Abstract
Purpose
The aim of this systematic review was to update the clinical practice guidelines for the use of anti-inflammatory agents in the prevention and/or treatment of oral mucositis.
Methods
A systematic review was conducted by the Multinational Association of Supportive Care in Cancer/ International Society of Oral Oncology (MASCC/ISOO) subcommittee on mucositis guideline update. The body of evidence for each intervention, in each cancer treatment setting, was assigned an evidence level. The findings were added to the database used to develop the clinical practice guidelines published in 2014. Based on the evidence level, one of the following three guideline determinations was possible: recommendation, suggestion, and no guidelines.
Results
A total of 11 new papers across five interventions were examined. The recommendation for the use of benzydamine mouthwash for the prevention of radiotherapy-induced mucositis remained unchanged. New suggestion for the use of the same for prevention of mucositis associated with chemoradiotherapy was made. No guideline was possible for any other anti-inflammatory agents due to inadequate and/or conflicting evidence.
Conclusions
Of the anti-inflammatory agents studied for oral mucositis, the evidence supports the use of benzydamine mouthwash in the specific populations listed above. Additional well-designed research is needed on other (class of agents) interventions and in other cancer treatment settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral mucositis (OM) associated with cancer therapy carries a significant morbidity. OM can be associated with radiotherapy (RT), chemotherapy (CT), radio-chemotherapy (RT-CT), or hematopoietic stem cell transplantation (HSCT) [1]. Patients with OM may experience difficulties in chewing, maintenance of oral hygiene, and nutrition and have negative effects on oral health–related quality of life [2, 3]. Patients with OM may experience significant pain and have an increased risk of infections and septicemia [4]. Management of OM may involve the use of narcotic analgesics, hospitalization, nutritional support, and treatment interruptions [5, 6]. Many agents were tested for the prevention, treatment, or relieve symptoms of OM [7, 8]. Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) has published clinical practice guidelines [1, 9, 10]. The latest update of clinical practice guidelines on the prevention and treatment of mucositis was published in 2014 [1]. This was based on the systematic reviews on various agents studied for the prevention and or treatment of mucositis from January 1966 to December 2010.
Due to significant increase in the clinical OM literature, a series of systematic reviews were undertaken to update the available guidelines. The agents reviewed in this process were categorized under broad topics based on their mode of action. This paper describes the results of the systematic review conducted on anti-inflammatory agents.
Inflammation is considered to be an important tissue reaction in radiotherapy and chemotherapy-induced OM [11]. Proinflammatory cytokines particularly tumor necrosis factor alpha (TNFα) and interleukin 1 beta (IL-1β) have been considered to play a pivotal role in the pathogenesis of OM [12,13,14]. Studies have shown that levels of cyclooxygenase-2 (COX-2) and nuclear factor kappa-B (NFkB) in the oral mucosa were significantly increased following cytotoxic chemotherapy [15]. Hence, the inhibition of these has been targeted in managing cancer therapy–induced OM, as can be viewed in the previous MASCC/ISOO guideline papers on anti-inflammatory agents [16, 17].
As part of a comprehensive update of the MASCC/ISOO clinical practice guidelines for mucositis, the aim of this project was to update the evidence-based clinical practice guidelines for the use of anti-inflammatory agents in the prevention and treatment of OM.
Methods
The methods are described in detail in Ranna et al. [18]. Briefly, a literature search for relevant papers indexed in the literature from 1 January 2011 to 30 June 2016 was conducted using PubMed and Web of Science. The list of intervention keywords used for the literature search of this section was as follows: amifostine, aminosalicylic acid, amlexanox, anti-TNF, anti-tumor necrosis factor, aspirin, benadryl, benzydamine, betamethasone, celecoxib, corticosteroid, dexamethasone, diphenhydramine, ethylol, flurbiprofen, flurbiprofen, histamine, hydrocortisone, ibuprofen, indomethacin, infliximab, irsogladine, lactoferrin, mesalazine, misoprostol, N-acetyl cysteine, non-steroidal anti-inflammatory agents (NSAIDs), orgotein, prednisone, prostaglandin, RK-02-02, salicylic acid, steroid, thalidomide, TNF antibody, TNF inhibitor, tumor necrosis factor (TNF), anti-inflammatory, human placental extract, and lactermin.
Studies on anti-inflammatory agents for the prevention and/or treatment of cancer therapy–induced OM were selected for review. The papers were selected using well-defined inclusion and exclusion criteria. Papers that were published based on testing an intervention for mucositis prevention or treatment, published in peer-reviewed journals in English language and interventions for all age groups, were included.
Papers that do not report the effects of an intervention on mucositis or on related outcomes such as mucositis-associated pain, animal studies or in vitro studies, non-English publications, and studies that did not assess mucositis directly were excluded from the review. Prior to the review process, the reviewers were calibrated. Papers were then reviewed by two independent reviewers, and data were entered to a predetermined standard electronic form. For papers reporting mixed cancer patient populations (e.g., mix of patients treated with RT and with RT-CT), they were considered as mixed only if there were more than 10% of patients receiving either one of the treatment options.
Studies were scored for their level of evidence (LoE) based on Somerfield criteria [19], and flaws were listed according to Hadorn criteria [20]. A well-designed study was defined as a study with no major flaws per the Hadorn criteria. Findings from the reviewed studies were merged with the evidence reviewed in the previous MASCC/ISOO guideline update. Then, data were integrated into guidelines based on LoE for each intervention. Guidelines were classified into three types: recommendation, suggestion, and no guideline possible.
Guidelines were separated based on (1) the aim of the intervention (prevention or treatment of mucositis), (2) the cancer treatment modality (RT, CT, RT-CT, or high-dose conditioning therapy for HSCT), and (3) the route of administration of the intervention.
Results
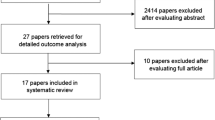
The literature searches yielded 692 papers (PubMed 292 and Web of Science 400). Of these, 654 were excluded after reading titles and abstracts. The remaining 38 papers were assessed against inclusion and exclusion criteria and excluded 27 papers yielding 11 papers for qualitative evidence synthesis (Fig. 1).
The 11 available studies reported for anti-inflammatory agents, overall level of evidence, and guideline determination are summarized in Table 1. To make an overall judgment for guideline determination, references from the previous guideline updates were integrated to this table as well. The agents assessed in the previous guideline updates, for which no new evidence was found and no guideline was possible, were also listed separately (Table 2) [42].
Benzydamine mouthwash
Based on the previous systematic review on anti-inflammatory agents [42], the latest guidelines recommended the use of benzydamine mouthwash for the prevention of OM in head and neck (H&N) cancer patients receiving moderate-dose RT (up to 50 Gy), without concomitant CT [1]. Benzydamine exhibits anti-inflammatory properties by inhibiting the production of proinflammatory cytokines such as TNFα and IL-1β [22, 43].
H&N cancer—radiotherapy—prevention of OM
Guideline: Benzydamine mouthwash is recommended for the prevention of OM in patients with H&N cancer receiving a moderate dose RT (< 50 Gy) (LoE I).
Placebo-controlled randomized controlled trials
The present review identified one new randomized controlled trial (RCT) [24]. This study compared the efficacy of 0.15% benzydamine mouthwash, natural honey, and 0.9% saline. The mucositis severity was lower in the honey group; however, there was no subanalysis for the severity of benzydamine vs. saline [24]. The difference in the onset of mucositis between benzydamine and saline was not statistically significant.
We also included three studies [21,22,23] from the previous review [42] for making an overall assessment on the efficacy of benzydamine mouthwash. Epstein and Stevenson-Moore found that benzydamine mouthwash reduced OM pain associated with RT dose of 45–60 Gy [21]. Another RCT reported similar results [22]. In another study, Epstein et al. found that the use of benzydamine mouthwash prevents RT-induced OM among patients receiving a cumulative dose up to 50 Gy [23]. Overall evidence from these three studies confirms that benzydamine mouthwash prevents mucositis among patients receiving RT up to 50 Gy (level I evidence). This confirms the previous guidelines published in 2014.
However, there was insufficient evidence on the efficacy of prevention of RT-induced OM among patients receiving higher doses of radiation.
RCTs—comparator studies
Review conducted in 2010 identified two RCTs that compared chlorhexidine mouthwash with benzydamine [42]. Samaranayake et al. compared benzydamine with chlorhexidine and found little difference between the two agents in controlling pain, mucositis, or oral carriage of microorganisms [44]. Similar study conducted by Cheng et al. reported that OM was less severe, among the users of benzydamine mouthwash although the difference was not statistically significant [45].
Head and neck cancer—radio-chemotherapy—prevention of OM
Guideline: Use of benzydamine mouthwash is suggested for the prevention of OM in patients with H&N cancer receiving RT and CT (LoE II).
Single RCT from the present review and two from the previous review were studied [25,26,27]. RCT conducted by Sheibani et al. compared the efficacy of benzydamine mouthwash against placebo using 51 patients. Fifteen and 17 patients received RT-CT in the test and the control groups, respectively. Although OM severity was not different between two groups at the end of the third week of treatment, they observed significant reduction (p = 0.01) of the mean severity score by the end of week 4 among those who received benzydamine mouthwash [27].
Prada et al. reported their study in which some patients (12%) received RT-CT while others received CT or RT alone [26]. Kazemain et al. [25] reported their RCT in which 60.5% of patients received RT-CT. They found efficacy of benzydamine mouthwash. Although all these RCTs reported efficacy for benzydamine, they did not carry out a separate analysis of the RT-CT group. Hence, we limited the guideline’s strength. Accordingly, the guideline panel only made a suggestion on the use of benzamine mouthwash for the prevention of OM in patients with H&N cancer receiving RT-CT.
RCTs—comparator studies
Putwatana et al. conducted a single-blinded study, which compared benzydamine mouthwash with papayor drops. Fifty percent in the benzydamine group and 57% in the papayor group received RT-CT. The study concluded that papayor was superior to benzydamine for preventing and relieving OM [46].
Head and neck cancer—radiotherapy—treatment of OM
Guideline: No guideline possible.
The present review identified one RCT for treatment of RT-induced OM [30]. This study included 38 patients undergoing RT at their third to fourth week of treatment. After RT, single dose of CAM2028-control or CAM2028-benzydamine 2 days apart was administered in a randomized crossover fashion. This was followed by assessment of pain after 8 h. Patients experienced 40% reduction in pain intensity at 6 h. Both treatments found to be equally effective in the relief of pain within 5 min of application. Same results were consistently evident during the entire 8-h assessment period indicating that benzydamine in combination with CAM2028 is not superior to CAM2028-control.
Previous review identified two studies [42] and was based on the same patient population published in 1985 and 1986 consecutively [28, 47]. We assessed only the latest paper [28]. This study reported significant control of pain and severity when benzydamine mouth rinse was used during RT.
In a non-RCT study, Roopashri et al. compared benzydamine, chlorhexidine, povidone iodine, and distilled water [29]. They found that benzydamine mouthwash was more efficient, safe, and well tolerated by the patients. Based on the results, establishment of guidelines for treatment of OM among patients undergoing RT for H&N cancer is not possible (LoE III).
Head and neck cancer—RT-CT—treatment of OM
Guideline: No guideline possible.
The present review did not identify RCTs or other studies on the treatment of RT-CT-induced OM. Hence, no guidelines are possible for the treatment of OM in with H&N cancer patients undergoing RT-CT.
RCTs—comparator studies
RCT conducted by Erdem and Gungormus compared royal jelly with benzydamine [48]. The results indicated that the mean resolution time of OM in the royal jelly group was significantly shorter than the benzydamine group.
Head and neck cancer—CT—prevention of OM
Prada et al. reported their preliminary study on the efficacy of benzydamine mouthwash among patients undergoing CT [31]. The study was based on 20 patients who were randomly divided into test and control (placebo) groups. This study has shown the efficacy of benzydamine mouthwash in the prevention of CT-induced OM.
Hematologic malignancies and solid tumors—chemotherapy—prevention
We did not identify RCTs or any other types of studies on the efficacy of benzydamine mouthwash. Hence, no guidelines are possible for the prevention of OM in this group of patients.
Hematologic malignancies and solid tumors—chemotherapy—treatment of OM
Guideline: No guideline possible.
We did not yield any new study on this intervention. However, the 2010 review identified one study [42]. Schubert and Newton reported that patients receiving benzydamime had less pain compared with placebo (p = 0.04) [32].
Two case series studies were identified by the 2010 review [42]. Sonis et al. reported that seven out of nine patients had significant palliation of symptoms by using benzydamine mouthwash [33]. Another crossover study compared the extent and duration of analgesic effects of benzydamine and Hospital for Sick Children mouthwash (nystatin, lidocaine viscous, and sodium chloride) [34]. They reported that both agents were effective in controlling pain for a duration of 1 hour.
Hematologic malignancies—chemotherapy—prevention of OM
We did not identify RCTs, and therefore, no guidelines are possible for the prevention of OM in this group of patients.
RCTs—comparator studies
Review conducted in 2010 [42] identified three RCTs based on the same cohort of pediatric patients, who received high-dose CT for hematological malignancies or solid tumors [49,50,51]. The original RCT was a non-blinded, two-period crossover trial that compared an oral care protocol containing benzydamine and chlorhexidine [50, 51]. The study concluded that benzydamine was inferior to chlorhexidine in the prevention of OM. Another study reported that benzydamine was acceptable and well tolerated by children over 6 years [49].
Systemic celecoxib
Celecoxib is an NSAID which inhibits cyclooxygenase-2 (COX-2), a key enzyme in the inflammatory process responsible for increased production of proinflammatory agents. Prostaglandin E2 (PGE2) and prostacyclin (PGI2) mediate tissue injury and pain. Pain scores associated with mucositis among patients receiving high-dose chemotherapy were correlated with tissue levels of COX-1 and PGE synthase and salivary prostaglandins [38]. Similarly, significant increase in tissue levels of nuclear factor kappa B (NFκB) and COX-2 in oral mucosa was found when chemotherapy is administered [15]. Therefore, it is hypothesized that inhibition of COX-2 could be a useful therapeutic target [35].
Head and neck cancer—chemotherapy and radiotherapy—prevention of OM
Guideline: No guideline possible (LoE II).
We identified one RCT assessing the effectiveness of systemic celecoxib in the prevention of OM associated with RT with or without CT [35]. Forty patients were randomized to daily use of 200-mg celecoxib or placebo. Seventeen and 15 subjects received RT-CT in the test and control groups, respectively. Celecoxib 200-mg tabs were given twice a day for four patients, and others received once daily and commenced 5 days prior to the beginning of RT-CT and continued 3 days following completion. The study found no difference between placebo and celecoxib for mean scores of OM, pain, normalcy of diet, or mean daily opioid dose. Due to the limitation of available evidence, no guideline determination is possible for this intervention.
Systemic irsogladine maleate
Irsogladine maleate (IM) has gastric mucosal protective properties. IM increases intracellular cAMP levels in the gastric mucosa and activates communication between cells [52]. IM inhibits production of reactive oxygen species and act as ROS scavenger [35].
Head and neck cancer—chemotherapy—prevention of OM
We identified one RCT for this intervention for prevention of CT (5-FU)-induced OM [36]. Sixty-six patients were randomized to test and control groups, and 2 mg of IM was given orally twice a day from the first day of CT and continued until 14th day. The incidence of OM was significantly lower for intervention than for placebo (p < 0.001). Having only a single study, no guideline determination is possible.
Misoprostol
Misoprostol is a synthetic analog of prostaglandin E1 with anti-inflammatory and mucosa-protecting properties [38, 53]. Due to the involvement of the cyclooxygenase pathway in OM, treatment using misoprostol was considered [54].
Hematologic and solid cancer—chemotherapy—prevention of OM
Guideline: No guideline possible (LoE III).
We found one new RCT [38] which assessed misoprostol mouth rinse among hematologic and solid cancer populations receiving CT. Twenty-two patients in the test and 26 in the control group were treated with 200 mcg of misoprostol or placebo in 15 ml of water to rinse 45 min–2 h before chemotherapy followed by 8 hourly until 24 h after treatment. There was no significant difference between test and placebo groups for OM or pain severity, length of hospital stay, and number of days on parenteral nutrition. This study was terminated prematurely before reaching the planned sample size. The study concluded that misoprostol is ineffective in the prevention of CT-induced OM.
We did not identify any new RCTs on the use of systemic misoprostol. The previous review (2010) identified one RCT [42]. This RCT tested the efficacy of systemic misoprostol in the prevention of high-dose chemotherapy-induced OM in 15 patients [37]. Patients were given misoprostol tablets 250 mcg or identical placebo tablets three times a day from the first day of chemotherapy prior to and until 16 days after HSCT.
The study prematurely terminated due to severe OM among test participants compared with placebo. The study concluded that the use of misoprostol tablets is not beneficial [37]. Therefore, no guidelines are possible both for topical or systemic administration of misoprostol for the prevention of CT-induced OM.
H&N cancer—radiotherapy—prevention of OM
Guideline: No guideline possible (LoE III).
We did not yield any new RCTs for this intervention. Review in 2010 identified one RCT on topical misoprostol [39]. In this study, 70 patients (35 in each group) were given misoprostol 250-mcg tablets or placebo dissolved in 15 ml of water. The study was conducted in two sites. Results from one site showed a decrease in the mean mucositis scores in the misoprostol group at fourth to fifth week of RT, with no significant decrease at sixth to seventh weeks. No benefit of misoprostol was seen at the second site.
H&N cancer—radio-chemotherapy—prevention of OM
Guideline: No guideline possible (LoE III).
We did not yield any new RCTs for this intervention. Review conducted in 2010 identified one study. This study assessed combined topical and systemic misoprostol [40]. Patients were randomized to receive misoprostol (n = 42) and placebo (n = 41). The patients were asked to rinse the mouth for 2 min before it is swallowed indicating the topical as well as systemic effects of this medication. Authors reported no beneficial effect from this intervention.
Although previous guidelines made a suggestion against the use misoprostol mouthwash [1], based on the separate analysis of RT and RT-CT studies, the panel concluded that no guidelines are possible for either RT-induced OM or RT-CT-induced OM.
Rebamipide
Rebamipide is a drug developed for the treatment of gastritis and gastric ulcers [41]. Multitude of mechanisms has been implicated for its anti-ulcer properties and cytoprotective effects. They include induction of PGE2 synthesis via COX-2 expression, upregulation of growth factors and their receptors, induction of mucus secretion, anti-free radical effects, and inhibition of the production of inflammatory cytokines such as IL-1, IL-8, and TNF-α [55]. Since the onset of OM is associated with production of free radicals, increase of inflammatory cytokines, and alteration of intracellular signal transduction, use of rebamipide considered a potential modality for treatment and prevention of OM associated with CT and RT.
H&N cancer—radio-chemotherapy—prevention
RCT was conducted by Yasuda et al. [41] to assess the efficacy of rebamipide for prevention of OM. Test group received rebamipide gargle (n = 12), and the control received placebo-gargle (n = 12). Participants were asked to gargle 2–3 min with 50 ml rebamipide solution or placebo solution six times per day. Gargling was started at the initiation of cancer therapy and continued until the end. Results of the study indicated that the number of patients with severe mucositis (WHO ≥ 3) was higher in the placebo group (83.3% vs. 33.3%, p = 0.036) than in the rebamipide group.
Small sample size and unavailability of other studies prevent developing any guidelines pertaining to this agent.
Discussion
This review analyzed the available literature on anti-inflammatory agents in the prevention or treatment of OM associated with RT, CT, RT-CT, or HSCT. Several studies focused on benzydamine mouthwash in the prevention and/or treatment of OM [21, 23,24,25,26,27,28, 30,31,32]. The 2014 mucositis guidelines have recommended benzydamine mouthwash for the prevention of OM among H&N cancer patients receiving RT up to 50 Gy [1]. The evidence from this review confirms the existing guideline.
Three RCTs yielded level II evidence on the effectiveness of benzydamine mouthwash for the prevention of RT-CT-induced OM [26, 27, 31]. Considering the overall evidence, the panel has decided to suggest the use of benzydamine mouthwash for the prevention of OM among H&N cancer patients receiving RT-CT.
No guideline was possible for other setting in which benzydamine mouthwash was studied. This includes H&N-RT in the treatment for OM [28, 30], H&N-CT in the prevention of OM [31], and hematological and solid cancers—CT in the treatment of OM [32].
The present review did not identify clear evidence on the effectiveness of other anti-inflammatory agents that were reviewed in the 2013 MASCC/ISOO mucositis guidelines update: celecoxib [35], irsogladine maleate (IM) [36], misoprostol [37,38,39,40], and rebamipide [41]. The evidence available for these agents will be described below.
The RCT about celecoxib failed to demonstrate its effectiveness in the prevention of OM and pain severity [35]. Although this study provides level II evidence, in the absence of any other studies, no guidelines are possible for the prevention of RT-CT-induced OM.
In another RCT, it was hypothesized that IM is effective in preventing CT-induced OM and tested for efficacy among H&N cancer patients [36]. Although this study provides strong evidence on effectiveness, we did not find other types of studies to confirm this observation. Therefore, development of a guideline on IM is currently not possible.
Including the study identified by the present review, we reviewed four studies on the use of misoprostol in the prevention of OM among H&N [39, 40] and hematologic and solid tumor patients [37, 38]. Of these studies, only one found that misoprostol mouthwash is effective in the prevention of radiotherapy-induced OM among H&N cancer patients [39]. However, no guidelines are possible on this agent in any mode of administration due to the limited evidence.
Rebamipide was considered a potential modality for treatment and prevention of OM. The present review identified one study which evaluated the use of rebamipide in the prevention of RT-CT-induced OM among H&N cancer patients [41]. Although the overall evidence was level II, in the absence of any other studies, establishment of guidelines is not possible.
Prevention and treatment of OM associated with H&N RT, CT, or combination are of paramount importance [1, 56]. OM may lead to significant morbidity, which includes pain, eating difficulties, nutritional compromise, and risk of infection. Symptoms may be severe enough needing hospitalization and even premature termination of cancer treatment [2]. Pathogenesis of mucositis is complex and involves the generation of damaging reactive oxygen species, activation of transcription factors such as nuclear factor-k beta and inflammatory pathway involving cyclooxygenase, and upregulation of proinflammatory cytokines including TNF-alpha and IL-1beta [11]. Involvement of inflammatory mechanism provides the rationale for testing anti-inflammatory agents for the prevention or treatment of OM.
Benzydamine mouthwash is the only anti-inflammatory agent with evidence in the prevention of OM to date. Although not within the databases included in this systematic review, there is a publically available clinical study report about benzydamine for the prevention of OM in a large H&N patient population that was posted on 2011 [57]. This large RCT did not confirm benzydamine’s effectiveness.
Although there is no clear evidence on the other anti-inflammatory agents, the studies conducted so far shed some light on their use in the prevention or treatment of OM. Design flaws, small sample size, and heterogeneous patient populations and treatment modalities weaken the quality of evidence. Therefore, well-designed RCTs are required to establish the evidence of benefits of anti-inflammatory agents for OM.
New information at the time the paper goes to press
After the completion of the review process for the present guideline update, we identified few more papers published in the recent past which reported the efficacy of benzydamine and rebamipide gargle for the prevention of OM.
One RCT found that benzydamine significantly reduces OM even at doses > 50 Gy in H&N cancer patients [58]. This study reported that RT group who received benzydamine rinses had significantly reduced OM (p = 0.038). However, the groups who received RT-CT did not show statistically significant results (p = 0.18).
Another recent RCT compared benzydamine mouth rinse with bicarbonate rinse among high-dose RT-CT for H&N cancer patients. They reported that benzydamine is superior in preventing OM (p < 0.001) [59]. A recent RCT found that rebamipide gargle prolongs the onset and reduces the severity of OM among RT-CT patients [60]. A similar study reported that rebamipide mouth rinse may be potentially effective and safe for patients undergoing RT-CT for H&N cancer [61].
In summary, benzydamine mouthwash is the only anti-inflammatory agent with evidence in prevention of OM to date. This systematic review confirmed the existing guideline on the use of benzydamine mouthwash for the prevention of OM caused by RT among H&N cancer patients. The panel also suggested the use of benzydamine mouthwash for patients receiving RT-CT among H&N cancer patients.
References
Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O, Peterson DE, Raber-Durlacher JE, Sonis ST, Elad S (2014) MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120:1453–1461
Barkokebas A, Silva IH, de Andrade SC, Carvalho AA, Gueiros LA, Paiva SM, Leao JC (2015) Impact of oral mucositis on oral-health-related quality of life of patients diagnosed with cancer. J Oral Pathol Med 44:746–751
Jensen SB, Jarvis V, Zadik Y, Barasch A, Ariyawardana A, Hovan A, Yarom N, Lalla RV, Bowen J, Elad S (2013) Systematic review of miscellaneous agents for the management of oral mucositis in cancer patients. Support Care Cancer 21:3223–3232
Sonis ST (2004) Oral mucositis in cancer therapy. J Support Oncol 2:3–8
Murphy BA, Beaumont JL, Isitt J, Garden AS, Gwede CK, Trotti AM, Meredith RF, Epstein JB, Le QT, Brizel DM, Bellm LA, Wells N, Cella D (2009) Mucositis-related morbidity and resource utilization in head and neck cancer patients receiving radiation therapy with or without chemotherapy. J Pain Symptom Manag 38:522–532
Vera-Llonch M, Oster G, Ford CM, Lu J, Sonis S (2007) Oral mucositis and outcomes of allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies. Support Care Cancer 15:491–496
Mallick S, Benson R, Rath GK (2016) Radiation induced oral mucositis: a review of current literature on prevention and management. Eur Arch Otorhinolaryngol 273:2285–2293
Jensen SB, Peterson DE (2014) Oral mucosal injury caused by cancer therapies: current management and new frontiers in research. J Oral Pathol Med 43:81–90
Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB, Hutchins RD, Peterson DE (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109:820–831
Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J, Elting LS, Fox PC, Cooksley C, Sonis ST (2004) Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 100:2026–2046
Kwon Y (2016) Mechanism-based management for mucositis: option for treating side effects without compromising the efficacy of cancer therapy. OncoTargets Ther 9:2007–2016
Sultani M, Stringer AM, Bowen JM, Gibson RJ (2012) Anti-inflammatory cytokines: important immunoregulatory factors contributing to chemotherapy-induced gastrointestinal mucositis. Chemother Res Prac 2012:490804
Ong ZY, Gibson RJ, Bowen JM, Stringer AM, Darby JM, Logan RM, Yeoh AS, Keefe DM (2010) Pro-inflammatory cytokines play a key role in the development of radiotherapy-induced gastrointestinal mucositis. Radiat Oncol (London, England) 5:22
Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, Syed A, Fritsche HA, Bruera E, Booser D, Valero V, Arun B, Ibrahim N, Rivera E, Royce M, Cleeland CS, Hortobagyi GN (2004) Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine 25:94–102
Logan RM, Gibson RJ, Sonis ST, Keefe DM (2007) Nuclear factor-kappaB (NF-kappaB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol 43:395–401
Barasch A, Elad S, Altman A, Damato K, Epstein J (2006) Antimicrobials, mucosal coating agents, anesthetics, analgesics, and nutritional supplements for alimentary tract mucositis. Support Care Cancer 14:528–532
Nicolatou-Galitis O, Sarri T, Bowen J, Di Palma M, Kouloulias VE, Niscola P, Riesenbeck D, Stokman M, Tissing W, Yeoh E, Elad S, Lalla RV (2013) Systematic review of amifostine for the management of oral mucositis in cancer patients. Support Care Cancer 21:357–364
Ranna V, Cheng K, Castillo D, Porcello L, Vaddi A, Lalla R, Bossi P, Elad S (2019) Development of the MASCC/ISOO Clinical Practice Guidelines for Mucositis: an overview of the methods. Support Care Cancer. In Press
Somerfield MPJ, Pfister D, Bennett C, Recht A, Smith T, Weeks J, Winn R, Durant J (2000) ASCO clinical practice guidelines: process, progress, pitfalls, and prospects. Class Pap Curr Comments 4:881–886
Hadorn DC, Baker D, Hodges JS, Hicks N (1996) Rating the quality of evidence for clinical practice guidelines. J Clin Epidemiol 49:749–754
Epstein JB, Stevenson-Moore P (1986) Benzydamine hydrochloride in prevention and management of pain in oral mucositis associated with radiation therapy. Oral Surg Oral Med Oral Pathol 62:145–148
Epstein JB, Stevenson-Moore P, Jackson S, Mohamed JH, Spinelli JJ (1989) Prevention of oral mucositis in radiation therapy: a controlled study with benzydamine hydrochloride rinse. Int J Radiat Oncol Biol Phys 16:1571–1575
Epstein JB, Silverman S Jr, Paggiarino DA, Crockett S, Schubert MM, Senzer NN, Lockhart PB, Gallagher MJ, Peterson DE, Leveque FG (2001) Benzydamine HCl for prophylaxis of radiation-induced oral mucositis: results from a multicenter, randomized, double-blind, placebo-controlled clinical trial. Cancer 92:875–885
Jayachandran S, Balaji N (2012) Evaluating the effectiveness of topical application of natural honey and benzydamine hydrochloride in the management of radiation mucositis. Indian J Palliat Care 18:190–195
Kazemian A, Kamian S, Aghili M, Hashemi FA, Haddad P (2009) Benzydamine for prophylaxis of radiation-induced oral mucositis in head and neck cancers: a double-blind placebo-controlled randomized clinical trial. Eur J Cancer Care 18:174–178
Prada A, Chiesa F (1987) Effects of benzydamine on the oral mucositis during antineoplastic radiotherapy and/or intra-arterial chemotherapy. Int J Tissue React 9:115–119
Sheibani KM, Mafi AR, Moghaddam S, Taslimi F, Amiran A, Ameri A (2015) Efficacy of benzydamine oral rinse in prevention and management of radiation-induced oral mucositis: a double-blind placebo-controlled randomized clinical trial. Asia Pac J Clin Oncol 11:22–27
Kim JH, Chu FC, Lakshmi V, Houde R (1986) Benzydamine HCl, a new agent for the treatment of radiation mucositis of the oropharynx. Am J Clin Oncol 9:132–134
Roopashri G, Jayanthi K, Guruprasad R (2011) Efficacy of benzydamine hydrochloride, chlorhexidine, and povidone iodine in the treatment of oral mucositis among patients undergoing radiotherapy in head and neck malignancies: a drug trail. Contemp Clin Dent 2:8–12
Hadjieva T, Cavallin-Stahl E, Linden M, Tiberg F (2014) Treatment of oral mucositis pain following radiation therapy for head-and-neck cancer using a bioadhesive barrier-forming lipid solution. Support Care Cancer 22:1557–1562
Prada A, Lozza L, Moglia D, Sala L, Chiesa F (1985) Effects of benzydamine on radio-polychemotherapeutic mucositis of the oral cavity. Int J Tissue React 7:237–239
Schubert MM, Newton RE (1987) The use of benzydamine HCl for the management of cancer therapy-induced mucositis: preliminary report of a multicentre study. Int J Tissue React 9:99–103
Sonis ST, Clairmont F, Lockhart PB, Connolly SF (1985) Benzydamine HCL in the management of chemotherapy-induced mucositis. I. Pilot study. J Oral Med 40:67–71
Lever SA, Dupuis LL, Chan HS (1987) Comparative evaluation of benzydamine oral rinse in children with antineoplastic-induced stomatitis. Drug Intell Clin Pharm 21:359–361
Lalla RV, Choquette LE, Curley KF, Dowsett RJ, Feinn RS, Hegde UP, Pilbeam CC, Salner AL, Sonis ST, Peterson DE (2014) Randomized double-blind placebo-controlled trial of celecoxib for oral mucositis in patients receiving radiation therapy for head and neck cancer. Oral Oncol 50:1098–1103
Nomura M, Kamata M, Kojima H, Hayashi K, Sawada S (2013) Irsogladine maleate reduces the incidence of fluorouracil-based chemotherapy-induced oral mucositis. Ann Oncol 24:1062–1066
Duenas-Gonzalez A, Sobrevilla-Calvo P, Frias-Mendivil M, Gallardo-Rincon D, Lara-Medina F, Aguilar-Ponce L, Miranda-Lopez E, Zinser-Sierra J, Reynoso-Gomez E (1996) Misoprostol prophylaxis for high-dose chemotherapy-induced mucositis: a randomized double-blind study. Bone Marrow Transplant 17:809–812
Lalla RV, Gordon GB, Schubert M, Silverman S Jr, Hutten M, Sonis ST, LeVeque F, Peterson DE (2012) A randomized, double-blind, placebo-controlled trial of misoprostol for oral mucositis secondary to high-dose chemotherapy. Support Care Cancer 20:1797–1804
Hanson WR, Marks JE, Reddy SP, Simon S, Mihalo WE, Tova Y (1997) Protection from radiation-induced oral mucositis by a mouth rinse containing the prostaglandin E1 analog, misoprostol: a placebo controlled double blind clinical trial. Adv Exp Med Biol 400b:811–818
Veness MJ, Foroudi F, Gebski V, Timms I, Sathiyaseelan Y, Cakir B, Tiver KW (2006) Use of topical misoprostol to reduce radiation-induced mucositis: results of a randomized, double-blind, placebo-controlled trial. Australas Radiol 50:468–474
Yasuda T, Chiba H, Satomi T, Matsuo A, Kaneko T, Chikazu D, Miyamatsu H (2012) Preventive effect of rebamipide gargle on chemoradiotherpy-induced oral mucositis in patients with oral cancer: a pilot study. J Oral Maxillofac Res 2:e3
Nicolatou-Galitis O, Sarri T, Bowen J, Di Palma M, Kouloulias VE, Niscola P, Riesenbeck D, Stokman M, Tissing W, Yeoh E, Elad S, Lalla RV (2013) Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients. Support Care Cancer 21:3179–3189
Chang JE, Min SW, Kim CS, Han SH, Kwon YS, Hwang JY (2015) Effect of prophylactic benzydamine hydrochloride on postoperative sore throat and hoarseness after tracheal intubation using a double-lumen endobronchial tube: a randomized controlled trial. Can J Anaesth 62:1097–1103
Samaranayake LP, Robertson AG, MacFarlane TW, Hunter IP, MacFarlane G, Soutar DS, Ferguson MM (1988) The effect of chlorhexidine and benzydamine mouthwashes on mucositis induced by therapeutic irradiation. Clin Radiol 39:291–294
Kin-Fong Cheng K, Ka Tsui Yuen J (2006) A pilot study of chlorhexidine and benzydamine oral rinses for the prevention and treatment of irradiation mucositis in patients with head and neck cancer. Cancer Nurs 29:423–430
Putwatana P, Sanmanowong P, Oonprasertpong L, Junda T, Pitiporn S, Narkwong L (2009) Relief of radiation-induced oral mucositis in head and neck cancer. Cancer Nurs 32:82–87
Kim JH, Chu F, Lakshmi V, Houde R (1985) A clinical study of benzydamine for the treatment of radiotherapy-induced mucositis of the oropharynx. Int J Tissue React 7:215–218
Erdem O, Gungormus Z (2014) The effect of royal jelly on oral mucositis in patients undergoing radiotherapy and chemotherapy. Holis Nurs Pract 28:242–246
Cheng KK (2004) Children’s acceptance and tolerance of chlorhexidine and benzydamine oral rinses in the treatment of chemotherapy-induced oropharyngeal mucositis. Eur J Oncol Nurs 8:341–349
Cheng KK, Chang AM (2003) Palliation of oral mucositis symptoms in pediatric patients treated with cancer chemotherapy. Cancer Nurs 26:476–484
Cheng KK, Chang AM, Yuen MP (2004) Prevention of oral mucositis in paediatric patients treated with chemotherapy; a randomised crossover trial comparing two protocols of oral care. Eur J Cancer (Oxford, England : 1990) 40:1208–1216
Kawano Y, Imamura A, Nakamura T, Akaishi M, Satoh M, Hanawa T (2016) Development and characterization of oral spray for stomatitis containing irsogladine maleate. Chem Pharm Bull 64:1659–1665
Haynes DR, Whitehouse MW, Vernon-Roberts B (1992) The prostaglandin E1 analogue, misoprostol, regulates inflammatory cytokines and immune functions in vitro like the natural prostaglandins E1, E2 and E3. Immunol 76:251–257
Lalla RV, Pilbeam CC, Walsh SJ, Sonis ST, Keefe DM, Peterson DE (2010) Role of the cyclooxygenase pathway in chemotherapy-induced oral mucositis: a pilot study. Support Care Cancer 18:95–103
Sun WH, Tsuji S, Tsujii M, Gunawan ES, Kawai N, Kimura A, Kakiuchi Y, Yasumaru M, Iijima H, Okuda Y, Sasaki Y, Hori M, Kawano S (2000) Induction of cyclooxygenase-2 in rat gastric mucosa by rebamipide, a mucoprotective agent. J Pharmacol Exp Ther 295:447–452
Jones JA, Avritscher EB, Cooksley CD, Michelet M, Bekele BN, Elting LS (2006) Epidemiology of treatment-associated mucosal injury after treatment with newer regimens for lymphoma, breast, lung, or colorectal cancer. Support Care Cancer 14:505–515
NIH (2011) Safety & efficacy study of benzydamine oral rinse for the treatment of oral mucositis (mouth sores) resulting from radiation therapy for cancer of the oral cavity, oropharynx, or nasopharynx
Rastogi M, Khurana R, Revannasiddaiah S, Jaiswal I, Nanda SS, Gupta P, Chufal KS, Bhatt ML (2017) Role of benzydamine hydrochloride in the prevention of oral mucositis in head and neck cancer patients treated with radiotherapy (>50 Gy) with or without chemotherapy. Support Care Cancer 25:1439–1443
Chitapanarux I, Tungkasamit T, Petsuksiri J, Kannarunimit D, Katanyoo K, Chakkabat C, Setakornnukul J, Wongsrita S, Jirawatwarakul N, Lertbusayanukul C, Sripan P, Traisathit P (2018) Randomized control trial of benzydamine HCl versus sodium bicarbonate for prophylaxis of concurrent chemoradiation-induced oral mucositis. Support Care Cancer 26:879–886
Chaitanya B, Pai KM, Yathiraj PH, Fernandes D, Chhaparwal Y (2017) Rebamipide gargle in preventive management of chemo-radiotherapy induced oral mucositis. Oral Oncol 72:179–182
Yokota T, Ogawa T, Takahashi S, Okami K, Fujii T, Tanaka K, Iwae S, Ota I, Ueda T, Monden N, Matsuura K, Kojima H, Ueda S, Sasaki K, Fujimoto Y, Hasegawa Y, Beppu T, Nishimori H, Hirano S, Naka Y, Matsushima Y, Fujii M, Tahara M (2017) Efficacy and safety of rebamipide liquid for chemoradiotherapy-induced oral mucositis in patients with head and neck cancer: a multicenter, randomized, double-blind, placebo-controlled, parallel-group phase II study. BMC Cancer 17:314
Acknowledgments
The authors would like to acknowledge the contribution of Lorraine Porcello, MSLIS, MSIM—Bibby Dental Library, Eastman Institute for Oral Health, University of Rochester Medical Center, Rochester, NY, USA and Daniel A. Castillo, MLIS—Edward G. Miner Library, University of Rochester Medical Center, Rochester, NY, USA in developing the search language and literature search and help throughout the project.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
According to the MASCC Guidelines Policy, employees of commercial entities were not eligible to serve on this MASCC Guidelines Panel. The authors disclose no conflict of interest (AA, KKFC, AK, VT, ARAZ, DG, KC, AV, VR, ONG, SE). PB has served an advisory role for AstraZeneca, Helsinn, and Kyowa Kyrin and received grants from Merck, Kyowa Kyrin, and Roche. RVL has served as a consultant for Colgate Oral Pharmaceuticals, Galera Therapeutics, Ingalfarma SA, Monopar Therapeutics, Mundipharma, and Sucampo Pharma; has received research support to his institution from Galera Therapeutics, Novartis, Oragenics, and Sucampo Pharma; and has received stock in Logic Biosciences.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ariyawardana, A., Cheng, K.K.F., Kandwal, A. et al. Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 27, 3985–3995 (2019). https://doi.org/10.1007/s00520-019-04888-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04888-w