Abstract
A pot experiment was conducted to examine the effect of the arbuscular mycorrhizal (AM) fungus, Glomus mosseae, on plant biomass and organic solute accumulation in maize leaves. Maize plants were grown in sand and soil mixture with three NaCl levels (0, 0.5, and 1.0 g kg−1 dry substrate) for 55 days, after 15 days of establishment under non-saline conditions. At all salinity levels, mycorrhizal plants had higher biomass and higher accumulation of organic solutes in leaves, which were dominated by soluble sugars, reducing sugars, soluble protein, and organic acids in both mycorrhizal and non-mycorrhizal plants. The relative abundance of free amino acids and proline in total organic solutes was lower in mycorrhizal than in non-mycorrhizal plants, while that of reducing sugars was higher. In addition, the AM symbiosis raised the concentrations of soluble sugars, reducing sugars, soluble protein, total organic acids, oxalic acid, fumaric acid, acetic acid, malic acid, and citric acid and decreased the concentrations of total free amino acids, proline, formic acid, and succinic acid in maize leaves. In mycorrhizal plants, the dominant organic acid was oxalic acid, while in non-mycorrhizal plants, the dominant organic acid was succinic acid. All the results presented here indicate that the accumulation of organic solutes in leaves is a specific physiological response of maize plants to the AM symbiosis, which could mitigate the negative impact of soil salinity on plant productivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salt-affected soils occupy approximately 7% of the global land surface (Ruiz-Lozano et al. 1996). In China alone, salinity affects about 3,630.5×104 hm2 land (Shi 1991). In general, salinity limits plant growth and productivity, especially in arid and semiarid regions (Apse et al. 1999). Arbuscular mycorrhizal (AM) fungi widely occur in saline environments (Sengupta and Chaudhuri 1990). Many researchers reported that AM fungi could enhance the ability of plants to cope with salt stress (Yano-Melo et al. 2003; Rabie 2005; Jahromi et al. 2008) in improving mineral nutrient absorption (Cantrell and Linderman 2001; Asghari et al. 2005), maintaining ion balance (Zandavalli et al. 2004; Giri et al. 2007), protecting enzyme activities (Giri and Mukerji 2004; Rabie and Almadini 2005), and facilitating water uptake (Berta et al. 1990; Ruiz-Lozano and Azcón 1995).
One of the most important responses of glycophytes to salinity is the accumulation of osmotically active organic solutes called osmolytes as a result of alterations in intermediary and secondary metabolism of nitrogen and carbon (Greenway and Munns 1980; Hasegawa et al. 2000; Hoque et al. 2007). This response is an important component of salinity tolerance in plants (Neocleous and Vasilakakis 2007). Organic solutes, such as proline, free amino acids, soluble protein, and sugars, maintain the osmotic balance and protect enzymes in presence of high cytoplasmic electrolyte concentrations (Greenway and Munns 1980; Hajlaoui et al. 2010). The regulation of organic acid metabolism also plays a key role in plant tolerance to saline conditions (Guo et al. 2010). The accumulation of organic acids may serve as counter ions to cations. They prevent toxic chloride accumulation in cells, contribute to cytosolic pH regulation (Yang et al. 2007; Hatzig et al. 2010), and are important osmolytes in plant vacuoles (Guo et al. 2010). Under salt stress, Francoise et al. (1991) found increased citric acid concentration in alfalfa roots, and Szalai and Janda (2009) found increased salicylic acid synthesis in the leaves and roots of young maize plants. It has been reported that AM fungi could modify the accumulation of soluble sugars in Trifolium alexandrinum (Khaled et al. 2003), soluble protein in T. alexandrinum (Khaled et al. 2003), Glycine max (Abdel-Fattah 2001), Vigna radiata (Rabie 2005), Vicia faba (Rabie and Almadini 2005), and Lotus glaber (Sannazzaro et al. 2006), and proline in Lactuca sativa (Ruiz-Lozano et al. 1996; Jahromi et al. 2008) and T. alexandrinum (Khaled et al. 2003).
Maize (Zea mays) is a very common crop in saline soils in China. Salt stress can inhibit the growth of maize, but this inhibition can be mitigated by the AM symbiosis (Feng et al. 2000, 2002; Sheng et al. 2008) through the modification of root morphology, and improvement of root activity and photosynthetic capacity (Sheng et al. 2008, 2009). Little is known, however, about the effect of the AM symbiosis on the regulation of organic solute levels in maize plants under saline conditions. Two studies reported an increase in maize shoot and root soluble sugar content (Feng et al. 2002), and a decrease in maize leaf proline content (Feng et al. 2000). In this study, we tested the effect of the AM symbiosis on the accumulation of organic solutes in maize leaves at different levels of soil salinity. We measured the concentrations of proline, free amino acids, reducing sugars, soluble sugars, soluble protein, and organic acids and interpreted modifications with relation to possible mechanisms involved in the mitigation of salt stress by the AM symbiosis in maize.
Materials and methods
Plant and soil
The soil (Eum-Orthic Anthrosols) used in this study was collected from the top layer (0–20 cm) of a field where maize (in summer) and wheat (in winter) were grown, in Yangling City, Shaanxi Province, China. The soil (pH 7.6, soil/water ratio of 1:2.5 w/v) contained 20 g kg−1 organic matter, 37 mg kg−1 available nitrogen, 12 mg kg−1 available phosphorus, and 207 mg kg−1 available potassium, measured as described by Bao (2000). The soil was ground, sieved through 2 mm, and mixed with fine sand (sand/soil, 1:2 v/v). The mixture was autoclaved at 121°C for 2 h.
Seeds of maize cultivar Shandan 16, which is sensitive to salinity, were surface-sterilized with 0.1% HgCl2 for 3 min, rinsed five times with sterile distilled water, and allowed to germinate on moist sterile filter paper at 28°C. Five pre-germinated seeds were sown in 3-l pot containing 2 kg of the sand/soil mixture. Seedlings were thinned to two per pot 10 days after sowing. Plants were supplemented once a week with 50 ml of a nutrient solution containing 6 mM KNO3, 1 mM NH4H2PO4, 2.6 mM MgSO4, 8 mM Ca(NO3)2, 10 μM H3BO3, 1.6 μM MnSO4, 1 μM ZnSO4, 0.5 μM CuSO4, 50 μM (NH4)6Mo2O4, and 20 μM Fe-EDTA. The solution pH was adjusted to 6.5 ± 0.3 (Li and Feng 2001).
AM inoculum
Glomus mosseae (Nicolson & Gerdemann) was isolated from the rhizosphere of Agropyron cristatum in a saline soil of the Inner Mongolia Autonomous Region, China, and multiplied in pot cultures using Z. mays as a host. The mycorrhizal inoculum consisted of soil containing spores (306–420 per 100 g dry soil), mycelium, and root fragments with a colonization level of 94%. Each pot received 30 g of inoculum or 30 g of sterilized inoculum plus 10 ml mycorrhizal fungi-free filtrate (1 μm) of inoculum washing as the non-mycorrhizal treatment. Live and sterile inocula were placed in a layer 3 cm below the maize seeds prior to sowing.
Experimental design
The experiment was conducted in a greenhouse under a temperature of 22–30°C, 12–14 h daylight, and 70–75% relative humidity, between July and September 2006. Pots were arranged in a randomized complete block design. Treatments were factorial combinations of two factors: (1) inoculation, with G. mosseae or non-mycorrhizal control, and (2) salinity, with 0, 0.5, and 1.0 g NaCl kg−1 dry soil mix. Ten pots were prepared for each treatment. In order to avoid an effect of salinity on fine plants and AM establishment, maize plants were grown for 15 days before application of the NaCl treatments, which was achieved by adding NaCl in irrigation water (0, 10, and 20 g l−1). To avoid osmotic shock, NaCl was introduced gradually by successively adding 20 ml of the NaCl solutions in each pot every day for 5 days, starting at day 15 after sowing. In total, each pot received 100 ml of a designated saline solution. This brought the electrical conductivity (EC) of saturated soil extracts to 0.8, 1.9, and 3.6 dS m−1 in the 0, 0.5, and 1.0 g kg−1 NaCl treatments, respectively. Leaching was prevented by keeping soil water below field capacity at all times. EC of soil extract was monitored using a conductivity meter (DDS-11A) and adjusted once a month. Plants were harvested 70 days after sowing. Five pots of each treatment were used to determine the biomass of maize plants. The other five pots were used to determine AM colonization rate and concentrations of soluble protein, soluble sugars, reducing sugars, proline, total free amino acids, and organic acids.
Measurement and analysis
The biomass of maize plants was determined after oven-drying their root and shoot at 70°C for 90 h according to the method described by Gao (2000).
To estimate AM colonization, roots were collected, washed gently with tap water, and dried with paper towels. A subsample of 0.5 g fresh roots was cleared 15 min in 10% KOH at 90°C, bleached in alkaline hydrogen peroxide for 20 min, acidified in 1% HCl, and stained in lactophenol blue (Phillips and Hayman 1970). Colonization was estimated using the gridline intercept method described by Giovannetti and Mosse (1980).
The reducing sugars, soluble sugars, total free amino acids, proline, and soluble protein of the second fully expanded leaf were measured according to the method described by Gao (2000).
Organic acids were measured on the second fully expanded leaf. A subsample of 1.5 g fresh leaf material was selected for organic acid measurement. Organic acids in maize leaves were extracted using the method described by Gao (2000) and measured using high performance liquid chromatography (Waters-510 equipped with a Variable UV/Vis detector model 481). A hypersil BDS column (C18, 4.6 × 250 mm, 10 μm) was used, and the mobile phase was a 0.5% diammonium phosphate solution (pH = 2.5). The column was held at 30°C, the flow rate of the mobile phase was 1.0 ml min−1, and the detection wavelength was 205 nm.
Statistical analysis
The data was subjected to analysis of variance. Treatment means were compared by Duncan's test at the 5% level (SAS version 8.0).
Results
AM fungal colonization
Non-inoculated plants had no AM colonization. AM fungal colonization of inoculated plants ranged from 99% to 79% root length.
Plant biomass
Maize plants produced more biomass when mycorrhizal, but plant productivity decreased with increasing salinity in all plants (Fig. 1).
Soluble sugars, reducing sugars, total free amino acids, proline, and soluble protein
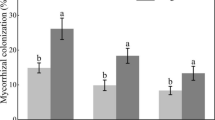
Concentrations of soluble and reducing sugars were significantly higher in mycorrhizal than in non-mycorrhizal plants regardless of salinity level and decreased with increasing soil salinity (Fig. 2a, b). At all salinity levels, total free amino acids and proline concentrations were lower in mycorrhizal than in non-mycorrhizal plants, although the difference in total free amino acid concentration was not significant in the 0 g kg−1 NaCl treatment. Concentrations of total free amino acids and proline increased with increasing salinity in non-mycorrhizal plants, but not in mycorrhizal plants (Fig. 2c, d). Regardless of salinity level, soluble protein concentration was higher in mycorrhizal than in non-mycorrhizal plants, although the difference between mycorrhizal and non-mycorrhizal plants was not significant (Fig. 2e).
Concentrations of soluble sugars (a), reducing sugars (b), total free amino acids (c), proline (d), and soluble protein (e) in leaves of maize plants inoculated (+M) or not (−M) with G. mosseae and grown at three NaCl levels. Means (±SD) labeled with different letters are significantly different (p < 0.05, n = 5) by Duncan's test
Organic acids
At all salinity levels, the concentration of total organic acids was higher in mycorrhizal than in non-mycorrhizal plants, although the difference was not significant in the 1.0 g kg−1 NaCl treatment. At 0, 0.5, and 1.0 g kg−1 NaCl levels, the concentration of total organic acids in mycorrhizal plants increased by 31.4%, 24.8%, and 8.1%, respectively, compared with the corresponding controls (Fig. 3).
Oxalic, fumaric, acetic, malic, citric, formic, lactic, and succinic acids were found in all plants, but the concentrations of these organic acids differed with plant mycorrhizal status. Differences were most striking for oxalic acid and succinic acid, especially in the 1.0 g kg−1 NaCl treatment. The dominant organic acid in mycorrhizal plants was oxalic acid, while in non-mycorrhizal plants, succinic acid was dominant.
At all salinity levels, the concentrations of oxalic, fumaric, acetic, malic, and citric acids were higher in mycorrhizal than in non-mycorrhizal plants, although the difference in concentrations of malic and citric acids was not significant in the 0.5 and 1.0 g kg−1 NaCl treatment. There were no significant differences in lactic acid concentration between mycorrhizal and non-mycorrhizal plants. At 0 and 0.5 g kg−1 NaCl levels, no significant effect of inoculation on the concentrations of formic and succinic acids was recorded, while at 1.0 g kg−1 NaCl level, non-mycorrhizal plants had higher concentrations of both organic acids (Fig. 4).
Concentrations of oxalic, formic, malic, lactic, fumaric, acetic, citric, and succinic acids in leaves of maize plants inoculated (+M) or not (−M) with G. mosseae and grown at three NaCl levels. Means (±SD) labeled with different letters are significantly different (p < 0.05, n = 5) by Duncan's test
Organic solute concentrations and relative abundance
The amount of total organic solutes was higher in mycorrhizal plants regardless of salinity level (Fig. 5). The AM symbiosis reduced the relative abundance of free amino acids and proline in total organic solutes, increased that of reducing sugars, but did not significantly affect the balance of soluble protein, organic acids, and soluble sugars (Fig. 6). The relative abundance of soluble protein was high in the organic solutes of all plants, while proline only accounted for 0.03–0.05% of solutes in mycorrhizal plants and for 0.27–0.33% in non-mycorrhizal plants. Soluble sugars, reducing sugars, soluble protein, and organic acids made up over 99% and 93% of the organic solutes of mycorrhizal plants and non-mycorrhizal plants, respectively (Fig. 6). Consequently, soluble sugars, reducing sugars, soluble protein, and organic acids were the dominant organic solutes in both mycorrhizal and non-mycorrhizal plants.
Discussion
Free amino acids are important osmolytes contributing to osmotic adjustment in plants (Hajlaoui et al. 2010). With increasing external salt concentration, free amino acids accumulate in the leaves and roots of maize (Abd-El Baki et al. 2000; Neto et al. 2009; Hajlaoui et al. 2010). We also observed the increase of free amino acid levels in maize leaves under salt stress, but to a lesser extent in AM plants. Among free amino acids, proline is a contributor to osmotic adjustment in salt-stressed maize plants (Hajlaoui et al. 2010). Reports on the effect of AM symbiosis on proline accumulation are somewhat contradictory. Some studies have shown an increase in proline accumulation in mycorrhizal plants subjected to salt stress (Khaled et al. 2003; Sharifi et al. 2007). Enhanced proline accumulation in plant cells can increase plant osmotic potentials (Hajlaoui et al. 2010) and abscissic acid level (Ober and Sharp 1994), thereby improving the tolerance of mycorrhizal plants to salinity. On the contrary, some studies have shown a reduction of proline levels in AM plants under salt stress (Duke et al. 1986; Ruiz-Lozano et al. 1996; Jahromi et al. 2008), as we have found in this study. This and the low relative abundance of proline in total organic solutes suggest that proline contributes little to osmotic balance and salt tolerance in mycorrhizal plants. The lower accumulation of proline in AM plants appears to indicate a lower level of salt stress in mycorrhizal plants.
We found higher soluble protein concentrations in mycorrhizal than in non-mycorrhizal plants under salt stress. Similar results were reported in L. glaber (Sannazzaro et al. 2006), V. radiata (Rabie 2005), V. faba (Rabie and Almadini 2005), T. alexandrinum (Khaled et al. 2003), and G. max (Abdel-Fattah 2001). Normally, the steady-state levels of soluble protein in plant cytoplasm depend on the rates of protein degradation and synthesis (Guo et al. 1999). Thus, AM-mediated increase in soluble protein has been attributed to AM-mediated activation of certain plant genes (Ouziad et al. 2006; Jahromi et al. 2008). However, the observation of reduced levels of total free amino acids concurrent with higher levels of soluble protein, in our study, suggests that the AM-mediated increase in soluble protein concentration is rather due to reduced protein degradation in AM maize.
In our study, sugar (soluble sugars and reducing sugars) accumulation in maize leaves decreased when salinity increased, but at the same NaCl level, the AM symbiosis favored sugar accumulation. Similar results were observed in the shoots of V. radiata (Rabie 2005) and in roots and shoots of maize (Feng et al. 2002). The high levels of sugars in mycorrhizal plants may be the result of an increase in photosynthetic capacity (Sheng et al. 2008; Wu et al. 2009). The accumulation of sugars induced by the AM symbiosis is a positive response to salt stress since sugars can prevent structural changes in soluble protein, maintain the osmotic equilibrium in plant cells, and protect membrane integrity (Abd-El Baki et al. 2000).
Zhang et al. (2003) reported that AM fungal colonization could change the concentrations of organic acids in root exudates, thus causing a decrease in soil pH, soil electrical conductivity, organic carbon, and an increase in soil availability of N, P, and K (Hoffland et al. 1992; Dinkelaker et al. 1997; Usha et al. 2004). With regard to the influence of AM fungi on organic acid accumulation in plant tissues under salt stress, our data firstly revealed that AM symbiosis increases the accumulation of organic acids in maize leaves. It is well known that organic acids, as metabolically active solutes, play a role in osmotic adjustment (Guo et al. 2010), in the balance of cation excess (Hatzig et al. 2010), and in pH homeostasis (Hasegawa et al. 2000; López-Bucio et al. 2000; Hatzig et al. 2010). Besides, high amounts of organic acids, especially malic acid, can enhance sugar synthesis through the C4 pathway since it plays an anaplerotic role in delivering CO2 to the Calvin cycle (Chollet et al. 1996). Thus, it appears that AM-mediated accumulation of organic acids is involved in the mitigation of deleterious effects of salt stress in maize plants.
In addition, our data also indicated that AM symbiosis changes the dominant organic acid in maize leaves, elevated concentrations of oxalic, fumaric, acetic, malic, and citric acids, decreased formic and succinic acid concentrations, but did not significantly affect lactic acid concentration. This suggests that the effect of AM symbiosis on organic acids differed according to the organic acid. Increases in the concentrations of oxalic, fumaric, acetic, malic, and citric acids could compensate for a reduction in formic and succinic acid levels in mycorrhizal maize and result in an overall increase in organic acid concentration in the plant. As we know, the AM symbiosis plays a key role in protecting enzyme activity under salt stress (Giri and Mukerji 2004; Rabie and Almadini 2005). This, together with the fact that the metabolic regulation of organic acids under saline conditions involves enzymes participating in basal metabolic pathways—such as the tricarboxylic acid cycle, glyoxylate cycle, or glycolysis—suggests that the observed changes in organic acid synthesis result from the influence of the AM symbiosis on enzyme activities in basal plant metabolic pathways. In order to clarify the mechanisms of AM-mediated changes in concentrations of organic acids, further investigations should be conducted to define the effect of AM symbiosis on enzyme activities regulating organic acid synthesis and utilization.
References
Abd-El Baki BGK, Siefritz F, Man HM, Weiner H, Kaldenhoff R, Kaiser WM (2000) Nitrate reductase in Zea mays L. under salinity. Plant Cell Environ 23:515–521. doi:10.1046/j.1365-3040.2000.00568.x
Abdel-Fattah GM (2001) Measurement of the viability of arbuscular-mycorrhizal fungi using three different stains; relation to growth and metabolic activities of soybean plants. Microbiol Res 156:359–367. doi:10.1078/0944-5013-00121
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285:1256–1258. doi:10.1126/science.285.5431.1256
Asghari HR, Marschner P, Smith SE, Smith FA (2005) Growth response of Atriplex nummularia to inoculation with arbuscular mycorrhizal fungi at different salinity levels. Plant Soil 273:245–256. doi:10.1007/s11104-004-7942-6
Bao SD (2000) Soil agricultural chemistry analysis, 3rd edn. Agriculture, Beijing
Berta G, Fusconi A, Trotta A, Scannerini S (1990) Morphogenetic modifications induced by the mycorrhizal fungus Glomus strain E3 in the root system of Allium porrum L. New Phytol 114:207–215. doi:10.1111/j.1469-8137.1990.tb00392.x
Cantrell IC, Linderman RG (2001) Preinoculation of lettuce and onion with VA mycorrhizal fungi reduces deleterious effects of soil salinity. Plant Soil 233:269–281. doi:10.1023/A:1010564013601
Chollet R, Vidal J, O'Leary MH (1996) Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol 47:273–298. doi:1040-2519/96/0601-0273
Dinkelaker B, Hengeler G, Neumann G, Eltrop L, Marschner H (1997) Root exudates and mobilization of nutrients. In: Rennenberg H, Eschrich W, Ziegler H (eds) Trees-contributions to modern tree physiology. Backhuys, Leiden, pp 441–452
Duke ER, Johnson CR, Koch KE (1986) Accumulation of phosphorus, dry matter and betaine during NaCl stress of split-root citrus seedlings colonized with vesicular-arbuscular mycorrhizal fungi on zero, one or two halves. New Phytol 104:583–590. doi:10.1111/j.1469-8137.1986.tb00658.x
Feng G, Li X, Zhang F, Li S (2000) Effect of AM fungi on water and nutrition status of corn plants under salt stress. Chin J Appl Ecol 11:595–598. doi: CNKI:SUN:YYSB.0.2000-04-025
Feng G, Zhang FS, Li XL, Tian CY, Tang C, Rengel Z (2002) Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 12:185–190. doi:10.1007/s00572-002-0170-0
Francoise F, Daniel LR, John G (1991) Effects of salt stress on amino acid, organic acid and carbohydrate composition of root, bacteroids, and cytosol of alfalfa (Medicago sativa L.). Plant Physiol 96:1228–1236. doi: 0032-0889/91/96/1228/09
Gao JF (2000) Techniques of plant physiology. World Publishing Corporation, Xi'an
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500. doi:10.1111/j.1469-8137.1980.tb04556.x
Giri B, Mukerji KG (2004) Mycorrhizal inoculant alleviates salt stress in Sesbania aegyptiaca and Sesbania grandiflora under field conditions: evidence for reduced sodium and improved magnesium uptake. Mycorrhiza 14:307–312. doi:10.1007/s00572-003-0274-1
Giri B, Kapoor R, Mukerji KG (2007) Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb Ecol 54:753–760. doi:10.1007/s00248-007-9239-9
Greenway H, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Physiol 31:149–190. doi:10.1146/annurev.arplant.59.032607.092911
Guo FQ, Zhou JM, Tang ZC (1999) Differences in the accumulation of some organic solutes and gene expression in leaves between the salt tolerant mutant and the wild type of wheat under NaCl stress. Acta Photophysiol Sin 25:263–268. cnki:ISSN:0257-4829.0.1999-03-009
Guo LQ, Shi DC, Wang DL (2010) The key physiological response to alkali stress by the alkali-resistant halophyte Puccinellia tenuiflora is the accumulation of large quantities of organic acids and into the rhyzosphere. J Agron Crop Sci 196:123–135. doi:10.1111/j.1439-037X.2009.00397.x
Hajlaoui H, Ayeb NE, Garrec JP, Denden M (2010) Differential effects of salt stress on osmotic adjustment and solutes allocation on the basis of root and leaf tissue senescence of two silage maize (Zea mays L.) varieties. Ind Crops Prod 31:122–130. doi:10.1016/j.indcrop.2009.09.007
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Bio 51:463–499. doi:10.1146/annurev.arplant.51.1.463
Hatzig S, Kumar A, Neubert A, Schubert S (2010) PEP-carboxylase activity: a comparison of its role in a C4 and a C3 species under salt stress. J Agron Crop Sci 196:185–192. doi:10.1111/j.1439-037X.2009.00403.x
Hoffland E, Boogaard R, Nelemans J, Findenegg G (1992) Biosynthesis and root exudation of citric and malic acids in phosphate-starved rape plants. New Phytol 122:675–680. doi:10.1111/j.1469-8137.1992.tb00096.x
Hoque MA, Okuma E, Banu Mst NA, Nakamura Y, Shimoishi Y, Murata Y (2007) Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J Plant Physiol 164:553–561. doi:10.1016/j.jplph.2006.03.010
Jahromi F, Aroca R, Porcel R, Ruiz-Lozano JM (2008) Influence of salinity on the in vitro development of Glomus intraradices and on the in vivo physiological and molecular responses of mycorrhizal lettuce plants. Microb Ecol 55:45–53. doi:10.1007/s00248-007-9249-7
Khaled LB, Gõmez AM, Ouarraqi EM, Oihabi A (2003) Physiological and biochemical responses to salt stress of mycorrhized and/or nodulated clover seedlings (Trifolium alexandrinum L.). Agronomie 23:571–580. doi:10.1051/agro:2003037
Li XL, Feng G (2001) Ecology and physiology of arbuscular mycorrhizae. Huawen, Beijing
López-Bucio J, Nieto-Jacobo MF, Ramírez-Rodríguez V, Herrera-Estrella L (2000) Organic acid metabolism in plants: from adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci 160:1–13. doi: S0168-9452(00)00347-2
Neocleous D, Vasilakakis M (2007) Effects of NaCl stress on red raspberry (Rubus idaeus L. ‘Autumn Bliss’). Sci Hortic 112:282–289. doi:10.1016/j.scienta.2006.12.025
Neto ADA, Prisco JT, Gomes-Filho E (2009) Changes in soluble amino-N, soluble proteins and free amino acids in leaves and roots of salt-stressed maize genotypes. J Plant Interact 4:137–144. doi:10.1016/j.indcrop.2009.09.007
Ober ES, Sharp RE (1994) Proline accumulation in maize (Zea mays L.) primary roots at low water potentials (I. Requirement for increased levels of abscisic acid). Plant Physiol 105:981–987
Ouziad F, Wilde P, Schmelzer E, Hildebrandt U, Bothe H (2006) Analysis of expression of aquaporins and Na+/H+ transporters in tomato colonized by arbuscular mycorrhizal fungi and affected by salt stress. Environ Exp Bot 57:177–186. doi:10.1016/j.envexpbot.2005.05.011
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Rabie GH (2005) Influence of arbuscular mycorrhizal fungi and kinetin on the response of mungbean plants to irrigation with seawater. Mycorrhiza 15:225–230. doi:10.1007/s00572-004-0345-y
Rabie GH, Almadini AM (2005) Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr J Biotechnol 4:210–222. http://www.academicjournals.org/AJB
Ruiz-Lozano JM, Azcón R (1995) Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol Plant 95:472–478. doi:10.1111/j.1399-3054.1995.tb00865.x
Ruiz-Lozano JM, Azcón R, Gómez M (1996) Alleviation of salt stress by arbuscular-mycorrhizal Glomus species in Lactuca sativa plants. Physiol Plant 98:767–772. doi:10.1111/j.1399-3054.1996.tb06683.x
Sannazzaro AI, Ruiz OA, Albertó EO, Menéndez AB (2006) Alleviation of salt stress in Lotus glaber by Glomus intraradices. Pant soil 285:279–287. doi:10.1007/s11104-006-9015-5
Sengupta A, Chaudhuri S (1990) Vesicular-arbuscular mycorrhiza (VAM) in pioneer saline marsh plants of the Ganges River Delta in west Bengal (India). Plant Soil 122:111–113. doi:10.1007/BF02851917
Sharifi M, Ghorbanli M, Ebrahimzadeh H (2007) Improved growth of salinity-stressed soybean after inoculation with salt pre-treated mycorrhizal fungi. J Plant Physiol 164:1144–1151. doi:10.1016/j.jplph.2006.06.016
Sheng M, Tang M, Chen H, Yang B, Zhang F, Huang Y (2008) Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 18:287–296. doi:10.1007/s00572-008-0180-7
Sheng M, Tang M, Chen H, Yang B, Zhang F, Huang Y (2009) Influence of arbuscular mycorrhizae on root system of maize plants under salt stress. Can J Microbiol 55:879–886. doi:10.1139/W09-031
Shi YL (1991) 1: 1000000 map of Chinese soil resource . Chinese People's University, Beijing
Szalai G, Janda T (2009) Effect of salt stress on the salicylic acid synthesis in young maize (Zea mays L.) plants. J Agric Crop Sci 195:165–171. doi:10.1111/j.1439-037X.2008.00352.x
Usha K, Saxena A, Singh B (2004) Rhizosphere dynamics influenced by arbuscular mycorrhizal fungus (Glomus deserticola) and related changes in leaf nutrient status and yield of Kinnow mandarin {King (Citrus nobilis) × Willow Leaf (Citrus deliciosa)}. Aust J Agric Res 55:571–576. doi:10.1071/AR03036
Wu QS, Zou YN, He XH (2009) Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiol Plant 32:297–304. doi:10.1007/s11738-009-0407-z
Yang CW, Chong JN, Kim CM, Li CY, Shi DC, Wang DL (2007) Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 294:263–276. doi:10.1007/s11104-007-9251-3
Yano-Melo AM, Saggin OJ, Maia LC (2003) Tolerance of mycorrhized banana (Musa sp. cv. Pacovan) plantlets to saline stress. Agric Ecosyst Environ 95:343–348. doi:10.1016/S0167-8809(02)00044-0
Zandavalli RB, Dillenburg LR, de Souza PVD (2004) Growth responses of Araucaria angustifolia (Araucariaceae) to inoculation with the mycorrhizal fungus Glomus clarum. Appl Soil Ecol 25:245–255. doi:10.1016/j.apsoil.2003.09.009
Zhang YF, Feng G, Li XL (2003) The effect of arbuscular mycorrhizal fungi on the components and concentrations of organic acids in the exudates of mycorrhizal red clover. Acta Ecol Sin 23:30–37. cnki:ISSN:1000-0933.0.2003-01-004
Acknowledgments
The study was supported by the Key Project of National Natural Science Foundation of China (30630054), the Program for Changjiang Scholars and Innovative Research Team in University of China (IRT0748), and the Ph.D. Program Foundation of Ministry of Education of China (20100204110033). We are indebted to Chantal Hamel, Semiarid Prairie Agricultural Research Centre, Agriculture and Agri-Food Canada, for editing this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheng, M., Tang, M., Zhang, F. et al. Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress. Mycorrhiza 21, 423–430 (2011). https://doi.org/10.1007/s00572-010-0353-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-010-0353-z