Abstract
Leaf gas-exchange, leaf and shoot anatomy, wood density and hydraulic conductivity were investigated in seedlings of Quercus suber L. grown for 15 months either at elevated (700 μmol mol−1) or normal (350 μmol mol−1) ambient atmospheric CO2 concentrations. Plants were grown in greenhouses in a controlled environment: relative humidity 50% (±5), temperature similar to external temperature and natural light conditions. Plants were supplied with nutrients and two water regimes (WW, well watered; WS, water stress). After 6 months exposure to CO2 enrichment an increase in photosynthetic rate, a decrease in stomatal conductance and a decrease in carbon isotope discrimination (Δ13C) were observed, along with enhanced growth and an increase in the number of branches and branch diameter. Over the same period, the shoot weight ratio increased, the root weight ratio decreased and the leaf weight ratio was unaffected. The specific leaf area increased due to an increase in total leaf thickness, mainly due to the palisade parenchyma and starch. However, after 9 and 15 months of elevated CO2 exposure, the above-mentioned physiological and morphological parameters appeared to be unaffected. Elevated CO2 did not promote changes in vessel lumen diameter, vessel frequency or wood density in stems grown in greenhouse conditions. As a consequence, xylem hydraulic efficiency remained unchanged. Likewise, xylem vulnerability to embolism was not modified by elevated CO2. In summary, elevated CO2 had no positive effect on the ecophysiological parameters or growth of water stressed plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cork oak (Quercus suber L., hereafter Q. suber) is a western Mediterranean evergreen oak tree. In the Iberian Peninsula it occurs mostly in savannah-type formations (“montados” in Portuguese, also known as “dehesas” in Spain) with sparse tree cover, intermingled with other evergreen oaks, mainly holm-oak (Quercus ilex spp. rotundifolia) (Vaz et al. 2010). Q. suber L. is a Mediterranean forest species with ecological, social and economic value in the Iberian Peninsula. The future climate scenario for that region, according to the Hadley Centre Regional Climate Model (Miranda et al. 2006), suggests that summer drought periods will be more severe and the CO2 concentration in the atmosphere will be twice that of the present at 2050.
An overall understanding of functional traits of this species and its response to climate change is crucial for future sustainable ecosystems management. In this context, natural regeneration of Q. suber seedlings in montados ecosystems is necessary to maintain forest equilibrium. Q. suber seedling establishment could be improved by modifying environmental conditions, such as the CO2 concentration in atmosphere, that affect physiological and morphological characteristics (Griffin 1971; McPherson 1993). Physiological responses to short-term exposure (usually less than 1 year) to elevated CO2 include an increase in photosynthetic carbon assimilation and a reduction in stomatal conductance, which improves the efficiency of water use (Curtis and Wang 1998). Thus, growth rate increases, not only due to the increase in photosynthetic carbon assimilation but also to increased carbon allocation to leaves versus non-photosynthetic tissues, leading to a higher leaf area (Callaway et al. 1994). However, these short-term responses could differ from responses to longer periods of elevated CO2 exposure since an acclimation process could occur. During long-term experiments, photosynthetic activity is often down-regulated in European forest species (Medlyn et al. 1999). Data on long-term exposure to elevated CO2 (e.g., photosynthesis and biomass partitioning) have mainly been obtained from deciduous Quercus species seedlings (e.g. Q. alba, Gunderson and Wullschleger 1994, Q. robur; Atkinson et al. 1997). Information from Mediterranean evergreen species is scarce; studies on Q. ilex were conducted by Tognetti et al. (1996) in a natural CO2 spring and by Faria et al. (1996) and Maroco et al. (2002) with Q. suber in greenhouse conditions. Tree regeneration in the montados is very low despite significant acorn production (168 g m2, Gomez-Gutierrez et al. 1981). Thus, the seedling stage is an important and usually critical phase in the regeneration of woody species under natural conditions since the risk of environmental stress is very high at this stage. In the Mediterranean environment, which is characterized by seasonal and episodic droughts, water availability is a limiting factor that is of prime importance in the regeneration of oaks and other woody species (Aranda et al. 2008).
In natural conditions, seedlings of Q. suber are generally exposed to soil drought, which will become more severe under future climate scenarios. In this context seedling establishment in these constrained environmental conditions will benefit not only from an improvement in stomatal control but also from an increment in the capacity to absorb and transport water. Hydraulic conductivity is an important factor regulating water flux through the soil–plant–atmosphere continuum (Tyree and Ewers 1991) and measurements of both stomatal conductance and hydraulic conductivity may provide a greater understanding of how water flux and uptake by trees will respond to elevated atmospheric CO2 concentrations (De Lucia et al. 2000). CO2 concentration in the atmosphere is known to affect anatomical structures during the process of wood formation, including wall vessel thickness and lumen diameter (Eckstein 2004; Fonti et al. 2009; Campelo et al. 2010). These play an important role in hydraulic conductivity and tolerance to drought (Leal et al. 2007). Only a few studies have examined changes in wood properties (Telewski et al. 1999; Yazaki et al. 2005) or hydraulic conductivity (Saxe et al. 1998) in evergreen Mediterranean species in response to elevated atmospheric CO2 (Corcuera et al. 2004; Campelo et al. 2007). Furthermore, the consequences of increasing atmospheric CO2 concentration on plant structure, an important determinant of physiological and competitive success, have not received sufficient attention in the literature. In order to provide more fundamental knowledge of Q. suber seedling establishment and the success of natural regeneration in elevated CO2, we studied the physiological, morphological and anatomical responses to such elevation in terms of photosynthetic performance, biomass allocation, water relations, wood structure and hydraulic conductivity. We tested the hypothesis that elevated CO2 will influence the development (ecophysiological and structural) of Q. suber seedlings and alleviate the effects of water stress.
Methods
Plant material and experimental conditions
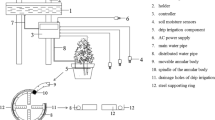
Acorns of Q. suber from a population at Tapada da Ajuda, Lisboa, Portugal (38°422 N, 9°112 W), were sown in 10-L pots containing a mixture of nursery soil, blond Sphagnum peat and sand (1:1:1, v/v). At 6 months of age the seedlings were transferred (at the end of April, a period of vegetative growth) from open-air conditions to greenhouses with homogeneous descending forced-air convection (0.3 m s−1) and two different CO2 concentrations (elevated CO2, 700 ppm and ambient CO2, 350 ppm), with 60 seedlings per CO2 atmospheric level. The temperature regime inside the greenhouses mimicked the local average temperatures recorded over the last 30 years in Lisbon (average maxima during the day and average minima during the night). The highest temperatures occurred in August (maximum temperatures of 28°C, minimum 16°C and average 22°C) and the lowest temperatures occurred in January (15, 7 and 11°C, respectively). The natural light within the greenhouses showed a ca. 25% mean reduction in relation to open-air conditions (observed on sunny days with maximum values of about 1,500 and 600 mol m−2 s−1 PAR in summer and winter months, respectively), and the relative humidity was 50% (±5). To avoid microenvironmental effects, the plants were rotated within and between greenhouses every week. Plants were watered twice a week until field capacity and fertilized once a week with a complete solution [N–NO3−, 120 g l−1; P, 13.6 g l−1; K, 60 g l−1 plus trace elements (Ca, Mg, Bo, Fe, Mn, Zn, Cu, Mo)].
After 12 months in the greenhouses, a subset of these plants was subjected to a water stress regime. Pots were covered with a plastic bag to prevent soil evaporation and water was supplied at 80, 70 or 60% of plant transpiration.
Ecophysiological and structural measurements were taken from 10 plants per CO2 level and soil water regime in autumn (October–November, after 6 months in the greenhouses), spring (March–April, after 9 months in the greenhouses) and summer (July–August, after 15 months in the greenhouses).
Structural parameters (biomass, leaf area, specific leaf area and branching)
Plant biomass (total, leaves, shoots and roots) and total leaf area were measured (10 plants per harvest date, CO2 treatment and water regime) at the start of the experiment (0 months) and after 6, 9 and 15 months in the greenhouses. Plant material was divided into leaves, shoots and roots and oven-dried at 80°C until constant weight was reached. Leaf area was determined by measuring the area of all the leaves using an area meter (LI-COR). Biomass and leaf area data were used to calculate the average relative growth rate (RGR, g g−1 week−1), leaf area ratio (LAR, cm −2 g−1), leaf weight ratio (LWR), shoot weight ratio (SWR), and root weight ratio (RWR) according to Evans (1972). The relative growth rate (RGR) was estimated using the equation RGR = NAR × LAR (where RGR is the relative growth rate, NAR is the net assimilation rate and LAR is the leaf area rate) (Callaway et al. 1994).
Specific leaf area (SLA, cm−2 g−1) was determined using five leaf discs (8.95 cm diameter) collected from recently expanded leaves (excluding the main vein) from 10 plants per treatment. These leaf discs were oven-dried at 80°C for 24 h and the ratio of leaf area per dry weight was determined.
The branching pattern was determined as follows: (a) first order branches: the average number of branches per 10 cm of the main stem; (b) second order branches: the average number of branches per 10 cm of the first order branches.
Hydraulic conductivity
Hydraulic conductivity was measured in saplings after 15 months exposure to 700 ppm CO2 or 350 ppm CO2 in the greenhouses. We focused our analysis on traits associated with both xylem hydraulic efficiency and safety. Hydraulic efficiency was estimated according to the maximum hydraulic conductivity (K max, kg ms−1 MPa−1) of xylem segments taken from different parts of the trees. Three representative saplings from each treatment were selected for this analysis. The trees were brought to the laboratory early in the morning and maintained under low light conditions until measurement. In the laboratory, 25 xylem segments were excised under tap water from the trunk and different branches of each tree. The segment length ranged from 10 to 100 mm, with longer segments being taken from the trunk. Xylem hydraulic conductivity (K) was measured according to the technique proposed by Sperry et al. (1988). In brief, segment ends were inserted into plastic tubing filled with distilled water and connected to two water reservoirs, one placed 40 cm above the sample and the other on the scale of an analytical balance (0.1 mg resolution). K was computed as the ratio between the water flow through the segment and the pressure gradient. We determined the initial conductivity (K init) and the saturated conductivity (K max) after successive 0.1 MPa flushes, which enabled calculation of the percentage loss of conductivity (PLC) as
Xylem hydraulic safety was assessed by constructing curves of xylem vulnerability to embolism according to Sperry et al. (1988). To construct the curves, 10 plants were selected for each treatment. The plants were removed from their pots, and the root system was gently washed with tap water to remove soil particles and then allowed to dehydrate on a bench. Leaf water potential was measured regularly using a pressure chamber (see below) and was assumed to equal xylem pressure because plant dehydration was very slow. When the target pressure was obtained, a branch was excised under tap water and xylem PLC was determined as above, using a minimum of six segments per branch. The variation in xylem PLC with xylem pressure enabled the construction of the vulnerability curves. The curves were fitted with the equation given by Pammenter and Vander Willigen (1998).
Anatomical parameters (leaves and shoots)
To investigate leaf anatomy, one recently expanded leaf was taken from each of 10 plants per treatment after 6 and 15 months exposure to 700 ppm CO2 or 350 ppm CO2. Two segments were cut from each leaf (approximately 1 × 0.5 cm): one parallel to the main vein and the other transversely. These segments were fixed in FAA (formaldehyde 37%, ethanol 70%, water, acetic acid 2:7:10:1) and dehydrated through a series of alcohol solutions of ascending concentrations. Plant material was embedded in a methacrylate-based plastic HistoResin (Jung-RFA), and sections were cut using a sliding microtome. Eight transverse leaf sections per treatment, with a thickness of 8 μm, were stained with a solution of toluidine blue O (0.005%) to obtain greater differentiation of cellular components. For cross-sectional measurements of palisade and spongy parenchyma and total leaf thickness two random locations were selected per leaf section. Total leaf thickness and spongy and palisade parenchyma thickness were measured twice per section. Leaf topographical analyses were conducted using a Leitz Dialux 20Eb microscope and a B.D. Elektronik camera linked to a Leitz ASM 68K measuring table.
To investigate shoot anatomy, radial sections were cut from the main stem (stems grown in greenhouse conditions) from three plants per treatment after 15 months exposure to 700 ppm CO2 or 350 ppm CO2 in well-watered conditions. Samples were fixed in FAA and embedded in polyethylene glycol DP 1500. Shoot sections were cut using a sliding microtome to a thickness of 8 μm and stained with the “triple stain”: Astra Blau Chrisoidin-Pyronin Y. For measurements of vessel lumen diameter, 30 random vessel cross-sections were selected. Shoot topographical analyses were conducted using a Leitz Dialux 20Eb microscope and a B.D. Elektronik camera linked to a Leitz ASM 68K measuring table. Vessel frequency was determined by counting the number of vessels in a mean area of 30 mm2 of a stem cross-section in three plants per treatment.
Water potential
Predawn leaf water potential (\( \Uppsi_{\text{lPD}} \)) and midday leaf water potential (\( \Uppsi_{\text{lMD}} \)) were measured after a period of 6, 9 and 15 months using a Scholander pressure chamber (PMS 1000, PMS Instruments, Corvallis, Ore.) (Scholander et al. 1965). Leaves (3 or 4) were sampled from the south-facing side of the crown just prior to sunrise and at midday (12–13 h, local time). Samples were taken at similar heights above ground to avoid variability due to hydrostatic water potential and were inserted into a plastic bag immediately after cutting to prevent further transpiration.
Gas exchange measurements
The assimilation rate (A) and stomatal conductance (g s) of fully expanded leaves from the current-year spring flush were measured after a period of 6, 9 and 15 months of growth at 350 and 700 ppm CO2 at 50% relative humidity with a photosynthetic photon flux density (PPFD) of 1,200 μmol m−2 s−1 using a Compact Minicuvette System (Heinz Walz, Germany) and a Bypass Humidity Control Unit.
Plant transpiration
Plant transpiration was measured by restoring soil water content to field capacity (in well-watered plants, WW) or to 80, 70 or 60% of field capacity (in water stressed plants, WS) twice a week. Soil water evaporation was limited by covering the pots with a plastic bag. The daily transpiration was estimated as the difference in pot weight between two subsequent days.
Starch concentration
Starch concentration was determined after 6 and 15 months exposure to 700 ppm CO2 or 350 ppm CO2, in leaf discs that were harvested at solar midday and immediately frozen in liquid nitrogen and stored at −80°C until analysis, as described by Stitt et al. (1989).
Carbon isotope composition in leaf
Leaf carbon isotope composition (δ13C) was measured via isotopic mass spectrometry (Finnigan Delta S mass spectrometer, Finnigan-Mat) and expressed as the 13C/12C ratio in the sample (R s) relative to that of the Pee Dee Belemnite standard (R PDB) (Eq. 1).
The resulting δ13C values were used to calculate isotopic discrimination (Δ, ‰) (Eq. 2).
δa and δp refer to the isotopic composition of atmospheric CO2 and the plant material, respectively. In our experimental conditions, δa differed between the two greenhouses as the predominant industrial (CO2 cylinder) origin of CO2 in the elevated CO2 greenhouse. The δa in the two greenhouses was assessed by measuring δp in Z. mays, a C4 plant that was grown at both CO2 levels during the experimental period. According to Marino and McElroy (1991), δp in Z. mays is linked to δa by the following equation (Eq. 3):
These measurements yielded δa values of −9.25 and −14.80‰ at 350 and 700 ppm CO2, respectively. The precision for isotopic measurements was ±0.2‰. Q. suber leaves and Z. mays leaves were sampled after 6 and 15 months exposure to elevated CO2.
Statistical analysis
All statistical analyses were conducted using the SPSS software package (Statistical Package for the Social Sciences for Windows 11 2003). One-way ANOVA was used to test the differences between means. Significant differences between means were evaluated using Tukey’s HSD tests at p = 0. 05. Descriptive statistics and the ANOVA (post hoc Student–Neuman–Keuls test at p = 0.05) were performed in order to assess the significance of differences observed between parameters. Before applying the ANOVA test, data were tested for normality using the Levene and Bartlett tests at a significance level of 0.05. When variances across groups were unequal, i.e. the usual ANOVA assumptions were not satisfied, the Welch-ANOVA test was applied (significance level p = 0.05).
Results
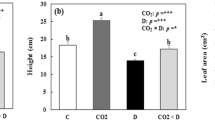
CO2 enrichment had significantly enhanced (p < 0.05) all biomass components (leaves, shoots, roots and total biomass) in well-watered plants (WW) 6 months after the start of the experiment (Table 1). During that period, total biomass increased by about threefold in elevated CO2 (Table 1) and leaf area increased by about 50% in plants exposed to elevated CO2 in comparison to ambient CO2 plants (Fig. 1).
During the same period, a significant decrease (p < 0.05) in root weight ratio (RWR) and an increase (p < 0.05) in shoot weight ratio (SWR) were observed in WW plants subjected to elevated CO2 in comparison to ambient CO2 plants, but no significant differences were observed in leaf weight ratio (LWR) (Table 2). However, the positive effects of elevated CO2 on biomass, leaf area and growth parameters were not apparent after longer periods of exposure to elevated CO2 (9 and 15 months) (Table 1 and Fig. 1). Soil water stress promoted a decrease in plant biomass (all components) and a slight increase in RWR. However, elevated CO2 did not alleviate the effect of drought on growth (Fig. 1; Table 1). The net assimilation rate (NAR), leaf area rate (LAR) and relative growth rate (RGR) decreased (p < 0.05) in the period from 6 to 9 and 15 months in WW plants subjected to elevated CO2 in comparison to ambient CO2 (p < 0.05, Table 2). The number of first order branches and branch diameter increased significantly (p < 0.05) in elevated CO2 in WW plants after 6 months of exposure, in comparison to ambient CO2 plants (Fig. 2; Table 3). However, after longer periods of exposure to elevated CO2, the positive effect of elevated CO2 on the number of first order branches disappeared. There were no significant differences in the number of second order branches between treatments at any point. The relation between shoot height and diameter was not modified by elevated CO2 in WW and WS plants (Fig. 3).
Stem wood anatomy showed no significant difference (p < 0.05) in terms of vessel lumen diameter, vessel frequency or wood density between the plants in the two CO2 regimes after 15 months exposure (Table 3).
After 6 months of exposure to elevated CO2 an increase (p < 0.05) in total leaf thickness as well as in palisade and spongy parenchyma was observed in comparison with ambient CO2 plants (Table 4). An additional cell layer was observed in the palisade parenchyma in elevated CO2 plants compared to the ambient CO2 plants, plus a decrease in specific leaf area (SLA) (p < 0.05) (Table 4). However, after 15 months of exposure to elevated CO2, total leaf thickness, palisade parenchyma thickness and specific leaf area (SLA) did not differ between CO2 treatments.
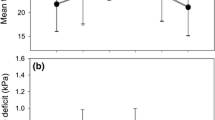
Predawn leaf water potential was similar in elevated CO2 WW plants and ambient CO2 WW plants (around −0.5 MPa) for the first 6 months (Fig. 4). Water stress led to a decrease in predawn leaf water potential (around −1.0 MPa) but elevated CO2 had no effect.
Predawn leaf water potential (ΨlPD, MPa); midday leaf water potential (ΨlMD, MPa); Photosynthetic carbon assimilation (A, μmol m−2 s−1); stomatal conductance (g s, mol m−2 s−1), of plants grown at 350 and 700 ppm of CO2 after a period of 6, 9 and 15 months in the greenhouses. Values are means of 10 plants ± SE, data sharing the same letter are not significantly different (p < 0.05). WW well watered, WS water stress
The net carbon assimilation rate (A), measured at the CO2 growth concentration, was higher in elevated CO2 WW plants (15 ± 0.8 μmol m−2 s−1) than in ambient CO2 WW plants (10 ± 0.6 μmol m−2 s−1) in the first 6 months. During the same period, stomatal conductance (g s) was lower in plants exposed to high CO2 (53 ± 1.1 mol H2O m−2 s−1) in relation to ambient CO2 plants (45 ± 1.9 mol H2O m−2 s−1) (Fig. 4). A and g s were lower in WS plants but no significant difference was found between ambient and elevated CO2 plants. Water use efficiency (A/g s) was higher in elevated CO2 plants (0.33 ± 0.08) than in ambient CO2 plants (0.18 ± 0.08). In addition, transpiration per plant (g of water transpired per day) was lower in elevated CO2 plants (Fig. 5) than in ambient CO2 plants for the first 6 months. Carbon isotope discrimination in leaves (Δ13C) was higher in WW plants grown at elevated CO2 (19.8‰) than ambient CO2 (18.7‰) and lower in WS plants (17.4 and 17.8‰ at ambient and elevated CO2, respectively) (Fig. 6). Soluble sugars and starch concentration in the leaf were higher in elevated CO2 plants than in ambient CO2 plants (Fig. 7). However, no significant difference (p > 0.05) was observed in terms of gas exchange and biochemical parameters after a longer period of exposure (15 months). Xylem hydraulic conductivity was strongly influenced by segment diameter (Fig. 8) but no differences were found between treatments. In other words, CO2 concentration had no effect on xylem hydraulic efficiency. The xylem vulnerability curves for trees grown at low and high CO2 concentrations are shown in Fig. 9. The curves had a typical sigmoid shape and did not differ between treatments. Embolisms started to form when xylem pressure dropped below −3.5 MPa and 50 PLC was measured at −5.02 and −4.97 MPa, respectively, at 350 and 700 ppm CO2.
Discussion
Quercus suber seedlings responded positively to the elevated CO2 treatment during the first 6-month period. The positive effect of CO2 on total biomass observed in this study (ca. 50%) was lower than that obtained by Damesin et al. (1996) (ca. 92%) for Q. suber seedlings after 4 months of exposure to elevated CO2, probably due to the different growth period during which the plants were transferred from open-air conditions to the greenhouses. In the present study Q. suber seedlings were transferred at the end of April (during the intense vegetative growth phase), and it has previously been demonstrated in several species that growth was stimulated by elevated CO2 at the beginning of the growing season (Retuerto and Woodward 1993; Vivin et al. 1996). Nevertheless, our value was in the range reported for trees: 20–120% (Eamus and Jarvis 1989), and similar to the average value of 40% reported by Poorter et al. (1996) in a review of 95 species (the average number of days of exposure to elevated CO2 was 112 days). The values obtained for deciduous Quercus seedlings of Q. petrea (Guehl et al. 1994) and Q. rubra (Vivin et al. 1996) were ca. 67 and 44%, respectively, after 3 and 11 months exposure to elevated CO2. The increase in plant biomass produced in elevated CO2 that was observed in our experiment was consistent with the increase in photosynthetic rate and the enhancement of sink capacity to use assimilates produced during the same period. Shoots were an important sink in the first 6 months, as shown by the increase in shoot weight ratio (SWR) due to an increase in the number of branches and their diameter. These results are consistent with other experiments in trees such as Liquidambar styraciflua and Malus hupehenis (Sionit et al. 1985) and Populus thrichocarpa (Radoglou and Jarvis 1990). Likewise, a high atmospheric CO2 concentration promoted the branching of Castanea sativa (El Kohen and Mousseau 1994), in which an increase in stem diameter was observed after 4 months of exposure to elevated CO2. The allocation of dry matter to the roots, in relation to total biomass (RWR), decreased in plants exposed to high CO2 in comparison to control plants after 6 months of exposure. However, contradictory results have been reported, with an increment in RWR in elevated CO2 conditions in Q. alba (Norby et al. 1995), and no effect of CO2 on Q. robur (Picon et al. 1996). Nevertheless, this effect occurred when water and/or nutrients (especially nitrogen) were limiting factors (Stulen and den Hertog 1993). Our results also indicate that the allocation of dry matter to leaves (leaf weight ratio, LWR) in Q. suber seedlings was not affected by CO2 and thus, the lower values of leaf area ratio (LAR) in the elevated CO2 plants were attributed to the lower values of specific leaf area (SLA). Such reduction in SLA in elevated CO2 is due to changes in leaf anatomy such as an increase in cell number in the palisade parenchyma (Lawlor and Mitchell 1991; Eamus and Jarvis 1989), an increase in cell size (Thomas and Harvey 1983; Conroy et al. 1990); changes in intercellular spaces (Radoglou and Jarvis 1990, Pettersson and MacDonald 1992) and/or accumulation of non-structural carbohydrates (Farrar and Williams 1991). In summary, elevated CO2 seems to alter plant structure by changing the allocation of biomass between plant organs. This suggests that elevated CO2 influences cell division, cell expansion, and cell patterning in both the primary and secondary meristems of shoots and roots, driven mainly by increased substrate (sucrose) availability and perhaps also by the differential expression of genes involved in cell cycling or cell expansion (Pritchard et al. 2001).
Water balance in Q. suber seedlings was improved by elevated CO2 in the short term (6 months), due to an increase in water use efficiency, not only instantaneous efficiency (A/g s) but also long-term efficiency (lower δ13C). This was related to lower stomatal conductance (g s) and a better leaf water status, as indicated by the higher leaf water potential at midday.
In the present study, the effects of CO2 in association with drought had no effect on carbon assimilation. Similar results were observed in other Quercus species (e.g., Q. robur, Picon et al. 1996). It should be noted that this was a short-term study on the effects of CO2 enhancement and drought, and that there is evidence that slow growing species (Picon et al. 1996) with a more robust leaf structure (Niinemets et al. 2011), such as Mediterranean evergreens, may cancel in the practice the responses to high CO2 (Niinemets et al. 2011).
The above-mentioned morphological and physiological responses did not persist for longer periods (9 and 15 months exposure). However, the relative growth rate (RGR) of plants exposed to high CO2 decreased by 62% in relation to control plants. This decrease in average RGR is compatible with the decrease of 52% in NAR and the decrease of 21% in LAR observed during the same period. It is unlikely that reduced growth during this period resulted from soil volume limitations given the large size of the pots and the observation that root systems at the time of harvest still had room for expansion. This growth acclimation to elevated CO2 might be related to a decrease in biomass partitioning between photosynthetic tissues and non-photosynthetic tissues (leading to a decrease in LAR), or the down-regulation of photosynthesis that we observed. Several explanations have been proposed for photosynthetic acclimation to elevated CO2, including a reduction in the activity of sinks for carbohydrate, and as a consequence a source–sink imbalance (Thomas and Strain 1991; Long and Drake 1992). Under elevated CO2, when photosynthesis greatly exceeds the capacity for photosynthate utilization, it has been shown that sugars accumulate and modulate the transcription of chloroplast proteins (van Oosten and Besford 1994; Willkins et al. 1994). In the present study, the increase in the pool of total soluble sugars and starch in leaves of plants grown at elevated CO2 suggests the down-regulation of photosynthesis in plants grown at elevated CO2. However, the occurrence of acclimation under elevated CO2 is not consensual since it depends on the species and growth conditions (Herrick and Thomas 2001; Ainsworth et al. 2003), including soil nitrogen (Maroco et al. 2002; Long et al. 2004). Nevertheless, in the present study, the Q. suber seedlings were grown with no limiting nutrient concentrations, and no N limitations seemed to occur (2.25 and 3.00% of N in leaves at 350 and 700 ppm of CO2 after 15 months, respectively), with similar values to those reported by Maroco et al. (2002) in Q. suber seedlings grown at a high N soil concentration (2.75 and 2.00% of N in leaves at 350 and 700 ppm of CO2, respectively).
Wood density in Q. suber, a diffuse-porous species in which wood density depends mostly on the frequency rather than diameter of vessels (Taylor and Wooten 1973), was not affected by elevated CO2. This is consistent with the results observed for vessel diameter, wall thickness and vessel frequency. However, contradictory results have been published on the effect of elevated CO2 on wood anatomy. For example, in Q. ilex seedlings (Gartner et al. 2003; Watanabe et al. 2008) elevated CO2 in the atmosphere promoted an increase in vessel diameter while no obvious effect was found in other diffuse-porous species such as Populus tremuloides (Kaakinen et al. 2004) and Betula pendula (Kostiainen et al. 2006). Consistent with the wood anatomical traits in Q. suber seedlings, no differences were found in xylem hydraulic efficiency between CO2 treatments. Likewise, in Q. ilex trees an increase in CO2 concentration (in a natural spring) did not affect hydraulic efficiency (Tognetti et al. 1996). The critical water potential value (CWP, at which loss of about 50% of hydraulic conductivity occurs) was similar in both CO2 treatments (−5.02 and −4.97 MPa at 350 and 700 ppm, respectively), as observed by Lo Gullo et al. (2003). However, our value was lower than that obtained by these authors in the same species (−2 Mpa).
In the present study, elevated CO2 had no clear effect on promoting a change in water relations in Q. suber seedlings. Likewise, the absence of any effect of CO2 on biomass growth observed here in water stressed plants refutes the notion that elevated CO2 will alleviate the inhibitory effects of drought on growth. These results were unexpected, especially as plants growing in elevated CO2 usually have decreased stomatal conductance (e.g. Uddling et al. 2008). However, they were in accordance with previous results in Q. ilex (Tognetti et al. 1996), and may be attributed to the morphological structure of leaves in evergreen sclerophylls in which the mesophyll conductance of CO2 has great importance as a limiting factor in photosynthesis, particularly under water stress conditions (Vaz et al. 2010).
In summary, our results confirm the hypothesis that Q. suber seedling development is positively influenced by an increase in CO2 air concentration in the short term (6 months), as indicated by an increment in photosynthesis, leaf area ratio and specific leaf area, which in turn induced the higher production of assimilates for growth. Concomitantly, a reduction in stomatal conductance and transpiration promoted higher water use efficiency in this period. However, the growth restrictions imposed by soil water stress were not improved in elevated CO2 conditions. Thus, an increase in the intensity and frequency of summer drought with climate change might have a negative impact on natural Q. suber regeneration, due to the annulment of the positive effects of elevated CO2 in the atmosphere.
References
Ainsworth EA, Davey PA, Hymus GJ, Osborne CE, Rogers A, Blum H, Nosberger J, Long SP (2003) Is stimulation of leaf photosynthesis by elevated carbon dioxide concentration maintained in the long term? A test with Lolium perenne grown for 10 years at two nitrogen fertilization levels under Free Air CO2 Enrichment (FACE). Plant Cell Environ 26:705–714
Aranda I, Puértolas J, Pardos M, Pardos JA (2008) Elevated atmospheric CO2 does not modify osmotic adjustment to light and drought in the Mediterranean oak Quercus suber L. Investigación Agraria: Sistemas y Recursos Forestales 17(1):3–9
Atkinson CJ, Taylor JM, Wilkins D, Besford RT (1997) Effects of elevated CO2 on chloroplast components, gas exchange and growth of oak and cherry. Tree Physiol 17:319–325
Callaway RM, DeLucia EH, Thomas M, Schlesinger WH (1994) Compensatory responses of CO2 exchange and biomass allocation and their effects on the relative growth rate of ponderosa pine in different CO2 and temperature regimes. Oecologia 98:159–166
Campelo F, Nabais C, Freitas H, Gutiérrez E (2007) Climatic significance of tree-ring width and intra-annual density fluctuations in Pinus pinea from a dry Mediterranean area in Portugal. Ann For Sci 64:229–238
Campelo F, Nabais C, Gutiérrez E, Freitas H, Garcıía-González I (2010) Vessel features of Quercus ilex L. growing under Mediterranean climate have a better climatic signal than tree-ring width. Trees 24:463–470
Conroy JP, Milham PJ, Mazur M, Barlow EWR (1990) Growth, dry weight partitioning and wood properties of Pinus radiata D. Don after 2 years of CO2 enrichment. Plant Cell Environ 13:329–337
Corcuera L, Camarero JJ, Gil-Pelegrín E (2004) Effects of a severe drought on growth and wood anatomical properties of Quercus faginea. IAWA J 25(2):185–204
Curtis PS, Wang X (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113:299–313
Damesin C, Galera C, Rambal S, Joffre R (1996) Effects of elevated carbon dioxide on leaf gas exchange and growth of cork-oak (Quercus suber L.) seedlings. Ann For Sci 53:61–467
De Lucia EH, Maherali H, Carey EV (2000) Climate-driven changes in biomass allocation in pines. Glob Change Biol 6(5):287–293
Eamus D, Jarvis PG (1989) The direct effects on increase in the global atmospheric CO2 concentration on natural and commercial temperate trees and forest. Adv Ecol Res 19:1–55
Eckstein D (2004) Change in past environments—secrets of the tree hydrosystem. New Phytol 163:1–4
El Kohen A, Mousseau M (1994) Interactive effects of elevated CO2 and mineral nutrition on growth and CO2 exchange of sweet chestnut seedlings (Castanea sativa). Tree Physiol 14((7-8-9)):679–690
Evans GC (1972) The quantitative analysis of plant growth. Blackwell, London
Faria T, Wilkins D, Besford RT, Vaz M, Pereira JS, Chaves MM (1996) Growth at elevated CO2 leads to down-regulation of photosynthesis and altered response to high temperature in Quercus suber L. seedlings. J Exp Bot 47:1755–1761
Farrar JF, Williams L (1991) The effects of increased atmospheric carbon dioxide and temperature on carbon partitioning, source–sink relations and respiration. Plant Cell Environ 14:819–830
Fonti P, von Arx G, García-González I, Eilmann B, Sass-Klaassen U, Gartner H, Eckstein D (2009) Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. New Phytol 185:42–53
Gartner BL, Roy J, Hue R (2003) Effects of tension wood on specific conductivity and vulnerability to embolism of Quercus ilex seedling grown at two atmospheric CO2 concentrations. Tree Physiol 23:387–395
Gomez-Gutierrez JM, Luis-Calabuig E, Escudero-Berian E (1981) Materiales aportados por la encina en la zona de dehesa salmantina. Studia Oecologica 2:181–221
Griffin JR (1971) Oak regeneration in the upper valley, California. Ecology 52:862–868
Guehl JM, Picon C, Aussenac G, Gross P (1994) The interactive effects of elevated CO2 and soil drought on growth and on transpiration efficiency and its determinants in two European forest tree species. Tree Physiol 14:707–724
Gunderson CA, Wullschleger SD (1994) Photosynthetic acclimation in trees to rising atmospheric CO2: a broader perspective. Photosynth Res 39:369–388
Herrick JD, Thomas RB (2001) No photosynthetic down-regulation in sweetgum trees (Liquidambar styraciflua L.) after three years of CO2 enrichment at the Duke Forest FACE experiment. Plant Cell Environ 24:53–64
Kaakinen S, Kostiainen K, Fredrik E, Saranpaa P, Kubiske ME, Sober J, Karnosky DF, Vapaavuori E (2004) Stem wood properties of Populus tremuloides, Betula papyrifera and Acer saccharum saplings after 3 years of treatments to elevated carbon dioxide and ozone. Glob Change Biol 10:1513–1525
Kostiainen K, Jalkanen H, Kaakinen S, Saranpaa P, Vapaavuori E (2006) Wood properties of two silver birch clones exposed to elevated CO2 and O3. Glob Change Biol 12:1230–1240
Lawlor DW, Mitchell RAC (1991) The effects of increasing CO2 on crop photosynthesis and productivity: a review of field studies. Plant Cell Environ 14:807–818
Leal S, Sousa BV, Pereira H (2007) Radial variation of vessel size and distribution in cork oak wood (Quercus suber L.). Wood Sci Technol 41:339–350
Lo Gullo MA, Salleo S, Rosso R, Trifil P (2003) Drought resistance of 2-year-old saplings of Mediterranean forest trees in the field: relations between water relations, hydraulics and productivity. Plant Soil 250:259–272
Long SP, Drake BG (1992) Photosynthetic CO2 assimilation and rising atmospheric CO2 concentrations. In: Baker NR, Thomas H (eds) Topics in photosynthesis. Crop photosynthesis: spatial and temporal determinants. Elsevier, Amsterdam, vol 2, pp 69–107
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants face the future. Annu Rev Plant Biol 55:591–628
Marino BD, McElroy MB (1991) Isotopic composition of atmospheric CO2 inferred from carbon in 4C plant cellulose. Nature 349:127–131
Maroco JP, Breia E, Faria T, Pereira JS, Chaves MM (2002) Effects of long-term exposure to elevated CO2 and N fertilization on the development of photosynthetic capacity and biomass accumulation in Quercus suber L. Plant Cell Environ 25:105–113
McPherson GR (1993) Effects of herbivory and herb interference on oak establishment in a semi-arid temperate savanna. J Veg Sci 4:687–692
Medlyn BE, Badeck FW, De Pury DG, Barton CVM, Broadmeadow M, Ceulemans R, De Angelis P, Forstreuter M, Jach ME, Kellomäki S, Laitat E et al (1999) Effects of elevated [CO2] on photosynthesis in European forest species: a meta-analysis of model parameters. Plant Cell Environ 22:1475–1495
Miranda PMA, Valente MA, Tomé AR, Trigo R, Coelho MFES, Azevedo EB (2006) O clima de Portugal nos séculos XX e XXI. In: Santos FD, Miranda P (eds) Alterações A Climáticas em Portugal. Cenários, Impactes e Medidas de Adaptação. Gradiva, Lisboa, pp 45–113
Niinemets U, Flexas J, Peñuelas J (2011) Evergreens favoured by higher responsiveness to increased CO2. Trends Ecol Evol 26:136–142
Norby RJ, Wullschleger SD, Gunderson CA, Nietch CT (1995) Increased growth efficiency of Quercus alba trees in a CO2-enriched atmosphere. New Phytol 131:91–97
Pammenter NW, Vander Willigen C (1998) A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol 18:589–593
Pettersson R, MacDonald AJS (1992) Effects of elevated carbon dioxide concentration on photosynthesis and growth of small birch plants (Betula pendula Roth.) at optimal nutrition. Plant Cell Environ 15:911–919
Picon C, Guehl JM, Aussenac G (1996) Growth dynamics, transpiration and water-use efficiency in Quercus robur plants submitted to elevated CO2 and drought. Ann Sci For 53:431–446
Poorter H, Roumet C, Campbell BD (1996) Interspecific variation in the growth response of plants to elevated CO2: a search for functional types. In: Körner C, Bazzaz FA (eds) Carbon dioxide, populations and communities. Academic Press, New York
Pritchard S, Rogers H, Prior SA, Peterson CM (2001) Elevated CO2 and plant structure: a review. Glob Change Biol 5(7):807–837
Radoglou M, Jarvis PG (1990) Effects of CO2 enrichment on four poplar clones. I. Growth and leaf anatomy. Ann Bot 65:617–626
Retuerto R, Woodward FI (1993) The influences of increased CO2 and water supply on growth, biomass allocation and water use efficiency of Sinapis alba L. grown under different wind speeds. Oecologia 94(3):415–427
Saxe H, Ellsworth DS, Heath J (1998) Tree and forest functioning in an enriched CO2 atmosphere. New Phytol 139:395–436
Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA (1965) Sap pressure in vascular plants. Science 148:339–346
Sionit N, Strain BR, Hellmers H, Reichers GH, Jaeger CH (1985) Long-term atmospheric CO2 concentration affects the growth and development of Liquidambar styraciflua and Pinus taeda seedlings. Can J For Res 15:468–471
Sperry JS, Donnelly JR, Tyree MT (1988) A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ 11:35–40
Stitt M, Lilley RM, Gerhardt R, Heldt HW (1989) Determination of metabolite in specific cells and subcellular compartments of plant leaves. Methods Enzymol 174:518–552
Stulen I, den Hertog J (1993) Root growth and functioning under atmospheric CO2 enrichment. Vegetatio 104(105):99–105
Taylor FW, Wooten TE (1973) Wood property variation of Mississippi Delta hardwoods. Wood Fiber Sci 5(1):2–13
Telewski FW, Swanson RT, Strain BR, Burns JM (1999) Wood properties and ring width responses to long-term atmospheric CO2 enrichment in field-grown loblolly pine (Pinus taeda L.). Plant Cell Environ 22:213–219
Thomas JF, Harvey CN (1983) Leaf anatomy of four species grown under continuous CO2 enrichment. Bot Gaz 144(3):303–309
Thomas RB, Strain BR (1991) Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon dioxide. Plant Physiol 96:627–634
Tognetti R, Giovannelli A, Longobucco A, Miglietta F, Raschi A (1996) Water relations of oak species growing in the natural CO2 spring of Rapolano (central Italy). Ann For Sci 56:371–377
Tyree M, Ewers FW (1991) Tansley Review No. 34. The hydraulic architecture of trees and other woody plants. New Phytol 119:345–360
Uddling J, Teclaw RM, Kubiske ME, Pregitzer KS, Ellsworth DE (2008) Sap flux in pure aspen and mixed aspen–birch forests exposed to elevated concentrations of carbon dioxide and ozone. Tree Physiol 28(8):1231–1243
van Oosten JJ, Besford RT (1994) Sugar feeding mimics effect of acclimation to high CO2-rapid down regulation of RuBisCO small subunit transcripts but not of the large subunit transcripts. Plant Cell Environ 17:913-923
Vaz M, Pereira JS, Gazarini LC, David TS, David JS, Rodrigues A, Maroco J, Chaves MM (2010) Drought-induced photosynthetic inhibition and autumn recovery in two Mediterranean oak species (Quercus ilex and Quercus suber). Tree Physiol 30:946–956
Vivin P, Guehl JM, Clement A, Aussenac G (1996) The effects of elevated CO2 and water stress on whole plant CO2 exchange, carbon allocation and osmoregulation in oak seedlings. Ann For Sci 53:2–3
Watanabe Y, Tobita H, Kitao M, Maruyama Y, Choi D, Sasa K, Funada R, Koike T (2008) Effects of elevated CO2 and nitrogen on wood structure related to water transport in seedlings of two deciduous broad-leaved tree species. Trees 22:403–411
Willkins D, van Oosten JJ, Besford RT (1994) Effects of elevated CO2 on growth and chloroplast proteins in Prunus avium. Tree Physiol 14:769–779
Yazaki K, Maruyama Y, Mori S, Koike T, Funada R (2005) Effects of elevated carbon dioxide concentration on wood structure and formation in trees. In: Omasa K, Nouchi I, De Kok LJ (eds) Plant responses to air pollution and global change. Springer, Tokyo
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Adams.
Rights and permissions
About this article
Cite this article
Vaz, M., Cochard, H., Gazarini, L. et al. Cork oak (Quercus suber L.) seedlings acclimate to elevated CO2 and water stress: photosynthesis, growth, wood anatomy and hydraulic conductivity. Trees 26, 1145–1157 (2012). https://doi.org/10.1007/s00468-012-0691-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-012-0691-x