturonic acid-induced saponin accumulation in suspension-cultured cells of Panax ginseng. Physiol Plant 118:414–421
Hu XL, Liu RX, Li YH, Wang W, Tai FJ, Xue RL, Li CH (2010) Heat shock protein 70 regulates the abscisic acid-induced antioxidant response of maize to combined drought and heat stress. Plant Growth Regul 60:225–235
Kai G, Xu H, Zhou C, Liao P, Xiao J, Luo X, You L, Zhang L (2011) Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metab Eng 13:319–327
Lacampagne S, Gagné S, Gény L (2010) Involvement of abscisic acid in controlling the proanthocyanidin biosynthesis pathway in grape skin: new elements regarding the regulation of tannin composition and leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) activities and expression. J Plant Growth Regul 29:81–90
Leshem Y, Golani Y, Kaye Y, Levine A (2010) Reduced expression of the v-SNAREs AtVAMP71/AtVAMP7C gene family in Arabidopsis reduces drought tolerance by suppression of abscisic acid-dependent stomatal closure. J Exp Bot 61:2615–2622
Lichtenthaler HK (2000) Non-mevalonate isoprenoid biosynthesis: enzymes, genes and inhibitors. Biochem Soc Trans 28:785–789
Liu H, Wang X, Wang D, Zou Z, Liang Z (2011) Effect of drought stress on growth and accumulation of active constituents in Salvia miltiorrhiza bunge. Ind Crop Prod 33:84–88
Mansouri H, Asrar Z, Szopa J (2009) Effects of ABA on primary terpenoids and Delta (9)-tetrahydrocannabinol in Cannabis sativa L. at flowering stage. Plant Growth Regul 58:269–277
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell Environ 33:453–467
Oh MM, Trick HN, Rajashekar CB (2009) Secondary metabolism and antioxidants are involved in environmental adaptation and stress tolerance in lettuce. J Plant Physiol 166:180–191
Preston TJ, Muller WJ, Singh G (2001) Scavenging of extracellular H2O2 by catalase inhibits the proliferation of HER-2/Neu-transformed rat-1 fibroblasts through the induction of a stress response. J Biol Chem 276:9558–9564
Rohmer M (1999) The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat Prod Rep 16:565–574
Sugiyama A, Zhu BM, Takahara A, Satoh Y, Hashimoto K (2002) Cardiac effects of Salvia miltiorrhiza/Dalbergia odorifera mixture, an intravenously applicable Chinese medicine widely used for patients with ischemic heart disease in China. Circ J 66:182–184
Sun Q, Hu JJ (2006) Reasearch technology of plant physiology. In: Lin L, Hu JJ (eds) Determination of superoxide anion in plants. Northwest A&F University Press, Yangling, pp 174–176
Wang DH, Du F, Liu HY, Liang ZS (2010) Drought stress increases iridoid glycosides biosynthesis in the roots of Scrophularia ningpoensis seedlings. J Med Plants Res 4:2691–2699
Wu SJ, Shi M, Wu JY (2009) Cloning and characterization of the 1-deoxy-D-xylulose 5-phosphate reductoisomerase gene for diterpenoid tanshinone biosynthesis in Salvia miltiorrhiza (Chinese sage) hairy roots. Biotechnol Appl Biochem 52:89–95
Xu MJ, Dong JF (2005) O2− from elicitor-induced oxidative burst is necessary for triggering phenylalanine ammonia-lyase activation and catharanthine synthesis in Catharanthus roseus cell cultures. Enzyme Microb Tech 36:280–284
Yang D, Yang S, Zhang Y, Liu Y, Meng X, Liang Z (2009a) Metabolic profiles of three related Salvia species. Fitoterapia 80:274–278
Yang DF, Liang ZS, Liu JL (2009b) LC fingerprinting for assessment of the quality of the lipophilic components of Salvia miltiorrhiza. Chromatographia 69:555–560
Zhang J, Jia W, Yang J, Ismail AM (2006) Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res 97:111–119
Zhao JL, Zhou LG, Wu JY (2010) Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl Microbiol Biotechnol 87:137–144
Zhu Z, Liang Z, Han R (2009) Saikosaponin accumulation and antioxidative protection in drought-stressed Bupleurum chinense DC plants. Environ Exp Bot 66:326–333
Acknowledgment
This work was supported by the Knowledge Innovation Project of the Chinese Academy of Science (KZCX2-YW-443).
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Yang, D. Sheng, and Q. Duan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, D., Sheng, D., Duan, Q. et al. PEG and ABA Trigger the Burst of Reactive Oxygen Species to Increase Tanshinone Production in Salvia miltiorrhiza Hairy Roots. J Plant Growth Regul 31, 579–587 (2012). https://doi.org/10.1007/s00344-012-9268-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-012-9268-6