Abstract
St. John’s Wort (Hypericum perforatum) is a perennial herb able to produce water-soluble active ingredients (a.i.), mostly in flowers, with a wide range of medicinal and biotechnological uses. However, information about the ability of arbuscular mycorrhizal fungi (AMF) to affect its biomass accumulation, flower production, and concentration of a.i. under contrasting nutrient availability is still scarce. In the present experiment, we evaluated the role of AMF on growth, flower production, and concentration of bioactive secondary metabolites (hypericin, pseudohypericin, and hyperforin) of H. perforatum under contrasting P availability. AMF stimulated the production of aboveground biomass under low P conditions and increased the production of root biomass. AMF almost halved the number of flowers per plant by means of a reduction of the number of flower-bearing stems per plant under high P availability and through a lower number of flowers per stem in the low-P treatment. Flower hyperforin concentration was 17.5% lower in mycorrhizal than in non-mycorrhizal plants. On the contrary, pseudohypericin and hypericin concentrations increased by 166.8 and 279.2%, respectively, with AMF under low P availability, whereas no effect of AMF was found under high P availability. These results have implications for modulating the secondary metabolite production of H. perforatum. However, further studies are needed to evaluate the competition for photosynthates between AMF and flowers at different nutrient availabilities for both plant and AM fungus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizal (AM) fungi (Glomeromycota) and most land plants act in a symbiosis which usually enhances the biomass accumulation of the host compared to a non-mycorrhizal counterpart. This occurs mostly because of the ability of the AM fungi to take up nutrients with low mobility or low concentration within the soil solution and under various stress conditions (Smith and Read 2008). The advantages of AM symbiosis to host plants have been demonstrated extensively in terms of enhancement of plant biomass and nutrient uptake, especially in cereals and legumes (Kaschuk et al. 2010; Saia et al. 2015a; Pellegrino et al. 2015; Bona et al. 2016a). In medicinal and aromatic plants (MAPs), most of the information about the effects of AM fungi has been derived from investigations about the family Lamiaceae (Khaosaad et al. 2006; Copetta et al. 2006; Zeng et al. 2013; López-García et al. 2014; Bona et al. 2016b; Rydlová et al. 2016; Varela-Cervero et al. 2016) in which the most important active compounds are liposoluble, whereas few studies have been performed on other MAPs bearing hydrosoluble active ingredients (a.i.) or on other plant taxa (Kapoor et al. 2004; Liu et al. 2007; Jurkiewicz et al. 2010; Zubek et al. 2012). In addition, the effects of AM fungi on the secondary metabolite (SM) production and storage in MAPs, of which the economic importance often relies upon their SM content and concentration, differed depending on the chemical classes, nutrient availability for the plant, AM fungus used in the experiment, and botanical taxon of the host plant (Brundrett 2009; Zeng et al. 2013; Bona et al. 2016b). In particular, it was shown that AM fungi can increase the content and concentration of SMs either by mediating nutrient uptake, mostly P, involved in the biosynthetic pathways of SMs (Kapoor et al. 2004) or irrespective of any effect on P uptake (Khaosaad et al. 2006; Nell et al. 2010). This latter likely depends on the direct intervention of the AM fungi in the biosynthesis of precursors of some SM constituents, as shown in cereals (Walter et al. 2000). Thus, the role of AM fungi in the accumulation of MAP a.i. depends on the host plant and target metabolite. In addition, the role of AM symbiosis in flowering date and flower amount, flowers frequently being the plant organ with highest SM concentration and content, is fragmented. It has been shown that AM fungi can induce earlier (Usha et al. 2005; Bona et al. 2015) or delayed flowering (Nowak 2004; Saia et al. 2014a) and either increase, reduce, or have no effect on flower number (Gaur and Adholeya 2005; Perner et al. 2007; Asrar et al. 2012; Bona et al. 2015). Such effects differed among host plants, AM species or consortium used, nutrient availability, and other growth conditions.
St. John’s Wort (Hypericum perforatum L.) is a perennial herb belonging to the Hypericaceae family (Ruhfel et al. 2013), native to Europe, Asia, and North Africa, introduced and naturalized in North America and in temperate areas of the Southern hemisphere (Carrubba and Scalenghe 2012). H. perforatum and related species have been used since ancient times as a local resource for medicinal purposes, due to their wound healing, mild sedative, antiviral, and antidepressant properties (Russo et al. 2014). These properties are associated with a group of hydrosoluble metabolites, mostly accumulated in flowers: the phenolic compounds naphthodianthrones, including hypericin and pseudohypericin, and the phloroglucinol derivative hyperforin (Lazzara et al. 2015).
Besides having been extensively studied for their medicinal applications, such compounds are recognized to possess antimicrobial and antifeedant properties and are involved in interaction mechanisms between plants and other organisms (Kirakosyan et al. 2004). However, scarce information is available about the effects of AM fungi and nutrient availability on plant biomass accumulation and flower content of hypericin, pseudohypericin, and hyperforin for H. perforatum.
The aim of the present experiment therefore was to evaluate the role of AM fungi on biomass, yield components, and flower production, as well as hypericin, pseudohypericin, and hyperforin content of H. perforatum grown under contrasting P availabilities.
Materials and methods
Plant material and experimental setup
The experiment was established at the CREA-SFM greenhouses in Bagheria (Palermo, Italy; 38°05′26″N, 13°31′15″E, 35 m a.s.l.). To ensure genetic uniformity of the plant material used, a unique clone of H. perforatum was employed, obtained from one single individual growing in the experimental farm “Sparacia” (Cammarata, AG, Italy, 37°38′ N, 13°46′ E, 415 m a.s.l). The mother plant chosen was among the most abundantly flowering plants available in the collection. Stem cuttings with four non-terminal buds were collected from the apical part of each branch. Each cutting was 2.5 cm long and weighted 3.2 g ± 0.86 g. Each cutting was placed in a 7 × 7 × 7-cm pot containing 140 g of a sterilized substrate, composed of 29% sand, 57% peat-based growth substrate, and 14% vermiculite (w/w). Sterilization was performed by exposing a thin (<5 cm thickness) layer of each substrate to UV-C radiation at 15 W per 4 h and stirring each substrate every hour. The peat-based commercial growth substrate used (Technic 3®) had the following main properties: 23% organic carbon (OC), 5% organic nitrogen (ON), 46% organic matter (OM), 410 kg m−3 bulk density, and 88% total porosity, pH 6.5. All pots were arranged in a heated-bed greenhouse (16 °C ± 1 °C, 95% RH ± 5%) irrigated by vaporization. After 40 days (May 4, 2013), the rooted cuttings were transplanted free of soil from the nursery pots to larger pots (18 cm diameter, 3 L volume) filled with the same growth substrate used in the nursery phase (3 kg per pot). After transplanting, the growth substrate in each pot was brought to water holding capacity. Throughout the experiment, a water amount corresponding to the evapotranspiration losses, measured by the gravimetric method, was added to each pot two to three times per week. The pots were arranged inside the greenhouse according to a two-factor, fully crossed factorial design with four replications. Each block contained only one replicate of each treatment. Each replicate consisted of an individual clone growing in one pot. Treatments were as follows: P fertilization (either P fertilized [+P] or not [−P]) and inoculation with AM fungi (either inoculated [+AMF] or not inoculated [−AMF]). In the P-fertilized treatments (also referred as high P), 140 mg P per pot in the form of Ca(H2PO4)2 (corresponding to 20 μg P2O5 g−1 substrate) was supplied at the beginning of the experiment in each pot. This amount was chosen because a fertilization of 100 kg P2O5 ha−1 constitutes a high dose according to a previous experiment (unpublished) and increases the soil P2O5 concentration by 20 μg P2O5 g−1 if considering a soil depth of 0.4 m and bulk density of 1.25 kg L−1. P was applied as dry powder thoroughly mixed throughout the substrate. In the +AMF treatments, arbuscular mycorrhizal fungi (AMF) were inoculated at a dose of 460 spores per pot by means of a commercial inoculum (2.15 g inoculum per pot) (Micronized Endo Mycorrhizae, Symbio, Wormley, Surrey, Great Britain, 95% AM spores, 5% organic material). The AMF inoculum included the following AM species: Scutellospora calospora, Acaulospora laevis, Gigaspora margarita, Glomus aggregatum, Rhizophagus irregularis (syn G. intraradices), Funneliformis mosseae (syn G. mosseae), Rhizophagus fasciculatus (syn G. fasciculatum), Claroideoglomus etunicatum (syn G. etunicatum), and G. deserticola. Total spore density in the inoculum was 225 spores g−1 (25 spores g−1 per AM species). Inoculum of AM fungi was inserted into the planting hole at the time of transplant. After transplanting, each pot received a microbial filtrate from both the substrate and the mycorrhizal inoculum. Substrate bacterial inoculum was extracted by suspending 1.0 kg unsterilized air-dried substrate in 5.0 L distilled water or 100 g AM inoculum in 1.0 L distilled water. After shaking and decanting, the suspensions were filtered (7 μm mesh) to discard AM fungi. Before starting the experiment, each pot received 200 mL of substrate filtrate and 30 mL of inoculum filtrate. After the addition of the filtrates, each pot was weighted and an amount of tap water needed to bring the substrate near to its field capacity (ca. 95% water holding capacity) was added.
When ca. 50% of flowers were fully expanded (July 2, 2013, see Supplementary Material Fig. 1 for temperature and relative humidity [RH] during the experiment), total plant biomass was collected from each pot and sorted by plant organ (roots, stems, and flowers). Each fraction was weighted separately, fully expanded flowers were counted, and a representative sample of each organ was oven-dried (65 °C until constant weight) in order to calculate the respective moisture levels. A subsample (3 g) of roots from each pot was stained with 0.05% trypan blue in lactic acid according to Phillips and Hayman (1970), and root colonization by AMF was measured according to Giovannetti and Mosse (1980) by counting at least 300 intersection at ×40 magnification under a microscope. An intersection was considered as positive if an intra-radical hypha and/or an arbuscule was present. Fifteen leaves per plant were randomly picked and leaf greenness/chlorophyll content index was immediately measured by SPAD (Minolta SPAD 502DL). Yield components (number of flower-bearing stems per plant, number of flowers per plant, mean flower weight) and flowers’ SM content (hyperforin, pseudohypericin, and hypericin) were measured. Mean number of flowers per stem was computed.
Secondary metabolite determination
SM determination was performed according to Tawaha et al. (2010). Briefly, 5 g of air-dried and powdered flowers for each treatment and replication were extracted in 50 mL ethanol for 72 h in the dark, constantly shaking the samples. Each extract was filtered and dried by a Rotavapor for yield determination in ethanol-extracted compounds. Secondary compound concentration was measured by HPLC (HPLC-DAD Thermo Scientific UltiMate 3000 equipped with an analytical HPLC column Phenomenex Gemini® 5 μm NX-C18 110 Å, 250 × 4.6 mm) on three technical replicates per biological replicate. In particular, 20 μL of ethanol extract was eluted with a gradient of 20 mM ammonium acetate (solution A) and acetonitrile (solution B) as follows: 0–25 min, 50% A; 25–35 min, 10% A; and 35–45 min, 50% A. Flow rate used was 1 mL min−1. Hyperforin, pseudohypericin, and hypericin were quantified using an external standard curve per each compound by means of their absorbance (287 nm for hyperforin, and 590 nm for pseudohypericin and hypericin).
Computations and statistical analysis
Data expressed as percentages were arcsine square-root transformed before running the statistical analyses. The analysis of variance was performed by means of the GLIMMIX procedure in SAS/STAT 9.2 statistical package (SAS Institute Inc., Cary, NC, USA). This procedure is capable of modeling non-normal data and correcting for heteroscedasticity (Schabenberger 2005). Block was treated as a random factor. Differences among means were compared by applying t-grouping with Tukey-Kramer correction at the 5% probability level to the LSMEANS p differences.
Results
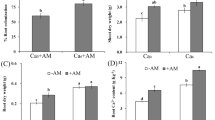
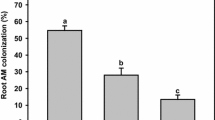
No AM fungi colonization was found in the roots of plants that were not inoculated with AM fungi. Phosphorus fertilization did not affect root colonization by AM fungi (Table 1), which was on average 42.5% ± 1.1 and 40.6% ± 1.9% in non-fertilized and P-fertilized treatments, respectively. P fertilization increased aboveground biomass (Table 1, Fig. 1a) and slightly reduced root biomass (Fig. 1b). AMF increased aboveground biomass in non-fertilized treatments by 16.7%, whereas they did not affect aerial biomass in fertilized treatments (Fig. 1a). In addition, AMF increased root biomass on average by 26.8% (Fig. 1b). The effect of treatments on total biomass and root to aboveground biomass ratio was similar to that observed for root biomass (Supplementary Material Table 1). The application of P reduced root to aboveground biomass ratio by 22.7%, which was on average 2.70 g g−1 in non-fertilized treatments and 2.08 g g−1 in P-fertilized treatments. Inoculation with AM fungi did not affect root to aboveground biomass ratio.
Dry weight of aboveground and root biomass of Hypericum perforatum grown under P-fertilized (+P) or non-fertilized (−P) conditions and inoculated with arbuscular mycorrhizal fungi (+AMF; gray bars) or not inoculated (−AMF; white bars). Data are means ± S.E., n = 4. Means among treatments were separated with t-grouping of the least square means differences; treatments with a letter in common are not different at p < 0.05. Please note that P fertilization × AMF interaction for root biomass was not significant (Table 1)
P fertilization reduced leaf chlorophyll content (SPAD values) by 26%, whereas no effects of AMF on SPAD values were observed (Supplementary Material Table 1).
AMF fungi almost halved the number of flowers per plant (Fig. 2a) in both P-fertilized and non-fertilized treatments; in the first case, this was due to a 42.3% reduction of the number of flower-bearing stems per plant (Fig. 2b), whereas in the non-fertilized treatments, this outcome may be attributed to a 58.7% reduction of the number of flowers per stem (Fig. 2c). Such effects resulted in a 49.3% lower flower dry matter (d.m.) yield in +AM than in −AM plants across both P fertilization treatments (Fig. 2d).
Number of flowers per plant, number of flower-bearing stems per plant, number of flowers per stem, and dry weight (d.w.) of flowers per plant of Hypericum perforatum grown under P-fertilized (+P) or non-fertilized (−P) conditions and inoculated with arbuscular mycorrhizal fungi (+AMF; gray bars) or not inoculated (−AMF; white bars). Data are means ± S.E., n = 4. Means among treatments were separated with t-grouping of the least square means differences; treatments with a letter in common are not different at p < 0.05. Please note that P fertilization × AMF interaction for flowers per plant, flowers per stem, and dry weight of flowers per plant was not significant (Table 1)
Extraction yield was on average 18.4% and did not differ among the treatments applied (Table 1 and Supplementary Material Table 1). However, the effect of AM fungi and P × AMF interaction for this trait was near significant (F = 4.68, p = 0.059 and F = 4.14, p = 0.072, respectively), which depended on a +8.2% extraction yield in +AMF compared to −AMF under low P availability and only a +0.3% under high P availability. Flowers’ hyperforin concentration (μg a.i. g−1 flower d.m.) decreased by 21.1% after P fertilization and by 17.7% after AMF inoculation, with no interaction between treatments (Fig. 3a).
Hyperforin, pseudohypericin, and hypericin concentration in flowers of Hypericum perforatum grown under P-fertilized (+P) or non-fertilized (−P) conditions and inoculated with arbuscular mycorrhizal fungi (+AMF; gray bars) or not inoculated (−AMF, white bars). Data are means ± S.E., n = 4. d.w., dry weight. Means among treatments were separated with t-grouping of the least square means differences; treatments with a letter in common are not different at p < 0.05. Please note that P fertilization × AMF interaction for hyperforin concentration was not significant (Table 1)
AMF enhanced by 166.8 and 279.2% pseudohypericin and hypericin concentrations, respectively, under low P availability (non-fertilized treatment) (Figs. 3b and 3c, respectively), whereas no effect of AMF was found on pseudohypericin and hypericin under high P availability (P-fertilized pots).
Discussion
Phosphorus availability did not affect root colonization by AMF, which was on average 41.5%. Such a level of root colonization by AMF is close to those found by Moora and Zobel (1998) in seedling (43–46%) and adult plants (45–48%) of H. perforatum. Davoodian et al. (2012) reported that the degree of root colonization by AMF in H. perforatum roots can range from 0 to 63% with a mean of 10%, and it is higher in post-flowering than pre-flowering and flowering stages. On the contrary, Zubek et al. (2012), by means of an AM measurement technique different from the one we used, found a higher mycorrhizal frequency than in the present study, with small differences among mycorrhizal inocula. The degree of AM colonization of roots may differ according to several factors, including plant species and genotype, phenological stage, AMF species, and soil fertility (Maron et al. 2004; Davison et al. 2011; Davoodian et al. 2012; Majewska et al. 2016), showing decreasing values with increasing P availability (Treseder 2004). However, the application of organic matter to the soil was found to stimulate the growth and activity of AM extra-radical mycelium (Joner and Jakobsen 1995) and root colonization by AMF (Saia et al. 2014b). Thus, it is likely that the high C content of the growth substrate that was employed for the present experiment reduced the negative effect of P availability on root AM colonization as already observed elsewhere (Alloush et al. 2000).
AM symbiosis increased the aboveground biomass in low P (non-fertilized) but not in high P (fertilized) pots and increased root biomass in both non-fertilized and P-fertilized treatments. Seifert et al. (2009) showed that the response of H. perforatum to root colonization by AMF usually is positive, but it can strongly differ depending on the plant genotype and clone. In contrast to the present study, Zubek et al. (2012) did not find any effect of the arbuscular mycorrhizal symbiosis on shoot biomass of H. perforatum, irrespective of using inocula of single or multiple AM species. However, Zubek et al.’s (2012) experimental conditions involved smaller plants, individuals obtained from seed, a higher plant density, and a lower amount of substrate per plant than the present study. Nonetheless, van der Heijden and Horton (2009) estimated by means of the data from Moora and Zobel (1998) that the mycorrhizal dependency (in term of biomass) of H. perforatum seedlings was negative, whereas that of adult plants was positive.
Arbuscular mycorrhizal symbiosis almost halved the number of flowers per plant. This consequence depended upon different effects on morphogenesis at high and low P availability. At high P availability (P-fertilized treatment), AMF reduced the number of flower-bearing stems per plant, whereas at low P availability (non-fertilized treatment), AMF mostly reduced the number of flowers per stem. Information on the effects of AM fungi on flowering is disparate. In particular, it has been shown that AM fungi usually increase flower amount, the number of flowering plants in a stand, or flowering earliness (Schenck and Smith 1982; Gaur et al. 2000; Scagel 2004; Perner et al. 2007; Bunn et al. 2009; Asrar et al. 2012; Bona et al. 2015). However, it also has been found that AM fungi can have no effects on flowering (Linderman and Davis 2004), delay its onset, or increase its duration (Schenck and Smith 1982; Dubský and Vosátka 2000; Saia et al. 2014a; Jin et al. 2015). Such effects could depend on both the competition for N and photosynthates between AM fungi and flowers (Johnson et al. 1982) and the ability of AM fungi to reduce nutrient deficiency or other stresses for the host plant. The number of flower-bearing stems is determined earlier than the number of flowers per stem (Slafer et al. 1996), and AM fungi retain most of the N taken up in organic form for their own growth (Hodge and Fitter 2010). The growth substrate used in the present study was rich in organic matter. Under such a condition, it is likely that at low P, competition between AM fungi and plant stems was partly balanced by the P uptake exerted by AMF, whereas at high P, where AM benefits were reduced, such competition also resulted in a reduced flower induction by the stems. The behavior of AM fungi can range from mutualism to commensalism and parasitism, and from this to amensalism or competition (Johnson et al. 1997), and such transitions strongly depend on the N/P ratio of the environment or experimental conditions (Johnson et al. 2014). In our experiment, the reduction of the SPAD reading at high P could reflect both a diminished N/P ratio and decreased N and P availabilities for the plant. Thus, the lack of AM effect on aboveground biomass and the greater number of yield components reduced by the AM symbiosis at high P suggest that P fertilization likely moved the phenotype of the AM symbiosis from partly mutualistic (for the aboveground biomass) to amensalistic. AMF reduced hyperforin content in both low and high P if compared to the non-inoculated controls. In contrast to the present study, other authors (Dias et al. 2001; Azizi and Omidbaigi 2002) found that N and P fertilization increased hyperforin content. Such a difference can depend on the different features of the growth substrate used in both experiments, as also suggested by Bruni and Sacchetti (2009). Hyperforin biosynthesis in H. perforatum starts from amino acid precursors and proceeds with prenylation (Karppinen et al. 2007). It has been shown that AMF can decrease free amino acid content and saturated fatty acid content in host plants (Rivero et al. 2015; Saia et al. 2015b) and that AMF depend on their host plants for the biosynthesis of some special fatty acids (Trepanier et al. 2005). Hence, it is likely that hyperforin biosynthesis decreases in mycorrhizal rather than in control plants due to a sequestration of precursors needed by the AMF. However, other fungal and plant-mediated mechanisms also can be involved in such a reduction. For example, the application of either a living or autoclaved cell suspension of the fungus Nomuraea rileyi to H. polyanthemum reduced the content of uliginosin B, a phloroglucinol derivative. Furthermore, hyperforin has an antimicrobial function in the plant (Kirakosyan et al. 2004), and thus its reduced accumulation in mycorrhizal compared to non-mycorrhizal plants could be related to the ability of the AM partner to suppress some of the plant’s defense mechanisms (Garcia-Garrido 2002). Finally, the unclear relationship between available sugars and hyperforin content in H. perforatum, as assessed in a bioreactor (Zobayed et al. 2003), and the demand for sugars by the AM fungi could be related to the reduced hyperforin content of the mycorrhizal plants.
Hypericin and pseudohypericin concentrations were higher under high than low P availability in the non-mycorrhizal controls. Similar results were found by other authors (Dias et al. 2001; Azizi and Omidbaigi 2002). On the other hand, AMF increased hypericin and pseudohypericin content only under low P availability. Similar results were found by Zubek et al. (2012), who found that an AMF mixture increased the content of naphthodianthrones more than single AM species did. The latter authors attributed this result to a plant regulation mechanism of the symbiosis with multiple AMF species through an increase in secondary metabolite production, as also observed by Pinior et al. (1999). Lingua et al. (2013) also found that AMF increased the content of many polyphenols in strawberry, and this result occurred at low nutrient availability. However, several mechanisms of the plant-fungi interaction can be involved in the stimulation by the AMF of naphthodianthrone synthesis. For example, methyl jasmonate or salicylic acid is involved in the mycorrhizal symbiosis (Pozo et al. 2004), positively affects hypericin content, and is involved in the plant-fungi interaction (Sirvent and Gibson 2002). Indeed, the activation in the arbuscular mycorrhizal symbiosis of molecular mechanisms common to those of plant pathogens already has been found (Pozo et al. 2010). In addition, as suggested above, AMF can reduce the N content of the plant (Saia et al. 2014a) or readdress amino acid metabolism to the biosynthesis of secondary compounds (Battini et al. 2016; Srivastava et al. 2016). Other authors (Briskin et al. 2000; Briskin and Gawienowski 2001) also showed that a reduction in N availability increased hypericin content without resulting in nitrogen deficiency symptoms. And indeed, we found that AM fungi did not alter SPAD values which are related to plant nutrient status. Finally, the reduced hyperforin content in the mycorrhizal compared to non-mycorrhizal treatments could indirectly have increased the availability of malonyl-coA, a common precursor to both hyperforin and naphthodianthrone biosynthetic pathways, and thus increased the latter in the mycorrhizal plant compared to the non-inoculated controls.
Conclusions
In the present experiment, AM fungi increased the concentration of the a.i. of H. perforatum flowers but strongly reduced the amount of flowers per plant, which consisted in a reduction of the total a.i. production per plant. Such an effect could have drawbacks when growing H. perforatum in semi-arid areas because high temperatures during flowering can reduce the timespan and intensity of flowering. Nonetheless, in temperate or cold environments, delaying flowering could result in a higher biomass accumulation and total flower production. The results of the present experiment also showed that AM fungi can play an important role in the accumulation of bioactive compounds in H. perforatum and that such effects could be related to the P uptake by the AMF partner. However, also other H. perforatum-fungi interactions could be involved in such effects, because both hyperforin and naphthodianthrones show antimicrobial and antifeedant activity (Kusari et al. 2013) and an endophytic fungus of H. perforatum was able to produce hypericin in a growth medium without any compound from its host plant (Kusari et al. 2008).
The reduction of the number of flowers of mycorrhizal than control plants could be related to competition for photosynthates between partners (Johnson et al. 1982), and such competition could be exacerbated when some mineral nutrient also is divided between AM symbiont and plant sinks (e.g., sprouts or flowering centers). At adequate resource availability for the AM fungus, mycorrhizal plants might instead contribute to compensatory increases in photosynthesis, and this would both compensate for photosynthates supplied to the AM partner and increase flower abundance.
References
Alloush GA, Zeto SK, Clark RB (2000) Phosphorus source, organic matter, and arbuscular mycorrhiza effects on growth and mineral acquisition of chickpea grown in acidic soil. J Plant Nutr 23:1351–1369. doi:10.1080/01904160009382105
Asrar AA, Abdel-Fattah GM, Elhindi KM (2012) Improving growth, flower yield, and water relations of snapdragon (Antirhinum majus L.) plants grown under well-watered and water-stress conditions using arbuscular mycorrhizal fungi. Photosynthetica 50:305–316. doi:10.1007/s11099-012-0024-8
Azizi M, Omidbaigi R (2002) Effect of NP supply on herb yield, hypericin content and cadmium accumulation of St. John’s Wort (Hypericum perforatum L.). Acta Hortic:267–271. doi:10.17660/ActaHortic.2002.576.39
Battini F, Bernardi R, Turrini A et al (2016) Rhizophagus intraradices or its associated bacteria affect gene expression of key enzymes involved in the rosmarinic acid biosynthetic pathway of basil. Mycorrhiza. doi:10.1007/s00572-016-0707-2
Bona E, Cantamessa S, Massa N et al (2016a) Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: a field study. Mycorrhiza. doi:10.1007/s00572-016-0727-y
Bona E, Lingua G, Manassero P et al (2015) AM fungi and PGP pseudomonads increase flowering, fruit production, and vitamin content in strawberry grown at low nitrogen and phosphorus levels. Mycorrhiza 25:181–193. doi:10.1007/s00572-014-0599-y
Bona E, Lingua G, Todeschini V (2016b) Effect of bioinoculants on the quality of crops. In: Arora NK, Mehnaz S, Balestrini R (eds) Bioformulations: for sustainable agriculture. Springer India, New Delhi, pp. 93–124
Briskin DP, Gawienowski MC (2001) Differential effects of light and nitrogen on production of hypericins and leaf glands in Hypericum perforatum. Plant Physiol Biochem 39:1075–1081. doi:10.1016/S0981-9428(01)01326-2
Briskin DP, Leroy A, Gawienowski M (2000) Influence of nitrogen on the production of hypericins by St. John’s wort. Plant Physiol Biochem 38:413–420. doi:10.1016/S0981-9428(00)00754-3
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77. doi:10.1007/s11104-008-9877-9
Bruni R, Sacchetti G (2009) Factors affecting polyphenol biosynthesis in wild and field grown St. John’s Wort (Hypericum perforatum L. Hypericaceae/Guttiferae). Molecules 14:682–725. doi:10.3390/molecules14020682
Bunn R, Lekberg Y, Zabinski C (2009) Arbuscular mycorrhizal fungi ameliorate temperature stress in thermophilic plants. Ecology 90:1378–1388. doi:10.1890/07-2080.1
Carrubba A, Scalenghe R (2012) The scent of Mare Nostrum: medicinal and aromatic plants in Mediterranean soils. J Sci Food Agric 92:1150–1170. doi:10.1002/jsfa.5630
Copetta A, Lingua G, Berta G (2006) Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 16:485–494. doi:10.1007/s00572-006-0065-6
Davison J, Öpik M, Daniell TJ et al (2011) Arbuscular mycorrhizal fungal communities in plant roots are not random assemblages. FEMS Microbiol Ecol 78:103–115. doi:10.1111/j.1574-6941.2011.01103.x
Davoodian N, Bosworth J, Rajakaruna N (2012) Mycorrhizal colonization of Hypericum perforatum L. (Hypericaceae) from serpentine and granite outcrops on the Deer Isles, Maine. Northeast Nat 19:517–526. doi:10.1656/045.019.0312
Dias ACP, Majid A, Reza O (2001) Effect of nitrogen and phosporous fertilisation in the cultivation of Hypericum perforatum L. variety Topas. In: International Congress and 49th annual meeting of the Society of Medicinal Plant Research. Erlangen, Germany, p short lecture
Dubský M, Vosátka M (2000) Inoculation of cyclamen (Cyclamen persicum) and poinsettia (Euphorbia pulcherrima) with arbuscular mycorrhizal fungi and Trichoderma harzianum. Rostlinna vyroba 48:63–68
Garcia-Garrido JM (2002) Regulation of the plant defence response in arbuscular mycorrhizal symbiosis. J Exp Bot 53:1377–1386. doi:10.1093/jexbot/53.373.1377
Gaur A, Adholeya A (2005) Diverse response of five ornamental plant species to mixed indigenous and single isolate arbuscular-mycorrhizal inocula in marginal soil amended with organic matter. J Plant Nutr 28:707–723. doi:10.1081/PLN-200052647
Gaur A, Gaur A, Adholeya A (2000) Growth and flowering in Petunia hybrida, Callistephus chinensis and Impatiens balsamina inoculated with mixed AM inocula or chemical fertilizers in a soil of low P fertility. Sci Hortic 84:151–162. doi:10.1016/S0304-4238(99)00105-3
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500. doi:10.1111/j.1469-8137.1980.tb04556.x
Hodge A, Fitter AH (2010) Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc Natl Acad Sci U S A 107:13754–13759. doi:10.1073/pnas.1005874107
Jin Z, Li J, Li Y (2015) Interactive effects of arbuscular mycorrhizal fungi and copper stress on flowering phenology and reproduction of Elsholtzia splendens. PLoS One 10:e0145793. doi:10.1371/journal.pone.0145793
Johnson CR, Graham JH, Leonard RT, Menge JA (1982) Effect of flower bud development in chrysanthemum on vesicular-arbuscular mycorrhiza formation. New Phytol 90:671–675. doi:10.1111/j.1469-8137.1982.tb03277.x
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–585. doi:10.1046/j.1469-8137.1997.00729.x
Johnson NC, Wilson GWT, Wilson JA et al (2014) Mycorrhizal phenotypes and the law of the minimum. The New phytologist. doi:10.1111/nph.13172
Joner EJ, Jakobsen I (1995) Growth and extracellular phosphatase activity of arbuscular mycorrhizal hyphae as influenced by soil organic matter. Soil Biol Biochem 27:1153–1159. doi:10.1016/0038-0717(95)00047-I
Jurkiewicz A, Ryszka P, Anielska T et al (2010) Optimization of culture conditions of Arnica montana L.: effects of mycorrhizal fungi and competing plants. Mycorrhiza 20:293–306. doi:10.1007/s00572-009-0280-z
Kapoor R, Giri B, Mukerji KG (2004) Improved growth and essential oil yield and quality in Foeniculum vulgare mill on mycorrhizal inoculation supplemented with P-fertilizer. Bioresour Technol 93:307–311. doi:10.1016/j.biortech.2003.10.028
Karppinen K, Hokkanen J, Tolonen A et al (2007) Biosynthesis of hyperforin and adhyperforin from amino acid precursors in shoot cultures of Hypericum perforatum. Phytochemistry 68:1038–1045. doi:10.1016/j.phytochem.2007.01.001
Kaschuk G, Leffelaar P, Giller KE et al (2010) Responses of legumes to rhizobia and arbuscular mycorrhizal fungi: a meta-analysis of potential photosynthate limitation of symbioses. Soil Biol Biochem 42:125–127. doi:10.1016/j.soilbio.2009.10.017
Khaosaad T, Vierheilig H, Nell M et al (2006) Arbuscular mycorrhiza alter the concentration of essential oils in oregano (Origanum sp., Lamiaceae). Mycorrhiza 16:443–446. doi:10.1007/s00572-006-0062-9
Kirakosyan A, Sirvent TM, Gibson DM, Kaufman PB (2004) The production of hypericins and hyperforin by in vitro cultures of St. John’s wort (Hypericum perforatum). Biotechnol Appl Biochem 39:71. doi:10.1042/BA20030144
Kusari S, Lamshöft M, Zühlke S, Spiteller M (2008) An endophytic fungus from Hypericum perforatum that produces hypericin. J Nat Prod 71:26–29
Kusari S, Pandey SP, Spiteller M (2013) Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites. Phytochemistry 91:81–87. doi:10.1016/j.phytochem.2012.07.021
Lazzara S, Napoli E, Carrubba A (2015) Hypericum spp.: a resource from wild Mediterranean flora for the treatment of mild depression. In: Gupta VK (ed) Bioactive phytochemicals—perspectives for modern medicine, vol 3. Daya Publishing House, New Delhi, pp. 337–354
Linderman RG, Davis EA (2004) Varied response of marigold (Tagetes spp.) genotypes to inoculation with different arbuscular mycorrhizal fungi. Sci Hortic 99:67–78. doi:10.1016/S0304-4238(03)00081-5
Lingua G, Bona E, Manassero P et al (2013) Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria x ananassa var. Selva) in conditions of reduced fertilization. Int J Mol Sci 14:16207–16225. doi:10.3390/ijms140816207
Liu J, Wu L, Wei S et al (2007) Effects of arbuscular mycorrhizal fungi on the growth, nutrient uptake and glycyrrhizin production of licorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul 52:29–39. doi:10.1007/s10725-007-9174-2
López-García Á, Palenzuela J, Barea JM, Azcón-Aguilar C (2014) Life-history strategies of arbuscular mycorrhizal fungi determine succession into roots of Rosmarinus officinalis L., a characteristic woody perennial plant species from Mediterranean ecosystems. Plant Soil 379:247–260. doi:10.1007/s11104-014-2060-6
Majewska ML, Rola K, Zubek S (2016) The growth and phosphorus acquisition of invasive plants Rudbeckia laciniata and Solidago gigantea are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza. doi:10.1007/s00572-016-0729-9
Maron JL, Vila M, Arnason J (2004) Loss of enemy resistance among introduced populations of St. John’s Wort (Hypericum perforatum). Ecology 85:3243–3253. doi:10.1890/04-0297
Moora M, Zobel M (1998) Can arbuscular mycorrhiza change the effect of root competition between conspecific plants of different ages? Can J Bot 76:613–619. doi:10.1139/b98-037
Nell M, Wawrosch C, Steinkellner S et al (2010) Root colonization by symbiotic arbuscular mycorrhizal fungi increases sesquiterpenic acid concentrations in Valeriana officinalis L. Planta Med 76:393–398. doi:10.1055/s-0029-1186180
Nowak J (2004) Effects of arbuscular mycorrhizal fungi and organic fertilization on growth, flowering, nutrient uptake, photosynthesis and transpiration of geranium (Pelargonium hortorum L.H. Bailey “Tango Orange”). Symbiosis 37:259–266
Pellegrino E, Öpik M, Bonari E, Ercoli L (2015) Responses of wheat to arbuscular mycorrhizal fungi: a meta-analysis of field studies from 1975 to 2013. Soil Biol Biochem 84:210–217. doi:10.1016/j.soilbio.2015.02.020
Perner H, Schwarz D, Bruns C et al (2007) Effect of arbuscular mycorrhizal colonization and two levels of compost supply on nutrient uptake and flowering of pelargonium plants. Mycorrhiza 17:469–474. doi:10.1007/s00572-007-0116-7
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–IN18. doi:10.1016/S0007-1536(70)80110-3
Pinior A, Wyss U, Piché Y, Vierheilig H (1999) Plants colonized by AM fungi regulate further root colonization by AM fungi through altered root exudation. Can J Bot 77:891–897. doi:10.1139/cjb-77-6-891
Pozo MJ, Jung SC, López-Ráez JA (2010) Impact of arbuscular mycorrhizal symbiosis on plant response to biotic stress: the role of plant defence mechanisms. In: Koltai H, Kapulnik Y (eds) Arbuscular mycorrhizas: physiology and function. Springer Netherlands, Dordrecht, pp. 193–207
Pozo MJ, Van Loon LC, Pieterse CMJ (2004) Jasmonates—signals in plant-microbe interactions. J Plant Growth Regul 23:211–222. doi:10.1007/BF02637262
Rivero J, Gamir J, Aroca R et al (2015) Metabolic transition in mycorrhizal tomato roots. Front Microbiol. doi:10.3389/fmicb.2015.00598
Ruhfel BR, Stevens PF, Davis CC (2013) Combined morphological and molecular phylogeny of the clusioid clade (Malpighiales) and the placement of the ancient rosid macrofossil Paleoclusia. Int J Plant Sci 174:910–936. doi:10.1086/670668
Russo E, Scicchitano F, Whalley BJ et al (2014) Hypericum perforatum : pharmacokinetic, mechanism of action, tolerability, and clinical drug-drug interactions. Phytother Res 28:643–655. doi:10.1002/ptr.5050
Rydlová J, Jelínková M, Dušek K et al (2016) Arbuscular mycorrhiza differentially affects synthesis of essential oils in coriander and dill. Mycorrhiza 26:123–131. doi:10.1007/s00572-015-0652-5
Saia S, Amato G, Frenda AS et al (2014a) Influence of arbuscular mycorrhizae on biomass production and nitrogen fixation of berseem clover plants subjected to water stress. PLoS One 9:e90738. doi:10.1371/journal.pone.0090738
Saia S, Benítez E, García-Garrido JM et al (2014b) The effect of arbuscular mycorrhizal fungi on total plant nitrogen uptake and nitrogen recovery from soil organic material. J Agric Sci 152:370–378. doi:10.1017/S002185961300004X
Saia S, Rappa V, Ruisi P et al (2015a) Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front Plant Sci 6:815
Saia S, Ruisi P, Fileccia V et al (2015b) Metabolomics suggests that soil inoculation with arbuscular mycorrhizal fungi decreased free amino acid content in roots of durum wheat grown under N-limited, P-rich field conditions. PLoS One 10:e0129591. doi:10.1371/journal.pone.0129591
Scagel CF (2004) Inoculation with vesicular-arbuscular mycorrhizal fungi and Rhizobacteria alters nutrient allocation and flowering of harlequin flower. HortTechnology 14:39–48
Schabenberger O (2005) Introducing the Glimmix Procedure for generalized linear mixed models. SUGI 30 Proceedings 1–20. doi: http://www2.sas.com/proceedings/sugi30/toc.html#fm
Schenck NC, Smith GS (1982) Responses of six species of vesicular-arbuscular mycorrhizal fungi and their effects on soybean at four soil temperatures. New Phytol 92:193–201
Seifert EK, Bever JD, Maron JL (2009) Evidence for the evolution of reduced mycorrhizal dependence during plant invasion. Ecology 90:1055–1062. doi:10.1890/08-0419.1
Sirvent T, Gibson D (2002) Induction of hypericins and hyperforin in Hypericum perforatum L. in response to biotic and chemical elicitors. Physiol Mol Plant Pathol 60:311–320. doi:10.1016/S0885-5765(02)90410-8
Slafer GA, Calderini DF, Miralles DJ (1996) Yield components and compensation in wheat: opportunities for further increasing yield potential. Increasing yield potential in wheat: Breaking the Barriers 101–133
Smith SE, Read D (2008) Mycorrhizas in agriculture, horticulture and forestry. In: Mycorrhizal symbiosis. Elsevier, p 611–XVIII
Srivastava S, Conlan XA, Cahill DM, Adholeya A (2016) Rhizophagus irregularis as an elicitor of rosmarinic acid and antioxidant production by transformed roots of Ocimum basilicum in an in vitro co-culture system. Mycorrhiza. doi:10.1007/s00572-016-0721-4
Tawaha K, Gharaibeh M, El-Elimat T, Alali FQ (2010) Determination of hypericin and hyperforin content in selected Jordanian Hypericum species. Ind Crop Prod 32:241–245. doi:10.1016/j.indcrop.2010.04.017
Trepanier M, Becard G, Moutoglis P et al (2005) Dependence of arbuscular-mycorrhizal fungi on their plant host for palmitic acid synthesis. Appl Environ Microbiol 71:5341–5347. doi:10.1128/AEM.71.9.5341-5347.2005
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355. doi:10.1111/j.1469-8137.2004.01159.x
Usha K, Mathew R, Singh B (2005) Effect of three species of arbuscular mycorrhiza on Bud sprout and ripening in grapevine ( Vitis vinifera L.) cv. Perlette. Biological Agriculture & Horticulture 23:73–83. doi:10.1080/01448765.2005.9755309
van der Heijden MGA, Horton TR (2009) Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J Ecol 97:1139–1150. doi:10.1111/j.1365-2745.2009.01570.x
Varela-Cervero S, López-García Á, Barea JM, Azcón-Aguilar C (2016) Differences in the composition of arbuscular mycorrhizal fungal communities promoted by different propagule forms from a Mediterranean shrubland. Mycorrhiza 26:489–496. doi:10.1007/s00572-016-0687-2
Walter MH, Fester T, Strack D (2000) Arbuscular mycorrhizal fungi induce the non-mevalonate methylerythritol phosphate pathway of isoprenoid biosynthesis correlated with accumulation of the “yellow pigment” and other apocarotenoids doi:10.1046/j.1365-313x.2000.00708.x. Plant J 21:571–578
Zeng Y, Guo L-P, Chen B-D et al (2013) Arbuscular mycorrhizal symbiosis and active ingredients of medicinal plants: current research status and prospectives. Mycorrhiza 23:253–265. doi:10.1007/s00572-013-0484-0
Zobayed SMA, Murch SJ, Rupasinghe HPV, Saxena PK (2003) Elevated carbon supply altered hypericin and hyperforin contents of St. John’s wort (Hypericum perforatum) grown in bioreactors. Plant cell. Tissue and Organ Culture 75:143–149. doi:10.1023/A:1025053427371
Zubek S, Mielcarek S, Turnau K (2012) Hypericin and pseudohypericin concentrations of a valuable medicinal plant Hypericum perforatum L. are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza 22:149–156. doi:10.1007/s00572-011-0391-1
Acknowledgements
The authors are grateful to Mrs. R. Cusimano, Mrs. I. Altobello, and Mr. D. Platia for their kind help during the experiment. The authors also thank the Editor-in-Chief and two anonymous reviewers for their constructive comments, which helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Silvia Lazzara and Marcello Militello equally contributed to this work.
Rights and permissions
About this article
Cite this article
Lazzara, S., Militello, M., Carrubba, A. et al. Arbuscular mycorrhizal fungi altered the hypericin, pseudohypericin, and hyperforin content in flowers of Hypericum perforatum grown under contrasting P availability in a highly organic substrate. Mycorrhiza 27, 345–354 (2017). https://doi.org/10.1007/s00572-016-0756-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-016-0756-6