Abstract
Purpose
Misoprostol, a synthetic analog of prostaglandin E1, has anti-inflammatory and mucosa-protecting properties. The objective of this study was to evaluate the efficacy of misoprostol oral rinse in reducing the severity of oral mucosal injury caused by high-dose chemotherapy.
Methods
The study used a randomized, double-blind, placebo-controlled, parallel-group design. Oncology patients receiving myeloablative high-dose chemotherapy, in preparation for a hematopoietic stem cell transplant, were randomized to misoprostol or placebo rinse. The primary outcome measure was the severity of oral mucositis, measured using the modified Oral Mucositis Index. Additional outcome measures included the severity of mouth pain (measured using a Visual Analog Scale and the Pain Affect Faces Scale), duration of hospital stay, and days on total parenteral nutrition.
Results
This study was originally planned to accrue 160 subjects but was terminated early due to revised sponsor research priorities. The intent-to-treat population consisted of 22 subjects randomized to misoprostol rinse and 26 subjects randomized to placebo rinse. There was no significant difference between the two groups in mucositis or pain severity. In both groups, duration of hospital stay was approximately 19 days, and number of days on total parenteral nutrition was 17–18 days. There were no serious adverse events attributable to misoprostol rinse.
Conclusions
Although this study did not find a beneficial effect of a misoprostol rinse in mucositis secondary to high-dose chemotherapy, the small sample size limits the strength of this conclusion. Given the proposed importance of the prostaglandin pathway in the pathogenesis of oral mucositis, additional studies are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Oral mucositis refers to erythematous, erosive, and ulcerative lesions of the oral mucosa that develop in patients receiving head and neck radiation therapy and/or chemotherapy for cancer [4, 12, 20]. Approximately 10–40% of conventional chemotherapy patients develop oral mucositis with a significant number developing severe mucositis that requires modification in their medical management [10]. In patients who receive chemotherapy as conditioning for hematopioetic stem cell transplantation, approximately 70% develop oral mucositis, with over 20% developing severe mucositis [28]. In these patients, mucositis causes significant pain, usually requiring parenteral narcotics for relief. It also compromises nutrition and overall quality of life with many patients requiring total parenteral nutrition. From the patient’s point of view, oral mucositis is often the single most debilitating complication of a transplant [2]. Since these patients are typically severely immunosuppressed, infections of the oral lesions have resulted in life-threatening systemic sepsis during myeloablation [24]. Mucositis severity is independently associated with increased risk for vancomycin-resistant enterococcal bloodstream infection in hospitalized cancer patients [11]. Moderate to severe oral mucositis has been correlated with bloodstream infections and transplant-related mortality [21]. A single point increase in peak mucositis scores in stem cell transplant patients is associated with one additional day of fever, a 2.1-fold increase in risk of significant infection, 2.7 additional days of total parenteral nutrition, 2.6 additional days of injectable narcotic therapy, 2.6 additional days in hospital, $25,405 in additional hospital charges, and a 3.9-fold increase in 100-day mortality risk [27]. Management of oral mucositis for most patients is focused on pain control and dietary support [13, 16]. Thus, there is a need to identify agents that may be effective in preventing oral mucositis or reducing its severity.
Misoprostol, a synthetic analog of prostaglandin E1 (PGE1), has anti-inflammatory and mucosa-protecting properties [9, 22]. Since the inflammatory cascade, including the cyclooxygenase pathway, plays a role in the pathogenesis of mucosal injury secondary to cancer therapy [14], there has been interest in studying the use of misoprostol for this indication. However, human clinical trials evaluating misoprostol in reducing severity of oral mucositis have had mixed results [3, 7, 8, 30]. To the best of our knowledge, a misoprostol oral rinse has not been previously evaluated in chemotherapy-induced oral mucositis. The objective of this proof-of- principle study was to evaluate the efficacy of a misoprostol oral rinse in reducing the severity of oral mucosal injury induced by high-dose chemotherapy.
Methods
Trial design and sample size
The study used a randomized, double-blind, placebo-controlled, parallel-group design. This was a multi-center study with six participating US sites: University of Connecticut Health Center (Farmington, CT), Fred Hutchinson Cancer Research Center (Seattle, WA), Wayne State University (Detroit, MI), Northwestern University (Chicago, IL), Dana Farber Cancer Institute (Boston, MA), and University of California at San Francisco (San Francisco, CA). The primary outcome measure was the mean modified Oral Mucositis Index (OMI) score, representing the severity of oral mucositis [1, 26]. A 3-point difference in total OMI scores between the two groups represents a clinically relevant change of at least 25% [1]. The sample size calculation assumed that the true standard deviation is 5.8 based on previously published work [1]. It was calculated that to detect a 3-point difference in total OMI scores with a power of 0.8 and assuming a two-sided significance level of 0.05 for an analysis of variance model would require a minimum of 64 subjects per group. After accounting for dropouts, this study was planned to enroll 80 subjects per group (total of 160 subjects). However, the study was terminated early, due to discontinuation of funding based on a change in sponsor priorities, after a total of 49 subjects were enrolled. Written informed consent was obtained from all subjects according to local Institutional Review Board protocols.
Participants
Inclusion criteria for eligibility for this study were the following: diagnosis of lymphoma, multiple myeloma or a solid tumor for which the patient was to receive a preparative conditioning chemotherapy regimen for autologous stem cell transplant of either marrow or peripheral blood origin, age ≥18 years, Karnofsky performance status of ≥60%, serum creatinine <2.5 mg/dL, bilirubin and AST less than or equal to three times the normal value, negative test for HIV and hepatitis B surface antigen, negative pregnancy test for women of child-bearing potential within 48 h of first study drug dose, and agreement by these subjects not to breastfeed and to use an effective method of contraception during the study.
Exclusion criteria were as follows: preparative conditioning regimen includes cisplatin/carboplatin or total body irradiation, scheduled conditioning regimen is >11 days, allergy to misoprostol or other prostaglandin analogs, patients receiving chlorhexidine, sucralfate, benzydamine, or any investigational agent during the study period.
Interventions
Subjects were randomized to either the misoprostol or placebo arm by the research pharmacist. Subjects and study personnel were blinded to group assignment. The study drug was provided to subjects as 200 mcg of misoprostol or placebo in 15 ml of water. This dose of misoprostol has been shown to be effective and is approved for reducing gastric mucosal injury secondary to nonsteroidal anti-inflammatory drug (NSAID) therapy [33]. Both misoprostol and placebo rinses were colorless and tasteless. Subjects were asked to swish and gargle with the 15-ml solution for 60 s and then swallow it. Study drug administration began 45 min to 2 h before the start of the conditioning regimen and continued every 8 h (±2 h) until 24 h after the end of the conditioning regimen (Fig. 1). All subjects were instructed with standard oral care and hygiene procedures. Subjects were instructed not to perform any oral care procedures (including toothbrushing and saline or bicarbonate rinses) for 1 h after study drug administration.
Outcome measures
Oral mucositis was graded using the modified Oral Mucositis Index. This is a validated and well-accepted scale for measuring oral mucositis [1, 26]. A baseline oral mucositis assessment was performed within 48 h before the first study drug administration. During the study period, mucositis assessments were performed every 2–3 days. Assessments were performed by calibrated examiners who were blinded as to group assignments. All clinical examiners were calibrated by Dr. Peterson or Dr. Schubert at in-person meetings. Calibrations were performed using a slide set developed by Dr. Schubert that showed representative clinical photographs of the various scoring categories of the Oral Mucositis Index. Additionally, a pocket reference card that showed the various shades of erythema and sizes of ulceration for each scoring category was used and left with the investigators at each site for ongoing calibration during the study.
Mouth pain was assessed on the same schedule as oral mucositis using a Visual Analog Scale (VAS) (range, 0–100) and a Pain Affect Faces Scale (PAFS) (range, 0–9). These are both validated instruments well accepted for pain assessment [19]. Pain scores were not collected within 30 min of topical anesthetic use in the oral cavity. Mucositis and pain assessments continued until one of the following occurred: 18 days following stem cell transplant, hospital discharge, both the OMI and VAS scores for pain reached zero.
The number of hospital days for each subject was recorded as the number of days from the day of transplant to the day of discharge (both inclusive). Days on total parenteral nutrition were recorded as the number of days each subject received nutrition solely by intravenous feeding.
Statistical analyses
Total OMI scores at each time point, for each subject, were used to calculate area-under-the-curve (AUC) values, using SPSS Statistics software (SPSS Inc, Chicago, IL). Mean AUC values were derived from the AUC values for all subjects in each of the misoprostol and placebo groups. Mean AUC values were similarly calculated for the two groups for pain scores (for both the VAS and PAFS). Mean AUC values were compared for each outcome variable between the two groups using the t test. Mean number of hospital days and mean number of days on total parenteral nutrition were each compared between the two groups using the t test. A p value of <0.05 was considered statistically significant for all comparisons.
Results
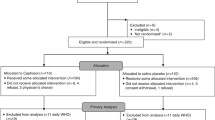
Forty-nine subjects were enrolled in this study. Subject flow is described in Fig. 2. The demographics and clinical characteristics for the intent-to-treat (ITT) population (n = 48) are summarized in Table 1. The breakdown of the intent-to-treat population by center was as follows: University of Connecticut Health Center, 9 subjects; Fred Hutchinson Cancer Center, 18 subjects; Wayne State University, 2 subjects; Northwestern University, 4 subjects; Dana Farber Cancer Center, 2 subjects; and University of California at San Francisco, 13 subjects. No analysis of outcomes by individual center was able to be performed due to the small number of subjects enrolled at each center. The experimental groups were comparable relative to age, gender, cancer diagnosis, and chemotherapy regimens. Thirty-nine subjects had no major deviations and constituted the “per protocol” group (18 in the misoprostol group and 21 in the placebo group). Protocol deviations involved either missed or delayed study drug doses or mucositis assessments. Study rinse use was supervised and timed in 99% of doses administered. Only 5% of planned study rinse doses were missed. Thus, 95% of planned study rinse doses were administered. In 19% of doses, the precise timing of study rinse use was outside the window specified by the protocol (every 8 ± 2 h). In 3% of doses, the duration of swish use was less than that specified by protocol (60 s).
Misoprostol, as used in this study, was safe in stem cell transplant patients. There were no serious adverse events attributable to the study drug. Diarrhea and emesis were the only adverse events attributable to the study drug; these events were infrequent and responded well to medical treatment.
Oral mucositis severity
Baseline OMI scores in both intent-to-treat groups approximated 0, peaked between days +4 and +9 post-transplant, and then gradually decreased. Peak mucositis scores were somewhat higher in the placebo group (Fig. 3a). However, mean AUC comparisons of total OMI scores for the ITT population revealed no statistically significant differences in mucositis severity for the total study period or for the period of peak mucositis severity only (p > 0.05 for both) (Fig. 3b).
Comparison of OMI scores between the per protocol groups revealed a larger difference in peak mucositis severity between the two groups, with lower peak scores in the misoprostol group (Fig. 3c). However, the comparison of mean AUC of total OMI scores did not achieve statistical significance for the total study period or for the period of peak mucositis severity only (p > 0.05 for both) (Fig. 3d).
Mouth pain severity
Mouth pain scores in the ITT population, as measured by the VAS and PAFS, started low, were highest during peak mucositis severity (between days +4 and +9), and then gradually declined (Fig. 4a and b). There were no significant differences in the mean AUC for either pain scale for the entire study duration or for the period of peak mucositis severity (p > 0.05 for both) (Fig. 4c and d). The per protocol analyses also revealed no significant differences (data not shown).
Number of hospital days and total parenteral nutrition
Approximately 19 days elapsed from the day of transplant to the day of hospital discharge, in both the misoprostol and placebo ITT groups. Subjects in both groups required feeding via total parenteral nutrition for approximately 17–18 days. There was no significant difference between the two groups in the number of hospital days or in the number of days for which total parenteral nutrition was required (p > 0.05 for both) (Fig. 5). The per protocol analyses also revealed no significant differences (data not shown).
Discussion
The prostaglandin pathways are considered to contribute an important role in the inflammatory component of mucositis [15]. Prostaglandin E2 (PGE2) and prostacyclin (PGI2) mediate tissue injury and pain at sites of inflammation. PGE2 contributes to tissue injury by upregulating the production of tissue-destroying matrix metalloproteinases via a cAMP-dependent pathway. PGE1, on the other hand, has anti-inflammatory effects. Misoprostol, being a synthetic analog of PGE1, also has similar biologic effects as PGE1. For example, misoprostol has mucosal-protective effects and has been approved for reducing the risk of gastric ulcers induced by NSAID use. Misoprostol also inhibits the release of TNF-α and IL-1β from activated human monocytes [31]. These pro-inflammatory cytokines have been implicated in contributing to the pathogenesis of oral mucositis [5, 32]. Animal studies have indicated that misoprostol reduces endotoxin-induced gastric mucosal injury. This protective effect may be mediated in part via inhibition of production of TNF-α, which is an important mediator of tissue injury [17]. Locally administered misoprostol was found to decrease hair loss resulting from systemic doxorubicin administration in mice [18]. Previous studies have shown that misoprostol is rapidly and extensively absorbed after oral administration with a T max of 12 ± 3 min and a terminal half-life of 20–40 min. Plasma levels have been found to be proportional to dose, and a plasma steady state is achieved within 2 days [33]. This collective science provided the rationale for examining misoprostol as a potential therapy for oral mucositis secondary to cancer therapy.
However, in our current study of patients receiving high-dose chemotherapy, no clear beneficial effect of a misoprostol rinse was observed on severity of mucositis and pain, on duration of hospital stay, or need for total parenteral nutrition. It is notable that the per-protocol analyses did show lower peak OMI scores (indicating lower mucositis severity) in the misoprostol group, although this difference did not achieve statistical significance. Data from this study should be considered in the context of other clinical investigations with misoprostol. For example, Duenas-Gonzalez et al. reported increased incidence and severity of mucositis in high-dose chemotherapy subjects treated with misoprostol tablets, as compared to a placebo group [3]. In the head and neck radiation therapy population, Veness et al. reported no benefit of a misoprostol rinse on the incidence of severe mucositis, as compared to placebo [30]. Hanson et al. conducted a two-center study of misoprostol tablet vs. placebo for oral mucositis secondary to head and neck radiation therapy [7, 8]. Data from one site when analyzed alone showed a statistically significant decrease in the mean mucositis scores at the 4th and 5th weeks of radiation, with no significant difference seen at weeks 6 and 7. No benefit of misoprostol was found at the other study site or when the data from the two sites were analyzed together. Sartori et al. conducted a randomized, placebo-controlled study testing systemic misoprostol, omeprazole, and placebo for the reduction of gastroduodenal mucositis secondary to chemotherapy [25]. Misoprostol was not superior to placebo in reducing gastric and duodenal ulcers, while a significant positive effect of omeprazole was seen. Taken together with the findings of our study, these data collectively suggest a lack of efficacy for misoprostol in mucositis.
A limitation of our study was that accrual was terminated before the planned sample size was achieved. Thus, the resultant study was underpowered; it is possible that a statistically significant effect may have been seen with a larger sample size as originally envisioned based on sample size calculations. Our planned sample size was based on detection of a 3-point different in OMI scores, representing a 25% change. However, the actual sample size achieved was only adequate to detect a significantly larger effect size of a 4.8-point difference in OMI scores (greater than a 40% difference). Thus, although this study indicates the absence of a large effect size, the possibility of a smaller effect cannot be eliminated. It should also be noted that subjects in our study, as well as in the study by Duenas-Gonzalez et al. [3], received high-dose myeloablative chemotherapy in preparation for stem cell transplant. Such high-dose chemotherapy causes significant oral mucositis and may have made it more difficult to see an effect of the intervention tested. It is possible that a different outcome may be achieved in subjects receiving lower dose chemotherapy that results in less severe oral mucositis, which may however also be less clinically significant. Our findings should be interpreted with these limitations in mind and in the context of the larger body of data on this subject.
This study did not investigate the mechanisms of action of misoprostol on oral mucositis, but some observations are in order that may have a bearing on the outcome. The mucosal-protective effects of misoprostol on the gastric mucosa are partly mediated via reducing gastric acid secretion and increasing bicarbonate and mucus production from gastric parietal cells [6]. The absence of these mechanisms in the oral mucosa may explain the lack of protective effect of misoprostol seen in this study. Furthermore, misoprostol suppresses neutrophil-mediated acute inflammation [23] and thus may interfere with wound healing of mucositis lesions [29].
In conclusion, in this study of patients receiving high-dose chemotherapy, misoprostol rinse was safe, but we did not find a beneficial effect on severity of oral mucositis, mouth pain severity, duration of hospital stay, and need for total parenteral nutrition. However, these findings should be interpreted with caution due to the limitations discussed above. Indeed, the rationale for publishing this prematurely terminated study is not to provide a definitive conclusion, but to contribute data to the published knowledge on the role of the prostaglandin pathways in mucositis. Additional research is warranted on the pathogenic inflammatory mechanisms involved in mucositis and the role of the prostaglandin pathways.
References
Barasch A, Peterson DE, Tanzer JM, D’Ambrosio JA, Nuki K, Schubert MM, Franquin JC, Clive J, Tutschka P (1995) Helium-neon laser effects on conditioning-induced oral mucositis in bone marrow transplantation patients. Cancer 76:2550–2556
Bellm LA, Epstein JB, Rose-Ped A, Martin P, Fuchs HJ (2000) Patient reports of complications of bone marrow transplantation. Support Care Cancer 8:33–39
Duenas-Gonzalez A, Sobrevilla-Calvo P, Frias-Mendivil M, Gallardo-Rincon D, Lara-Medina F, Aguilar-Ponce L, Miranda-Lopez E, Zinser-Sierra J, Reynoso-Gomez E (1996) Misoprostol prophylaxis for high-dose chemotherapy-induced mucositis: a randomized double-blind study. Bone Marrow Transplant 17:809–812
Epstein JB (2007) Mucositis in the cancer patient and immunosuppressed host. Infect Dis Clin North Am 21:503–522, vii
Fall-Dickson JM, Ramsay ES, Castro K, Woltz P, Sportes C (2007) Oral mucositis-related oropharyngeal pain and correlative tumor necrosis factor-alpha expression in adult oncology patients undergoing hematopoietic stem cell transplantation. Clin Ther 29(Suppl):2547–2561
Garris RE, Kirkwood CF (1989) Misoprostol: a prostaglandin E1 analogue. Clin Pharm 8:627–644
Hanson WR, Marks JE, Reddy SP, Simon S, Mihalo WE, Tova Y (1995) Protection from radiation-induced oral mucositis by misoprostol, a prostaglandin E(1) analog: a placebo-controlled double-blind clinical trial. Am J Ther 2:850–857
Hanson WR, Marks JE, Reddy SP, Simon S, Mihalo WE, Tova Y (1997) Protection from radiation-induced oral mucositis by a mouth rinse containing the prostaglandin E1 analog, misoprostol: a placebo controlled double blind clinical trial. Adv Exp Med Biol 400B:811–818
Haynes DR, Whitehouse MW, Vernon-Roberts B (1992) The prostaglandin E1 analogue, misoprostol, regulates inflammatory cytokines and immune functions in vitro like the natural prostaglandins E1, E2 and E3. Immunology 76:251–257
Jones JA, Avritscher EB, Cooksley CD, Michelet M, Bekele BN, Elting LS (2006) Epidemiology of treatment-associated mucosal injury after treatment with newer regimens for lymphoma, breast, lung, or colorectal cancer. Support Care Cancer 14:505–515
Kuehnert MJ, Jernigan JA, Pullen AL, Rimland D, Jarvis WR (1999) Association between mucositis severity and vancomycin-resistant enterococcal bloodstream infection in hospitalized cancer patients. Infect Control Hosp Epidemiol 20:660–663
Lalla RV, Peterson DE (2005) Oral mucositis. Dent Clin North Am 49:167–184
Lalla RV, Peterson DE (2006) Treatment of mucositis, including new medications. Cancer J 12:348–354
Lalla RV, Pilbeam CC, Walsh SJ, Sonis ST, Keefe DM, Peterson DE (2010) Role of the cyclooxygenase pathway in chemotherapy-induced oral mucositis: a pilot study. Support Care Cancer 18:95–103
Lalla RV, Schubert MM, Bensadoun RJ, Keefe D (2006) Anti-inflammatory agents in the management of alimentary mucositis. Support Care Cancer 14:558–565
Lalla RV, Sonis ST, Peterson DE (2008) Management of oral mucositis in patients who have cancer. Dent Clin North Am 52:61–77
Mahatma M, Agrawal N, Dajani EZ, Nelson S, Nakamura C, Sitton J (1991) Misoprostol but not antacid prevents endotoxin-induced gastric mucosal injury: role of tumor necrosis factor-alpha. Dig Dis Sci 36:1562–1568
Malkinson FD, Geng L, Hanson WR (1993) Prostaglandins protect against murine hair injury produced by ionizing radiation or doxorubicin. J Invest Dermatol 101:135S–137S
McGrath PA (1990) Pain in children-nature, assessment, and treatment. Guiilford Publications, New York
Raber-Durlacher J, Barasch A, Peterson DE, Lalla RV, Schubert MM, Fibbe WE (2004) Oral complications and management considerations in patients treated with high-dose cancer chemotherapy. Supportive Cancer Therapy 1:219–229
Rapoport AP, Miller Watelet LF, Linder T, Eberly S, Raubertas RF, Lipp J, Duerst R, Abboud CN, Constine L, Andrews J, Etter MA, Spear L, Powley E, Packman CH, Rowe JM, Schwertschlag U, Bedrosian C, Liesveld JL (1999) Analysis of factors that correlate with mucositis in recipients of autologous and allogeneic stem-cell transplants. J Clin Oncol 17:2446–2453
Reimann HJ, Lewin J, Schmidt U, Wendt P, Blueml G, Dajani EZ (1987) Misoprostol prevents damage to the gastric mucosa by stabilizing the mast cells. Prostaglandins 33(Suppl):105–116
Rossetti RG, Seiler CM, Brathwaite K, Zurier RB (1995) Effect of misoprostol on acute and chronic inflammation. Am J Ther 2:600–606
Ruescher TJ, Sodeifi A, Scrivani SJ, Kaban LB, Sonis ST (1998) The impact of mucositis on alpha-hemolytic streptococcal infection in patients undergoing autologous bone marrow transplantation for hematologic malignancies. Cancer 82:2275–2281
Sartori S, Trevisani L, Nielsen I, Tassinari D, Abbasciano V (1996) Misoprostol and omeprazole in the prevention of chemotherapy-induced acute gastroduodenal mucosal injury. A randomized, placebo-controlled pilot study. Cancer 78:1477–1482
Schubert MM, Williams BE, Lloid ME, Donaldson G, Chapko MK (1992) Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplantation. Development of an oral mucositis index. Cancer 69:2469–2477
Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, Hayden V, Eilers J, Epstein JB, LeVeque FG, Miller C, Peterson DE, Schubert MM, Spijkervet FK, Horowitz M (2001) Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol 19:2201–2205
Vagliano L, Feraut C, Gobetto G, Trunfio A, Errico A, Campani V, Costazza G, Mega A, Matozzo V, Berni M, Alberani F, Banfi MM, Martinelli L, Munaron S, Orlando L, Lubiato L, Leanza S, Guerrato R, Rossetti A, Messina M, Barzetti L, Satta G, Dimonte V (2011) Incidence and severity of oral mucositis in patients undergoing haematopoietic SCT-results of a multicentre study. Bone Marrow Transplant 46:727–732
Vandervoort JM, Nieves MA, Fales-Williams A, Evans R, Mason DR (2006) An investigation of misoprostol in the promotion of wound healing. Vet Comp Orthop Traumatol 19:191–195
Veness MJ, Foroudi F, Gebski V, Timms I, Sathiyaseelan Y, Cakir B, Tiver KW (2006) Use of topical misoprostol to reduce radiation-induced mucositis: results of a randomized, double-blind, placebo-controlled trial. Australas Radiol 50:468–474
Widomski D, Fretland DJ, Gasiecki AF, Collins PW (1997) The prostaglandin analogs, misoprostol and SC-46275, potently inhibit cytokine release from activated human monocytes. Immunopharmacol Immunotoxicol 19:165–174
Xanthinaki A, Nicolatou-Galitis O, Athanassiadou P, Gonidi M, Kouloulias V, Sotiropoulou-Lontou A, Pissakas G, Kyprianou K, Kouvaris J, Patsouris E (2008) Apoptotic and inflammation markers in oral mucositis in head and neck cancer patients receiving radiotherapy: preliminary report. Support Care Cancer 16:1025–1033
Prescribing information for cytotec. Available at www.accessdata.fda.gov/drugsatfda_docs/label/2002/19268slr037.pdf Accessed on 20 July 2011
Acknowledgments
This study was supported by G.D. Searle and Company, now a component of Pfizer, Inc. The authors acknowledge the contributions of the subjects who participated in this study.
Conflict of interest
Dr. Gary B. Gordon was an employee of G.D. Searle and Company during the study period. None of the other authors have any conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lalla, R.V., Gordon, G.B., Schubert, M. et al. A randomized, double-blind, placebo-controlled trial of misoprostol for oral mucositis secondary to high-dose chemotherapy. Support Care Cancer 20, 1797–1804 (2012). https://doi.org/10.1007/s00520-011-1277-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-011-1277-7