Abstract
Purpose
The purpose of the study is to compare the efficacy of benzydamine HCl with sodium bicarbonate in the prevention of concurrent chemoradiation-induced oral mucositis in head and neck cancer patients.
Methods
Sixty locally advanced head and neck cancer patients treated with high-dose radiotherapy concurrently with platinum-based chemotherapy were randomly assigned to receive either benzydamine HCl or sodium bicarbonate from the first day of treatment to 2 weeks after the completion of treatment. The total score for mucositis, based on the Oral Mucositis Assessment Scale (OMAS), was used for the assessment, conducted weekly during the treatment period and at the fourth week of the follow-up. Pain score, all prescribed medications, and tube feeding needs were also recorded and compared.
Results
The median of total OMAS score was statistically significant lower in patients who received benzydamine HCl during concurrent chemo-radiotherapy (CCRT) than in those who received sodium bicarbonate, (p value < 0.001). There was no difference in median pain score, (p value = 0.52). Nineteen percent of patients in sodium bicarbonate arm needed oral antifungal agents whereas none in the benzydamine HCl arm required such medications, (p value = 0.06). Tube feeding needs and the compliance of CCRT were not different between the two study arms.
Conclusions
For patients undergoing high-dose radiotherapy concurrently with platinum-based chemotherapy, using benzydamine HCl mouthwash as a preventive approach was superior to basic oral care using sodium bicarbonate mouthwash in terms of reducing the severity of oral mucositis and encouraging trend for the less need of oral antifungal drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Concurrent chemo-radiotherapy (CCRT) has confirmed benefits to improve treatment outcomes and survival rates over radiation therapy alone in treating locally advanced head and neck cancer patients. One of the common and major side effects of CCRT is oral mucositis. Radiation-induced mucositis was found to be as high as 70% when adding concurrent chemotherapy [1,2,3,4]. Radiation therapy-induced oral mucositis produces oral pain, swallowing difficulties, loss of taste, nausea, vomiting, loss of appetite, fatigue, weight loss, and decrease in quality of life. One or a combination of these symptoms can lead to delay in radiation treatment and poor treatment outcomes [5].
Radiation and chemotherapy directly injure DNA resulting in clonogenic death of basal epithelial cells. The primary damage responses then initiate a series of interacting biological events and transcription factors, such as NF-kB, Wnt, cytokines, tumor necrosis factor (TNF α), and p53, resulting in cell apoptosis and indirect cell death. In response, oral flora aggravates cytokine responses causing more inflammation [6]. Despite the development of radiation technology, three-dimensional conformal radiation therapy, or intensity-modulated radiation therapy (IMRT), oral mucositis has not been significantly alleviated. Oral mucositis can develop even at low dose to the oral cavity (a cumulative point dose less than 32 Gy) [7]. Typically, the therapeutic radiation therapy doses for curative aim range from 60 to 70 Gy for either postoperative treatment or definitive treatment [8,9,10].
The Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO) and National Comprehensive Cancer Network (NCCN) have recommended utilizing benzydamine hydrochloride mouthwash to prevent oral mucositis in head and neck cancer patients receiving moderate doses of radiation up to 50 Gy without concomitant chemotherapy (level I evidence) [11]. Other interventions such as basic oral care including teeth brushing, oral gaggle with normal saline or sodium bicarbonate solution, systemic zinc supplements, oral cryotherapy, and low-level laser therapy were suggested to prevent oral mucositis (level II–III evidences [11]. However, antimicrobial paste or lozenges and other mouthwashes such as antiseptic oral wash, sucralfate mouthwash, and G-CSF mouthwash were not recommended [11,12,13,14].
Benzydamine hydrochloride mouthwash has a component of non-steroidal anti-inflammatory drugs (NSAIDs) for local anti-inflammation, analgesia, and non-specific antibacterial [12, 15]. There have been several studies where benzydamine hydrochloride mouthwash was used to prevent oral mucositis in head and neck cancer patients receiving radiation therapy alone. Benzydamine hydrochloride mouthwash has been confirmed to decrease severity of oral mucositis in patients who received radiation therapy less than 50 Gy [12, 16, 17]. However, there has been no evidence to support using benzydamine hydrochloride mouthwash to prevent oral mucositis in patients who received CCRT for curative intent. Therefore, this study aims to evaluate the efficacy of benzydamine hydrochloride mouthwash with the intent to decrease severities of oral mucositis in head and neck cancer patients receiving CCRT with mean radiation doses of at least 60 Gy to their oral cavities. The effectiveness of the mouthwash was measured weekly using the total Oral Mucositis Assessment Scale (OMAS) in each week of CCRT. In this study, another group of patients who received basic oral care together with sodium bicarbonate mouthwash was used as a baseline for comparison. The second objective of this study is to compare the pain score between the two groups. For each week, any drugs prescribed e.g., analgesic drugs and antifungal agents, tube feeding insertion as well as the compliance of CCRT was also recorded.
Material and methods
Patients and treatment
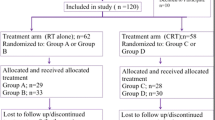
This study was a multicenter, randomized study conducted among three university hospitals and one cancer hospital, i.e., Chiang Mai University Hospital (CMUH), King Chulalongkorn Memorial Hospital (KCMH), Siriraj Hospital (SH), and Udonthani Cancer Hospital (UCH). It was conducted in compliance with principles of good clinical practice and all subjects provided the institutional approved informed consents. Tumor staging was based on the 7th American Joint Committee on Cancer recommendation. A total of 60 head and neck cancer patients were accrued as follows: 15 from CMUH, 10 from KCMH, 10 from SH, and 25 from UCH.
The study enrolled non-metastatic head and neck cancer patients, aged between 18 and 70 years old, with ECOG performance status between 0 and 2. They were scheduled for platinum-based CCRT at least 60 Gy with treatment volume affecting bilateral oral mucosa with any of the following techniques: two-dimensional (2D) radiotherapy, three-dimensional conformal radiotherapy (3D CRT), or intensity-modulated radiotherapy (IMRT). Pregnant and breast feeding patients were excluded from the study. Also excluded were patients who were unable to attend the protocol follow-up visit, and those with known hypersensitivity to benzydamine hydrochloride or NSAIDs.
Basic oral care protocols at each institution were permitted. Patients were encouraged to brush their teeth regularly at least twice a day and to consistently rinse with either benzydamine hydrochloride or sodium bicarbonate mouthwash as predefined by their randomized groups. One daily floss and use of fluoride were allowed. Commercial mouthwashes such as chlorhexidine or other oral hygiene products were prohibited.
Patients were randomly assigned to receive either 0.15% benzydamine hydrochloride or sodium bicarbonate mouthwash using block method with the allocation ratio of 1:1. The study statisticians and nurses dispensed the study drugs and maintained the confidential records of group assignment. Study participants and clinical providers were all blinded to subject assignment until the end of study.
Sodium bicarbonate mouthwash was prepared by the study nurse by mixing of 1 l of water with two teaspoons of baking soda. Patients were instructed to rinse four times daily with 15 ml of the solutions for 2 min to ensure that the solutions were well absorbed before expectorating as recommended in a previous study [12]. If burning or stinging sensation occurs with benzydamine mouthwash, a 1:1 dilution of water is permitted. The mouthwash regimens were initiated prior to radiation and continued until 2 weeks after the end of radiation.
Both groups of patients were provided measuring cups to measure the quantity of oral mouthwash. The mouthwash was given to each patient for 1-week use. All bottles of mouthwash in both groups were returned each week for the quality and compliance control.
Assessment
Patients were evaluated by the radiation oncologists at baseline before CCRT, weekly during CCRT, at the end of CCRT, and at 4 weeks after CCRT. The Oral Mucositis Assessment Scale (OMAS) was utilized to assess the severity of mucositis [18]. This validated scale provides grading of ulceration and erythema at nine oral sites: the upper lip, lower lip, right buccal mucosa, left buccal mucosa, right lateral and ventral tongue, left lateral and ventral tongue, floor of mouth, soft palate/fauces, and hard palate. Ulceration score was 0 for no lesion, 1 for lesion less than 1 cm2, 2 for lesion 1–3 cm2, and 3 for lesion more than 3 cm2. Erythema score was 0 for no lesion, 1 for mild to moderate erythema, and 3 for severe erythema.
Pain score (0–10), all prescribed medications including analgesic, artificial saliva supplement, and anti-infection medications, the need for hospitalization, treatment interruption, and the addition of nutritional support or feeding tube were also recorded. Oral candidiasis assessment was based on the physical examination of pseudomembranous lesions or angular cheilitis in all our centers except at the CMUH center where confirmation by the microscopic identification of Candida was used.
Statistical analysis
In order to examine the effectiveness of benzydamine HCl on the severity reduction of mucositis in head and neck cancer patients receiving CCRT, null hypothesis was generated stating that the mean mucositis score of study group (benzydamine hydrochloride) is less than the mean mucositis score of control group (sodium bicarbonate). Sample size calculations were performed to achieve high power for detection of a one-point difference in mean of total OMAS score (range 0–5). Published data for peak OMAS scores in patients (no intervention) indicated a standard deviation of ± 1.1 points [19]. Assuming 10% early drop-out or loss of follow-up, a minimum sample size of 30 patients for each treatment group was needed to obtain 90% power in order to apply a one-tailed, two-sample t test at 5% level of significance.
Categorical demographics and tumor characteristics data were compared between groups using Fisher’s exact and Wilcoxon rank-sum test for continuous characteristics. For patients with poor CCRT compliance, the Fisher’s exact test was used to compare the proportion of patients received regimen (i.e., weekly cisplatin, weekly carboplatin, and cisplatin every 3 months) between the study group and the control group. Comparison of group of other treatments during CCRT between study group and control group was done using the Fisher exact test. The OMAS and pain scores for each assessment were compared between groups, using Wilcoxon rank-sum test. All analyses were done using STATA (version 10).
Results
A total of 60 patients were enrolled in the study. As shown in Table 1, patients and treatment characteristics showed no statistically significant difference between the study group and the control group. Most of the patients had locally advanced stage disease (stages III–IVB); 90 and 83% in the study group and the control group, respectively. Nasopharyngeal carcinoma was the most common primary site in both groups. The patients had received a median total dose of 69.96 and 67.98 Gy in the study and the control group, respectively. Most of our patients were treated with IMRT technique in 48%. Mean oral doses for patients who received 3D/IMRT were 51.8 Gy.
As shown in Fig. 1, all patients developed mucositis onset at second week during CCRT and most of them recovered at the follow-up period. Statistically, the median for OMAS scores was significantly lower in the study group at every week between the second and eighth week of CCRT. The corresponding p values for those weeks in chronological order were 0.003, < 0.001, < 0.001, < 0.001, < 0.001, 0.01, and 0.04. We found the maximum of the third quartile of OMAS scores to be around the fifth to the sixth week in both groups. In addition, the maximum OMAS score across the whole period of CCRT in the benzydamine HCl group was 25, substantially lower than the maximum of 37 in the sodium bicarbonate group.
As depicted in Fig. 2, there was no significant difference in the median of pain scores between the two groups during CCRT and at the follow-up. The corresponding p values from the second to the eighth week were 0.88, 0.59, 0.96, 0.73, 0.63, 0.92, and 0.15 and the p value at the follow-up after CCRT was 0.80. We have observed the maximum of the third quartile of pain scores at the seventh week of CCRT in the study group and eighth week in the control group. The maximum pain score across the period of CCRT was 10 in both groups. Four weeks after the CCRT, the median of pain score decreased to 2 in both groups.
Compliance of CCRT was shown in Table 2, with poor compliance defined as patients in postoperative CCRT group and in definitive CCRT group who received weekly chemotherapy less than 3 cycles and 4 cycles, respectively. We found more patients with poor compliance in the control group than in the study group i.e., 13 patients versus 10 patients. However, no differences were found between the two groups.
As shown in Table 3, most of the patients in both groups need analgesic drugs; 89% in the control group and 90% in the study group, p value = 1.0. The most common analgesic drugs used were opioids (syrup morphine/morphine sulfate tablet; MST/morphine sulfate sustain released; kapanol®), the prescribed usage rates of which were 48% in the control group and 33% in the study group. For the antifungal agents used, we found that none of the patients in the benzydamine HCl group needed these agents, whereas 19% of the patients in the sodium bicarbonate group were prescribed antifungal drugs such as nystatin oral suspension or clotrimazole tablet. The rate of feeding tube placement was essentially the same in both groups during CCRT, i.e., 24 and 22% in the study group and the control group, respectively. Clinical pictures of oral mucositis and candidiasis are shown in Fig. 3a, b.
Oral retention and compliance for the mouthwash products were better in the sodium bicarbonate arm than in the benzydamine HCl arm. Four patients (6.75%) in the study arm could not tolerate the full dose of benzydamine HCl due to burning and stinging sensation. For these patients, the mouthwash was diluted at 1:1 with water.
Discussions
Strengths of our trial include the multicenter randomized study in platinum-based chemotherapy with high-dose RT which is the current standard of care in locally advanced head and neck cancer. Moreover, our study is the first study that enrolled both patients treated with definitive CCRT and postoperative CCRT. To our best knowledge, only one study has explored the efficacy of benzydamine HCl in high-dose radiotherapy with or without chemotherapy [20]. In that study, the investigators found a significant reduction in the rates of grade 3 mucositis in patients receiving benzydamine HCl and treated with high-dose RT alone (> 50 Gy), but not in patients treated with CCRT.
The present study has demonstrated that prophylaxis rinsing with benzydamine HCl can be more effective in reducing the severity of oral mucositis induced by CCRT, when compared to basic care with sodium bicarbonate mouthwash. The median of OMAS scores at every weekly assessment during CCRT was lower in the benzydamine HCl group compared to that of the control group. In both groups of patients, we found the maximum of the third quartile of OMAS scores around the fifth week to seventh week of CCRT. The scores dramatically decreased at 4 weeks after CCRT.
Data from several studies demonstrated that benzydamine HCl rinses have a positive impact on the prevention of oral mucositis in patients undergoing radiotherapy alone with the radiation dose of less than or equal to 50 Gy [12, 16, 17, 20]. Rooparshri et al. [21] reported that benzydamine HCl (0.15%) can help in delaying the progression of mucositis and in reducing the intensity of pain and that it can be more effective than 0.2% chlorhexidine and povidone iodine oral rinses. Despite the fact that they used higher dose RT at 6600 cGy, the exclusion criteria in their study were the patients who received CCRT. While the previous research by Rastogi M et al. [20] has not shown the benefit of prophylaxis rinsing benzydamine HCl in CCRT group, our study reports the benefit of this drug in the prevention of oral mucositis induced by CCRT. The patients in the benzydamine HCl group in their study received 100% cisplatin concurrently with RT, while ours had 73%. Moreover, the oral cavity was the most common primary site (57%) in their study, whereas in our study, it was not, i.e., only 17%. Although we had lower number of oral cavity cancer, all of our patients received at least 60 Gy with treatment volume affecting the bilateral oral mucosa. Another difference that we found was in the radiotherapy technique, i.e., 100% of 3D CRT in their study, but 43% of 2D RT, 3% of 3D CRT, and 54% of IMRT in ours for the benzydamine HCl group. All differences could have been the reasons for their CCRT patients not getting the benefit of prophylaxis rinsing.
The distinguishing feature of benzydamine HCl in comparison with basic sodium bicarbonate mouth rinses is that it has a component of non-steroidal anti-inflammatory mouthwash which also poses analgesic, antimicrobial, antifungal, and anesthetic properties [22,23,24]. Although benzydamine HCl has the advantage on reducing the severity of mucositis, it does not have any effect on the severity of pain, the compliance of CCRT, or the need for analgesics as shown in Table 3.
The reason of ineffectiveness in reducing pain score in the study group despite the lower OMAS score might be because of the high percentage of nasopharyngeal cancer patients in both groups, i.e., 50 and 53%. As we know, the radiation field of locally advanced stage nasopharyngeal cancer covers all the pharyngeal mucosa. As such, oral pain and throat pain would be the two main symptoms already experienced by this group of patients and oral rinse with the study drugs could not cover the whole pharyngeal areas. From the fifth week of CCRT, it seemed that benzydamine HCl was ineffective in pain reduction. Especially around the seventh to eighth week, the third quartile of pain score of the study group was 9 and 8 in the control group. However, during the fifth to eighth week, the median pain score was 3.8–5 in the benzydamine HCl group, slightly lower than 5–6 in the control group.
Another advantage of benzydamine HCl that we found in this study is the reduction in the need of antifungal agents. The antiseptic, antimicrobial, and antifungal efficacy of this mouthwash may be the explanation. Pina-Vaz et al. [25] evaluated the activity of benzydamine, lidocaine, and bupivacaine, three drugs with local anesthetic activity, against Candida albicans and non-albicans strains and explained their mechanism of activity. They concluded that at lower concentrations, all three tested drugs had a fungistatic activity, due to yeast metabolic impairment, while at higher concentrations, they were fungicidal, due to direct damage to the cytoplasmic membrane. The three drugs possess an antifungal activity through a membrane-damaging action because of their lipophilic molecules. Benzydamine HCl was found to be the most active of the three drugs. Moreover, it was reported that benzydamine solutions for oral applications contain 0.15% w/v mouthwash, a concentration 30 times higher than the minimal inhibitory concentration of the least susceptible Candida strain. A few limitations of our study should be noted. Although oral hygiene is a contributor to the score of oral mucositis, we did not investigate the influence of this parameter on the study endpoints. Second, we did not attempt to impose any specific scale to the erythema score assessment. Its severity (mild, moderate, or severe) was determined by the site investigators. And third, most of our patients were diagnosed of oral candidiasis based on clinical examination only, not confirmed by the microscopic identification of Candida.
In conclusion, this study found that prophylaxis oral rinsing with benzydamine HCl for patients undergoing high-dose radiotherapy concurrently with platinum-based chemotherapy was superior to sodium bicarbonate mouthwash in terms of alleviating the severity of oral mucositis and encouraging trend for reducing the need of oral antifungal agents use.
References
Baujat B, Audry H, Bourhis J, Chan AT, Onat H, Chua DT, Kwong DL, Al-Sarraf M, Chi KH, Hareyama M, Leung SF, Thephamongkhol K, Pignon JP, Group M-NC (2006) Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys 64(1):47–56. https://doi.org/10.1016/j.ijrobp.2005.06.037
Bourhis J, Sire C, Graff P, Gregoire V, Maingon P, Calais G, Gery B, Martin L, Alfonsi M, Desprez P, Pignon T, Bardet E, Rives M, Geoffrois L, Daly-Schveitzer N, Sen S, Tuchais C, Dupuis O, Guerif S, Lapeyre M, Favrel V, Hamoir M, Lusinchi A, Temam S, Pinna A, Tao YG, Blanchard P, Auperin A (2012) Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): an open-label phase 3 randomised trial. Lancet Oncol 13(2):145–153. https://doi.org/10.1016/S1470-2045(11)70346-1
Pignon JP, le Maitre A, Maillard E, Bourhis J, Group M-NC (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92(1):4–14. https://doi.org/10.1016/j.radonc.2009.04.014
Calais G, Alfonsi M, Bardet E, Sire C, Germain T, Bergerot P, Rhein B, Tortochaux J, Oudinot P, Bertrand P (1999) Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst 91(24):2081–2086
Hansen O, Overgaard J, Hansen HS, Overgaard M, Hoyer M, Jorgensen KE, Bastholt L, Berthelsen A (1997) Importance of overall treatment time for the outcome of radiotherapy of advanced head and neck carcinoma: dependency on tumor differentiation. Radiother Oncol 43(1):47–51
Sonis ST (2009) Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol 45(12):1015–1020. https://doi.org/10.1016/j.oraloncology.2009.08.006
Narayan S, Lehmann J, Coleman MA, Vaughan A, Yang CC, Enepekides D, Farwell G, Purdy JA, Laredo G, Nolan K, Pearson FS, Vijayakumar S (2008) Prospective evaluation to establish a dose response for clinical oral mucositis in patients undergoing head-and-neck conformal radiotherapy. Int J Radiat Oncol Biol Phys 72(3):756–762. https://doi.org/10.1016/j.ijrobp.2008.01.060
Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, Garden AS, Ridge JA, Cooper JS, Ang KK (2000) A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 48(1):7–16
Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, Giralt J, Maingon P, Rolland F, Bolla M, Cognetti F, Bourhis J, Kirkpatrick A, van Glabbeke M, European Organization for R, Treatment of Cancer T (2004) Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 350(19):1945–1952. https://doi.org/10.1056/NEJMoa032641
Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, Machtay M, Ensley JF, Chao KS, Schultz CJ, Lee N, Fu KK, Radiation Therapy Oncology Group I (2004) Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 350(19):1937–1944. https://doi.org/10.1056/NEJMoa032646
Lalla RV, Ashbury FD (2013) The MASCC/ISOO mucositis guidelines: dissemination and clinical impact. Support Care Cancer 21(11):3161–3163. https://doi.org/10.1007/s00520-013-1924-2
Epstein JB, Silverman S Jr, Paggiarino DA, Crockett S, Schubert MM, Senzer NN, Lockhart PB, Gallagher MJ, Peterson DE, Leveque FG (2001) Benzydamine HCl for prophylaxis of radiation-induced oral mucositis: results from a multicenter, randomized, double-blind, placebo-controlled clinical trial. Cancer 92(4):875–885
Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, DB MG, Hutchins RD, Peterson DE, Mucositis Study Section of the Multinational Association of Supportive Care in C, the International Society for Oral O (2007) Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109(5):820–831. https://doi.org/10.1002/cncr.22484
Rosenthal DI, Trotti A (2009) Strategies for managing radiation-induced mucositis in head and neck cancer. Semin Radiat Oncol 19(1):29–34. https://doi.org/10.1016/j.semradonc.2008.09.006
Quane PA, Graham GG, Ziegler JB (1998) Pharmacology of benzydamine. Inflammopharmacology 6(2):95–107. https://doi.org/10.1007/s10787-998-0026-0
Diwan AK, Meshram SD (2016) Role of benzydamine in radiation-induced oral mucositis in head and neck cancer patients. Int J Med Res Prof 2(4):23–26. 10.21276/ijmrp.2016.2.4.006
Kazemian A, Kamian S, Aghili M, Hashemi FA, Haddad P (2009) Benzydamine for prophylaxis of radiation-induced oral mucositis in head and neck cancers: a double-blind placebo-controlled randomized clinical trial. Eur J Cancer Care (Engl) 18(2):174–178. https://doi.org/10.1111/j.1365-2354.2008.00943.x
Sonis ST, Eilers JP, Epstein JB, LeVeque FG, Liggett WH Jr, Mulagha MT, Peterson DE, Rose AH, Schubert MM, Spijkervet FK, Wittes JP (1999) Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group. Cancer 85(10):2103–2113
Epstein JB, Gorsky M, Guglietta A, Le N, Sonis ST (2000) The correlation between epidermal growth factor levels in saliva and the severity of oral mucositis during oropharyngeal radiation therapy. Cancer 89(11):2258–2265
Rastogi M, Khurana R, Revannasiddaiah S, Jaiswal I, Nanda SS, Gupta P, Chufal KS, Bhatt ML (2017) Role of benzydamine hydrochloride in the prevention of oral mucositis in head and neck cancer patients treated with radiotherapy (>50 Gy) with or without chemotherapy. Support Care Cancer 25(5):1439–1443. https://doi.org/10.1007/s00520-016-3548-9
Roopashri G, Jayanthi K, Guruprasad R (2011) Efficacy of benzydamine hydrochloride, chlorhexidine, and povidone iodine in the treatment of oral mucositis among patients undergoing radiotherapy in head and neck malignancies: a drug trail. Contemp Clin Dent 2(1):8–12. https://doi.org/10.4103/0976-237X.79292
Cheng KK (2004) Children’s acceptance and tolerance of chlorhexidine and benzydamine oral rinses in the treatment of chemotherapy-induced oropharyngeal mucositis. Eur J Oncol Nurs 8(4):341–349. https://doi.org/10.1016/j.ejon.2004.04.002
Epstein JB, Stevenson-Moore P, Jackson S, Mohamed JH, Spinelli JJ (1989) Prevention of oral mucositis in radiation therapy: a controlled study with benzydamine hydrochloride rinse. Int J Radiat Oncol Biol Phys 16(6):1571–1575
Kim JH, Chu FC, Lakshmi V, Houde R (1986) Benzydamine HCl, a new agent for the treatment of radiation mucositis of the oropharynx. Am J Clin Oncol 9(2):132–134
Pina-Vaz C, Rodrigues AG, Sansonetty F, Martinez-De-Oliveira J, Fonseca AF, Mardh PA (2000) Antifungal activity of local anesthetics against Candida species. Infect Dis Obstet Gynecol 8(3–4):124–137. https://doi.org/10.1155/S1064744900000168
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
It was conducted in compliance with principles of good clinical practice and all subjects provided the institutional approved informed consents.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chitapanarux, I., Tungkasamit, T., Petsuksiri, J. et al. Randomized control trial of benzydamine HCl versus sodium bicarbonate for prophylaxis of concurrent chemoradiation-induced oral mucositis. Support Care Cancer 26, 879–886 (2018). https://doi.org/10.1007/s00520-017-3904-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-017-3904-4