Abstract
Elevated atmospheric CO2 concentration and changes in precipitation patterns affect plant physiological processes and alter ecosystem functions. In combination, the interactions between these factors result in complex responses that challenge our current understanding. We aimed to investigate the effects of elevated CO2 and drought stress on the growth and physiology traits of One-year-old Pistacia atlantica seedlings. Seedlings of P. atlantica were grown at two different CO2 concentrations (ambient 380 ppm and elevated 700 ppm) and the two irrigation regimes (100% and 50% of field capacity) for one growing season. Seedlings collar diameter, height, leaf area, biomass accumulation, root length and volume, photosynthetic parameters, pigment content, and relative water content increased at elevated CO2. At the same time, the amounts of proline, electrolyte leakage, malondialdehyde, and antioxidant enzymes decreased at elevated CO2. Drought stress had negative effects on the measured growth parameters. These, however, ameliorate in the presence of elevated CO2 through enhanced photosynthesis performance and maintaining better water status, and possibly also by a reduction of oxidative stress. Increased CO2, as expected in a future climate, might thus mitigate the negative effects of drought in P. atlantica trees under natural conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atmospheric CO2 concentration has increased since the onset of the industrial revolution, and a future increase in CO2 concentration is expected (IPCC 2018). Increasing CO2, along with other greenhouse gases, is supposed to trigger global warming, changes in precipitation patterns (IPCC 2013), and more frequent, intense, and erratic drought (Sippel et al. 2018; Jiang et al. 2021). Global warming impacts eco-physiological processes in terrestrial plants and ecosystems (Jentsch and Beierkuhnlein 2008; Albert et al. 2011a). The simultaneous drought and warming occurrence emphasize the need to investigate their impact on plants and ecosystems (Adams et al. 2009; Allen et al. 2010). Considering co-occurrences of climate features, studying each factor alone and in combination with others’ effects on environmental changes is necessary, especially in interaction investigations.
Available water is a major limiting factor for plant growth because the water restriction induces changes in various physiological and biochemical processes (Farooq et al. 2009; Sippel et al. 2018). Stomata close progressively with increased drought stress, followed by reduced net photosynthetic rates (Reddy et al. 2004). Drought stress also reduces the contents and activities of photosynthetic carbon reduction cycle enzymes, including the critical enzyme, ribulose-1,5-bisphosphate carboxylase/oxygenase (Reddy et al. 2004). In addition, drought stress-induced generation of reactive oxygen species (ROS) is well recognized at the cellular level and is tightly controlled at both the production and consumption levels in vivo through increased antioxidative systems (Reddy et al. 2004). Antioxidant enzyme activity is an adaptive mechanism in plants to reduce ROS damage. In other words, the activity of the antioxidant enzyme scavenges the accumulated Hydrogen peroxide (H2O2) and reduces it to non-toxic levels, and in this way, alleviates oxidative stress (Gill and Tuteja 2010; Lotfi et al. 2019). Long-term exposure of plants to elevated CO2 leads to several physiological effects, many of which are interpreted in the context of ameliorating the negative impacts of drought stress (Wullschleger et al. 2002). The direct effects of elevated CO2 are typically increased photosynthesis and water use efficiency in vegetation (Ainsworth et al. 2020). Acclimation of photosynthesis during long-term exposure to elevated CO2 reduces critical enzymes of the photosynthetic carbon reduction cycle, increasing nutrient use efficiency (Drake et al. 1997).
More recently, observations of widespread drought-induced tree mortality (Allen et al. 2015) have sharpened the focus on CO2-induced changes in plant water use as a mechanism to maintain vegetation function during drought (De Kauwe et al. 2021). Numerous studies have reported the mitigation effects of elevated CO2 on drought stress in different plant species (Drake et al. 1997; Wullschleger et al. 2002). Most elevated CO2 field studies have addressed the potential for system-wide water savings under mild or moderate drought conditions (De Kauwe et al. 2021). On the contrary, elevated CO2 may enhance plant performance during drought while water storage is depleted similarly to plants in control environments. For example, Jiang et al. (2021) found that elevated CO2-grown eucalypts exhibited less drought stress during short-term drought, with less negative leaf water potentials despite having larger biomass and no change in soil moisture content. The positive and extended photosynthetic response to elevated CO2 during drought stress may provide plants with additional nonstructural carbohydrates (NSC) to maintain lower osmotic potential and sustain metabolic activity (Jiang et al. 2021). Other studies, show a shift of biomass allocation into more roots in coffee plants exposed to elevated CO2 during moderate drought (e.g. Avila et al. 2020). They suggested that this was associated with a higher transcript abundance of aquaporin genes (Avila et al. 2020). However, studies show different effects of elevated CO2 on plant water relationships during drought, possibly influenced by experimental treatment (e.g. duration of CO2 exposure, nutrient availability, and drought severity) as well as species-specific morphological, physiological, and biomass adjustments to the growth conditions (Zhou et al. 2013; Becklin et al. 2017). For example, it was shown that the elevated CO2 benefits on plant growth, photosynthesis and nonstructural carbohydrates diminished with increasing aridity (Albert et al. 2011b; Duan et al. 2013). In addition, spring and early season leaf responses are most susceptible to elevated CO2 and are followed by a down-regulation towards the onset of autumn. At the whole-tree level, CO2 fertilization only causes consistent biomass increments in young seedlings, whereas mature trees show a variable response (Lauriks et al. 2021). Overall, it is necessary to consider various influencing factors to reconcile the disparate experimental evidence on the possible ameliorating role of elevated CO2 during drought stress.
Atlas mastic tree, Beneh in Iran (Pistacia atlantica Desf.) is one of the most important native species distributed extensively in Zagros forests located in western Iran. These forests, characterized by a semi-Mediterranean climate, are one of Iran's most important and sensitive ecosystems (Ahmadi et al. 2014). Many studies have shown the ecological flexibility and tolerance of P. atlantica to challenging environmental conditions in Iran. Therefore, natural forests of this species are found throughout Iran but are particularly common in the western and southern parts of the country (Heydari et al. 2016). Although it tolerates and adapts to diverse ecological conditions, the natural regeneration and reforestation of P. atlantica have become difficult (Mirzaei and Karamshahi 2015; Sadeghzadeh Hallaj et al. 2022). The harsh climate of Zagros prohibited the natural regeneration of P. atlantica (Mirzaei and Karamshahi 2015). In recent years, the mortality of P. atlantica has been increasing rapidly and has become a public concern (Attarod et al. 2016; Hosseini et al. 2017). These extreme events are attributed to regional consequences of global climate change and are projected to further increase in intensity and frequency. In order to better adapt forest management, we aim to better understand the functional traits and physiological responses of this species to the expected combined changes in climate and CO2 concentration.
This study aims to elucidate whether elevated CO2 concentration mitigates or exacerbates the negative effects of drought stress in P. atlantica seedlings. We are particularly interested in responses that increase or decrease the vulnerability of P. atlantica in the Zagros region. It is hypothesized that elevated CO2 concentration leads to increased assimilation and improved water use efficiency due to a higher photosynthetic pigment concentration, resulting in increased diameter and height growth, as well as a relative increase of the root system. These effects will be more expressed in drought stressed plants. In addition, increased CO2 concentration mitigates the drought stress-induced proline concentration, electrolyte leakage (EL), malondialdehyde, and antioxidant enzyme activity.
Materials and methods
Plant material
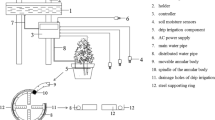
The seeds of P. atlantica were grown for one year in 5-L pots containing a mixture of natural soil, sand, and manure (1:3:1, v/v). The pot’s soil properties are shown in Table 1. The seedlings were watered twice every week at field capacity in the nursery. One-year-aged seedlings in the nursery were transferred to the two growth chambers (3. 3. 2 m, L, W, H) with the two different CO2 concentrations (the ambient CO2, 380 ppm, and the elevated CO2, 700 ppm) for one growing season from April 15 to December 10, 2019. We considered the other climatic factors fixed in the two chambers (170 μmol m−2 s−1 photosynthetically active radiation (PAR), 60 ± 5% humidity, 27/16 °C day/night air temperature, and 14/10, 15/9, and 10/14 h day/night photoperiod in spring, summer, and autumn, respectively). Pistacia atlantica's natural regeneration commonly occurs under the canopy shade of nurse trees (Sadeghzadeh Hallaj et al. 2022; Jahanpour et al. 2010; Negahdarsaber and Abbasi 2010). Reforestation attempts by Pistacia atlantica also could be successful if the seedlings were treated with the shade of nurse trees (Hamzepour et al. 2006). A study on the growth and development of Pistacia atlantica seedlings also revealed that full sunlight inhibits growth even for well-watered seedlings (Sadeghzadeh Hallaj et al. 2022). Field observations in the Zagros forest confirm that the early development of Pistacia atlantica seedlings depends on the low light intensity. This species grows solely under the shade of light-tolerant trees. It is unclear whether improved performance under shade is due to protection against severe sunlight, moderation of drought stress or both. According to the literature and experiences, we thus applied a very low light intensity (170 μmol m−2 s−1) for the experiment. The seedlings were rotated within the growth chambers every week to avoid micro-environmental effects.
Experimental design and treatments
The experiment was conducted in the two growth chambers located at the Faculty of Agriculture and Natural Resources, Lorestan University (33° 26′ 14.4″ N, 48° 15′ 38.7″ E). The experiment was done as a factorial based on a completely randomized design. The experimental treatments consisted of the control or ambient conditions (C), drought stress (D), elevated CO2 concentration (CO2), and the two factorial combinations (CO2 × D) with four replicates. We considered ten seedlings in each replicate, which resulted in 40 seedlings for each treatment. At each CO2 concentration, seedlings were divided into two groups. One group was subjected to well-watered treatment (100% of field capacity), and the other was subjected to drought stress (50% of field capacity).
Growth measurements
Each seedling's stem diameter and height were measured using a digital calliper and ruler at the end of the growing season. Then, the seedlings were carefully dug out of the pots. The roots were hand-washed to remove all soil particles. Roots' lengths were carefully measured using a ruler. Also, root volume was estimated directly through the transposition of water in the graded container cylinders (Norouzi Haroni et al. 2019). Scanned leaf images were used to determine the leaf area for each seedling using the software Image Tool 3.0 (Wilcox et al. 2002). The seedlings shoot and root were dried at 80 °C for 48 h, and then biomass was measured.
Measurements of gas exchange parameters
Leaf gas exchange parameters were measured using an LC4 portable gas exchange system (ADC Bioscientific, Ltd., Hoddesdon, UK). Net CO2 assimilation (Anet, μmol CO2 m−2 s−1), stomatal conductance (gs, mol H2O m−2 s−1), and transpiration (E, mmol H2O m−2 s−1) were simultaneously measured of the first fully expanded leaves. Leaf temperature was held at 27 °C at a relative humidity of 60%. Measurements were made under a PAR of 170 μmol m−2 s−1. The CO2 concentration in the leaf chamber was the same as in the growth conditions. The Anet/gs ratio was used as intrinsic water use efficiency (WUEi, μmol mol−1), according to Farquhar et al. (1989).
Measurements of photosynthetic pigments
At first, frozen leaves (0.1 g) were extracted at − 80 °C with 0.1 g calcium carbonate and 4 ml 80% acetone in the dark. Since chlorophyll is light-sensitive, the extraction took place in a dark room (Bergstrasser et al. 2015). The resulting extract was centrifuged at 4000 rpm for 10 min at 4.0 °C. The light absorption was measured at 470, 662, and 645 nm wavelengths using a spectrophotometer (Mapada UV-1800, Shanghai, People’s Republic of China). The contents of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids were calculated according to Lichtenthaler (1987).
Relative water content measurement
First, the fresh weights (FW) of the sample leaves were recorded, and the leaves were floated in distilled water in Petri dishes. After 24 h, the leaves were removed, the leaf surface was gently wiped, and the turgid weight (TW) was measured. The samples were dried in an oven at 80 °C for 48 h to measure dry weight (DW). Relative water content (RWC) of leaf tissue was determined using the equation RWC = 100[(FW − DW)/(TW − DW)] (Ritchie et al. 1990).
Proline, electrolyte leakage, and malondialdehyde determination
Free proline content in leaves was quantified following Bates et al. (1973). 0.5 g of fresh leaf tissue was removed and mixed with sulfosalicylic acid and acetic acid. After adding the ninhydrin solution, samples were placed in hot water. Light absorbance was read at 520 nm. 100 mg fresh leaf samples were cut into 5 mm lengths and placed in test tubes containing 10 mL distilled deionized water, then EL was determined. The tubes were placed in a water bath maintained at a constant 32 °C. After two hours, the initial electrical conductivity of the medium (EC1) was measured using an electrical conductivity meter. Then, the samples were put in an oven at 120 °C for 120 min. Then, samples were cooled to 25 °C, and the final electrical conductivity (EC2) was measured. We used the equation suggested by Nayyar (2003) to calculate El.
Malondialdehyde (MDA) as a measure for oxidative destruction of lipids was determined by forming a pink dye when reacting with thiobarbituric acid (TBA). First, 0.5 g of the fresh leaf was mixed with 0.5% (w/v) thiobarbituric acid solution containing 20% (w/v) trichloroacetic acid. The mixture was heated at 95 °C for 25 min, and the reaction was stopped by quickly placing it in an ice bath. The absorbance of the supernatant was read by spectrophotometer at 532 nm (Valentovic et al. 2006).
Activities of antioxidant enzymes in leaf extracts
Catalase (CAT) activity was determined in leaf extracts according to Chance and Maehly (1955). Leaf tissue (0.3 g FW) was ground in liquid N2, homogenized with 1.5 mL of K phosphate buffer (containing 1 mM EDTA and 2% PVPP), and centrifuged at 14,000 rpm for 20 min at 4 °C. The CAT activity in the supernatant was calculated from the decrease in A240 and expressed as μmol H2O2 reduced min−1 g FW−1. Peroxidase (POD) activity was assayed in leaf extracts as in MacAdam et al. (1992). Leaf tissue (0.3 g FW) was ground in liquid N2, homogenized with 1.5 mL K phosphate buffer (pH 7.0), and centrifuged at 14,000 rpm and 4 °C for 20 min. The POD activity in the supernatant was calculated from the decline in A475 and expressed as μmol H2O2 reduced min−1 g FW−1. Ascorbate peroxidase (APX) activity was assayed in leaf extracts as in Nakano and Asada (1981). Leaf tissue (0.3 g FW) was homogenized with 3 mL 0.05 mM sodium phosphate buffer (pH 7.8) containing 1 mM EDTA and 2% PVPP and centrifuged at 14,000 rpm for 20 min. The APX activity in the supernatant was calculated from the decline in A290 and expressed as μmol H2O2 reduced min−1 g FW−1.
Statistical analysis
We checked the data’s normality with the Kolmogorov–Smirnov test. Results indicated that the normality assumption was met for all variables, and no transformation was necessary. Two-way analysis of variance (ANOVA) was used to test the effects of CO2 concentrations, drought stress, and their interaction on all dependent variables. The Duncan test at P ≤ 0.05 was used to compare means. All statistical analyses were conducted using the SPSS software version 21.0.
Results
Growth parameters
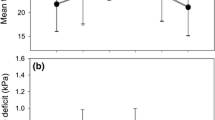
Elevated CO2 concentration and drought stress significantly affected the diameter growth of P. atlantica seedlings, while CO2 × D had no effect (Table 2). Elevated CO2 concentration enhanced the mean diameter growth by 19% compared to the control seedlings, whereas the diameter of drought stressed seedlings only reached 57% (Fig. 1a). Height growth and leaf area also increased under elevated CO2 concentration (Table 2). The height and leaf area increments were 38% and 27% greater under elevated CO2 than the control seedlings (Fig. 1b, c). Under CO2 × D, height growth and leaf area decreased by 6% and 30%, respectively, compared to the control (Table 2, Fig. 1b, c). The positive effect of CO2 could be better revealed when we compare CO2 × D with drought stress alone. Under CO2 × D, height growth and leaf area increased by 24% and 17%, respectively, compared to drought stress alone (Table 2, Fig. 1b, c).
Mean (± SE) diameter (a), height (b), and leaf area (c) of P. atlantica seedlings after 8 months of growth under control (C), elevated CO2 concentration (CO2), drought stress (D), and CO2 × D conditions (Duncan test; P ≤ 0.05; n = 4). Asterisks (*) denote the level of significance (*P < 0.05, **P < 0.01, ***P < 0.001)
Root length and volume tended to increase relative to the control when P. atlantica seedlings were exposed to elevated CO2 (by 30% and 43%, respectively), while drought stress reduced the root length and volume by 12% and 69%, respectively (Table 2, Fig. 2a, b). Root length and volume were considerably higher under CO2 × D than under drought stress alone (13% and 23%, respectively) (Table 2, Fig. 2a, b).
Mean (± SE) root length (a) and volume (b) of P. atlantica seedlings after 8 months of growth in control (C), elevated CO2 concentration (CO2), drought stress (D), and CO2 × D conditions (Duncan test; P ≤ 0.05; n = 4). Asterisks (*) denote the level of significance (*P < 0.05, **P < 0.01, ***P < 0.001)
Elevated CO2 concentration and drought stress also affected shoot, root, and total biomass (Table 2). As expected, elevated CO2 concentration increased shoot biomass by 73%, root biomass by 47%, and total biomass by 58% in comparison to the control, while drought stress reduced the shoot, root, and total biomass by 76%, 84%, and 80%, respectively (Fig. 3a–c). Under CO2 × D, shoot and total biomass increased by 50% and 56%, respectively, compared to drought stress alone (Table 2, Fig. 3a, c).
Mean (± SE) shoot (a), root (b), and total (c) biomass of P. atlantica seedlings after 8 months of growth under control (C), elevated CO2 concentration (CO2), drought stress (D), and CO2 × D conditions (Duncan test; P ≤ 0.05; n = 4). Asterisks (*) denote the level of significance (*P < 0.05, **P < 0.01, ***P < 0.001)
Gas exchange
The net photosynthesis (Anet), stomatal conductance (gs), and transpiration (E) rate of P. atlantica seedlings were influenced by elevated CO2, drought stress, and CO2 × D treatments (Table 2). The Anet (by 81%), gs (by 43%), and E (by 61%) increased under elevated CO2 concentration, while their rates decreased under drought stress by 82%, 56%, and 62%, respectively, in comparison with the control treatment (Fig. 4a–c). In the combined treatment, elevated CO2 concentration alleviated the effect of drought stress so that the reductions in Anet, gs, and E were improved by 84%, 22%, and 51%, respectively (Table 2, Fig. 4a–c). In addition, elevated CO2 concentration also enhanced intrinsic water use efficiency (WUEi) by 27% compared to the control seedlings, while its rate did not differ between the two water regimes (Table 2, Fig. 4d). In the CO2 × D treatment, although the WUEi was increased in comparison with drought stress alone (by 55%), it was not significant (Table 2, Fig. 4d).
Mean (± SE) net photosynthesis (Anet) (a), stomatal conductance (gs) (b), transpiration (E) (c), and intrinsic water use efficiency (WUEi) (d) rates of P. atlantica seedlings after 8 months of growth under control (C), elevated CO2 concentration (CO2), drought stress (D), and CO2 × D conditions (Duncan test; P ≤ 0.05; n = 4). Asterisks (*) denote the level of significance (*P < 0.05, **P < 0.01, ***P < 0.001)
Pigment content
Exposure to elevated CO2 concentration significantly increased chlorophyll a by twofold, chlorophyll b by 86%, chlorophyll a + b by twofold, and carotenoids by 87% compared to the control seedlings. In contrast, their concentrations were reduced with increasing drought stress by 46%, 44%, 45%, and 24%, respectively (Table 2, Fig. 5a–d). When elevated CO2 concentration was combined with drought stress, the chlorophyll a, chlorophyll b, chlorophyll a + b, and carotenoids increased 86%, 77%, 83%, and 68%, greater than drought stress alone treatments, respectively (Table 2, Fig. 5a–d).
Mean (± SE) concentrations of chlorophyll a (a), chlorophyll b (b), chlorophyll a + b (c), and carotenoids (d) in leaves of P. atlantica seedlings after 8 months of growth under control (C), elevated CO2 concentration (CO2), drought stress (D), and CO2 × D conditions (Duncan test; P ≤ 0.05; n = 4). Asterisks (*) denote the level of significance (*P < 0.05, **P < 0.01, ***P < 0.001)
Water status, proline, electrolyte leakage, and malondialdehyde
RWC increased by 13% under elevated CO2 concentration compared to the control treatment, while its level decreased under drought stress by 20% (Table 2, Fig. 6a). Increasing CO2 levels generally reduced or reversed the impact of drought stress (by 22%; Table 2, Fig. 6a).
Mean (± SE) relative water content (RWC) (a), Proline (b), electrolyte leakage (EL) (c), and malondialdehyde (MDA) (d) contents of P. atlantica seedlings after 8 months of growth in control (C), elevated CO2 concentration (CO2), drought stress (D), and CO2 × D conditions (Duncan test; P ≤ 0.05; n = 4). Asterisks (*) denote the level of significance (*P < 0.05, **P < 0.01, ***P < 0.001)
In leaves, drought stress increased the mean proline, EL, and malondialdehyde (MDA) concentrations by 85%, 33%, and 84% compared to the control seedlings. However, their contents decreased by 10%, 12%, and 23% with increasing CO2 concentration, respectively (Table 2, Fig. 6b–d). Elevated CO2 significantly alleviated the effect of drought stress so that proline, EL, and MDA contents were considerably lower under CO2 × D than under drought stress alone treatment (by 53%, 23%, and 51%, respectively; Table 2, Fig. 6b–d).
Enzyme activities
Drought stress also promoted an increase in CAT (twofold), POD (27%), and APX (66%) activities in comparison to control seedlings (Table 2, Fig. 7a–c). In contrast, the CAT, POD, and APX activities, measured at the CO2 growth concentration, were lower in elevated CO2 seedlings than in ambient CO2 seedlings (by 7%, 42%, and 13%, respectively) (Table 2, Fig. 7a–c). Compared to the activity of the antioxidant enzymes of leaves between drought stress treatments, CAT and APX activities were significantly reduced under elevated CO2 (by 67% and 55%, respectively). However, POD activity remained unaffected (Table 2, Fig. 7a–c).
Mean (± SE) catalase (CAT) (a), peroxidase (POD) (b), and ascorbate peroxidase (APX) (c) activities of P. atlantica seedlings after 8 months of growth under control (C), elevated CO2 concentration (CO2), drought stress (D), and CO2 × D conditions (Duncan test; P ≤ 0.05; n = 4). Asterisks (*) denote the level of significance (*P < 0.05, **P < 0.01, ***P < 0.001)
Discussion
Results show that increasing CO2 up to 700 ppm increased growth, including diameter, height, leaf area, root length, root volume, shoot-, root-, and total biomass. Similar results also were observed in the previous studies with other plant species (Vaz et al. 2012; Arab et al. 2018; Song et al. 2020; Lauriks et al. 2021). Increased drought or CO2 also affects root allocation in many cases. Effects on root allocation are often observed regarding drought (e.g. Santos et al. 2021; Jeong et al. 2021), and sometimes also regarding CO2 (e.g. Norby et al. 2004). However, the latter response depends on other soil and weather conditions (e.g. Handa et al. 2008) and plant strategies, which also might favour exudation instead of root biomass (Fahey et al. 2005). Many studies have reported that elevated CO2 stimulates root growth (Crookshanks et al. 1998; Norby et al. 2004; De Graaff et al. 2006). In a meta-analysis, root biomass exhibited more significant increases than shoot biomass with elevated CO2; therefore, increasing root biomass with elevated CO2 may enhance the potential to store C (De Graaff et al. 2006; Luo et al. 2006; Nie et al. 2013). In addition, these effects are expected to cause increased amounts of C input into the soil (De Graaff et al. 2006). The observed plant growth increment in elevated CO2 in our experiment was consistent with the increase in photosynthetic rate during the same period.
We found that drought stress reduced all the measured growth parameters, similar to the findings of Guo et al. (2010), Deligoz and Gur (2015), and Jafarnia et al. (2017). The reductions in the aboveground growth of seedlings under increasing water deficit are well-known adaptations. Optimal partitioning theory suggests that “plants preferentially allocate biomass to acquire the resource that most limits growth” (Kobe et al. 2010). Therefore, under drought stress, plants tend to invest in root growth at the expense of diameter and height growth resulting in aboveground biomass reductions (see, for example, Schall et al. 2012; Jeong et al. 2021; Santos et al. 2021). In our study, elevated CO2 concentration alleviated the negative effects of drought and promoted plant growth under stress. Owensby et al. (1997) have successfully used a combination of approaches (measurements of leaf water potential to whole-ecosystem gas exchange) to show that reduced water use in a C4 tallgrass prairie exposed to elevated CO2 was sufficient to increase above- and below-ground biomass production in years when drought stress was frequent.
Elevated CO2 is often reported to increase photosynthesis (Anet) and intrinsic water use efficiency (WUEi) in C3 plants. In this study, Anet and WUEi also increased with increasing CO2. The same results are observed for Vitis vinifera (Moutinho-Pereira et al. 2009), Kalopanax septemlobus (Watanabe et al. 2010), Quercus mongolica (Yan et al. 2010), Deschampsia flexuosa (Albert et al. 2011c), Quercus suber (Vaz et al. 2012), and Phragmites australis (Mozdzer and Caplan 2018).
Despite many studies that have shown a reduction in stomatal conductance (gs) and transpiration (E) rates under elevated CO2 (Wullschleger et al. 2002; Ainsworth and Rogers 2007; Mozdzer and Caplan 2018), the results of this study showed that the rate of gs and E of P. atlantica seedlings increased under elevated CO2, which is in accordance to (Albert et al. 2011c; Zinta et al. 2014; Sreeharsha et al. 2015; Monda et al. 2016). Thus, the decrease in gs due to findings in some other species elevated CO2 is not a universal response but may be due to a species-specific strategy. The different response of gs due to elevated CO2 is particularly found in specific species or ecotypes, plant functional types (PFTs), and development stages in cotrast to others (Xu et al. 2016). In addition, Medlyn et al. (2001) reported that gs response to elevated CO2 was significantly stronger in young trees than in old trees, deciduous compared to coniferous trees, and drought-stressed to nutrient-stressed trees.
According to the current findings, drought stress significantly decreased Anet, gs, and E rates in P. atlantica. This indicates a stomatal closure in response to a reduction in relative water content (Reddy et al. 2004). Stomatal closure decreases the foliar photosynthetic rate and internal CO2 concentration. In the current study, increasing CO2 levels generally alleviated the negative effects of drought stress on gas exchange. Previous studies also observed this finding (Zeppel et al. 2012; Bauweraerts et al. 2013; AbdElgawad et al. 2015; Miranda-Apodaca et al. 2015; Jiang et al. 2021).
Drought stress significantly decreased the concentrations of photosynthetic pigments (chlorophyll a, chlorophyll b, chlorophyll a + b, and carotenoids (Dutta et al. 2015; Zhang et al. 2018; and Mahmoudian et al. 2021). The chloroplast and thylakoid structures are usually injured under increased oxidative stress due to drought, leading to decreases in chlorophyll and carotenoid content (Asrar and Elhindi 2011). Our findings also support the role of elevated CO2 in the photosynthetic pigments concentration enhancement of seedlings under drought stress. It may be due to less oxidative stress and less damage to photosynthetic pigments, which is consistent with the findings of AbdElgawad et al. (2015).
RWC decreased under drought stress. Similar results were obtained by Wang (2014) and Cui et al. (2019). The foliar photosynthetic rate of higher plants is known to decrease as the relative water content and leaf water potential decrease (Cornic 2000). In our study, drought stress led to stomata closure, but in the presence of elevated CO2, while the stomata remained open, the RWC reduction was mitigated. Similar results were shown by Atwell et al. (2007) and Cui et al. (2019). It may be due to better water supply through more root biomass. With increasing drought intensity and decreasing relative water content, plants try to absorb maximum moisture from the soil through osmotic adjustment mechanisms and reduced stem water potential (Sanchez-Blanco et al. 2004). Some authors have affirmed that elevated CO2 would permit the plant to increase the fine roots and, in general, the root biomass, raising the root-to-shoot ratio and boosting drought tolerance (Xu et al. 2013; Miranda-Apodaca et al. 2018).
In our study, the accumulation of proline, malondialdehyde and EL concentrations increased with drought stress (Wang 2014; Jafarnia et al. 2017; Chiappero et al. 2019; Zhang et al. 2019) and a significant increase was seen in antioxidant enzyme activities such as CAT, POD, and APX (Xu et al. 2008; Patel and Hemantaranjan 2012; Wang 2014). While the seedlings grown under drought stress conditions and simultaneously exposed to elevated CO2 showed reduced proline, MDA, and EL contents, similar results were obtained by Xu et al. (2014) and AbdElgawad et al. (2015). A significant alleviation was seen in the negative effects of drought stress on CAT and APX activities in the pistacia seedlings exposed to elevated CO2 (see also Schwanz et al. 1996). In fact, under conditions of oxidative stress, the peroxidation of unsaturated fatty acids increases and various aldehydes, including MDA, are produced by the attack of free radicals on lipids (Gharibi et al. 2016). EL is also an indicator of cell integrity and cellular membrane stability, reflecting the degree of damage to the plant by stress factors (Kocheva et al. 2004). A reduction in oxidative stress effects under elevated CO2 may originate from reduced ROS generation, with a concomitant reduction of stress impact (relaxation) and/or at the level of increased ROS scavenging (antioxidant) capacity (AbdElgawad et al. 2015). A reduction of ROS levels by elevated CO2 is biochemically explained by increased rubisco carboxylation capacity, reducing photorespiratory H2O2 production. As a consequence of the reduced ROS generation, also antioxidant levels may remain low under stress conditions in elevated CO2 (AbdElgawad et al. 2015).
Conclusions
The obtained results in this study largely supported our hypotheses. We found that elevated CO2 positively provokes marked changes in the physio-morphological traits of P. atlantica seedlings. On the other hand, drought stress negatively affected the studied traits. We also observed that elevated CO2 could potentially mitigate the negative effects of drought stress by improving photosynthesis and mitigating drought stress. In summary, plants exposed to drought stress may benefit from future elevated CO2 conditions.
Data availability
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
References
AbdElgawad H, Farfan-Vignolo ER, de Vos D, Asard H (2015) Elevated CO2 mitigates drought and temperature-induced oxidative stress differently in grasses and legumes. Plant Sci 231:1–10. https://doi.org/10.1016/j.plantsci.2014.11.001
Adams HD, Guardiola-Claramonte M, Barron-Gafford GA, Camilo Villegas J, Breshears DD, Zou CB, Troch PA, Huxman TE (2009) Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc Natl Acad Sci USA 106:7063–7066. https://doi.org/10.1073/pnas.0901438106
Ahmadi R, Kiadaliri H, Mataji A, Kafaki S (2014) Oak forest decline zonation using AHP model and GIS technique in Zagros forests of Ilam province. J Biodivers Environ Sci 4:141–150
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30:258–270. https://doi.org/10.1111/j.1365-3040.2007.01641.x
Ainsworth EA, Lemonnier P, Wedow JM (2020) The influence of rising tropospheric carbon dioxide and ozone on plant productivity. Plant Biol 22:5–11. https://doi.org/10.1111/plb.12973
Albert KR, Mikkelsen TN, Michelsen A, Ro-Poulsen H, van Der Linden L (2011a) Interactive effects of drought, elevated CO2 and warming on photosynthetic capacity and photosystem performance in temperate heath plants. J Plant Physiol 168:1550–1561. https://doi.org/10.1016/j.jplph.2011.02.011
Albert KR, Ro-Poulsen H, Mikkelsen TN, Michelsen A, van Der Linden L, Beier C (2011b) Effects of elevated CO2, warming and drought episodes on plant carbon uptake in a temperate heath ecosystem are controlled by soil water status. Plant Cell Environ 34:1207–1222. https://doi.org/10.1111/j.1365-3040.2011.02320.x
Albert KR, Ro-Poulsen H, Mikkelsen TN, Michelsen A, van der Linden L, Beier C (2011c) Interactive effects of elevated CO2, warming, and drought on photosynthesis of Deschampsia flexuosa in a temperate heath ecosystem. J Exp Bot 62:4253–4266. https://doi.org/10.1093/jxb/err133
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T et al (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259:660–684. https://doi.org/10.1016/j.foreco.2009.09.001
Allen CD, Breshears DD, McDowell NG (2015) On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6:1–55. https://doi.org/10.1890/ES15-00203.1
Arab L, Seegmueller S, Kreuzwieser J, Eiblmeier M, Rennenberg H (2018) Atmospheric pCO2 impacts leaf structural and physiological traits in Quercus petraea seedlings. Planta 249:481–495. https://doi.org/10.1007/s00425-018-3016-5
Asrar AA, Elhindi KM (2011) Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J Biol Sci 18:93–98. https://doi.org/10.1016/j.sjbs.2010.06.007
Attarod P, Sadeghi SMM, Taheri Sarteshnizi F, Saroyi S, Abbasian P, Masihpoor M, Kordrostami F, Dirikvandi A (2016) Meteorological parameters and evapotranspiration affecting the Zagros forests decline in Lorestan province. Iran J For Range Protect Res 13:97–112. https://doi.org/10.22092/ijfrpr.2016.106018
Atwell BJ, Henery ML, Rogers GS, Seneweera SP, Treadwell M, Conroy JP (2007) Canopy development and hydraulic function in Eucalyptus tereticornis grown in drought in CO2-enriched atmospheres. Funct Plant Biol 34:1137–1149. https://doi.org/10.1071/FP06338
Avila RT, de Almeida WL, Costa LC, Machado KLG, Barbosa ML, de Souza RPB, Martino PB, Juarez MAT, Marcal DMS, Martins SCV et al (2020) Elevated air CO2 improves photosynthetic performance and alters biomass accumulation and partitioning in drought-stressed coffee plants. Environ Exp Bot 177:104137. https://doi.org/10.1016/j.envexpbot.2020.104137
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bauweraerts I, Wertin TM, Ameye M, Mcguire MA, Teskey RO, Steppe K (2013) The effect of heat waves, elevated [CO2] and low soil water availability on northern red oak (Quercus rubra L.) seedlings. Glob Change Biol 19:517–528. https://doi.org/10.1111/gcb.12044
Becklin KM, Wallker SM II, Way DA, Ward JK (2017) CO2 studies remain key to understanding a future world. New Phytol 214:34–40. https://doi.org/10.1111/nph.14336
Bergstrasser S, Fanourakis D, Schmittgen S, Cendrero-Mateo MP, Jansen M, Scharr H, Rascher U (2015) HyperART: non-invasive quantification of leaf traits using hyperspectral absorption-reflectance-transmittance imaging. Plant Methods 11:1. https://doi.org/10.1186/s13007-015-0043-0
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775. https://doi.org/10.1016/S0076-6879(55)02300-8
Chiappero J, Cappellari LDR, Alderete LGS, Palermo TB, Banchio E (2019) Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind Crops Prod 139:111553. https://doi.org/10.1016/j.indcrop.2019.111553
Cornic G (2000) Drought stress inhibits photosynthesis by decreasing stomatal aperture–not by affecting ATP synthesis. Trends Plant Sci 5:187–188. https://doi.org/10.1016/S1360-1385(00)01625-3
Crookshanks M, Taylor G, Broadmeadow M (1998) Elevated CO2 and tree root growth: contrasting responses in Fraxinus excelsior, Quercus petraea and Pinus sylvestris. New Phytol 138:241–250. https://doi.org/10.1046/j.1469-8137.1998.00109.x
Cui Q, Li Y, He X, Li S, Zhong X, Liu B, Zhang D, Li Q (2019) Physiological and iTRAQ based proteomics analyses reveal the mechanism of elevated CO2 concentration alleviating drought stress in cucumber (Cucumis sativus L.) seedlings. Plant Physiol Biochem 143:142–153. https://doi.org/10.1016/j.plaphy.2019.08.025
De Graaff MA, Van Groenigen KJ, Six J, Hungate B, Van Kessel C (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Change Biol 12:2077–2091. https://doi.org/10.1111/j.1365-2486.2006.01240.x
De Kauwe MG, Medlyn BE, Tissue DT (2021) To what extent can rising [CO2] ameliorate plant drought stress? New Phytol 231:2118–2124. https://doi.org/10.1111/nph.17540
Deligoz A, Gur M (2015) Morphological, physiological and biochemical responses to drought stress of Stone pine (Pinus pinea L.) seedlings. Acta Physiol Plant 37:243. https://doi.org/10.1007/s11738-015-1998-1
Drake BG, Gonzalez-Meler MA, Long SP (1997) More efficient plants: a consequence of rising atmospheric CO2? Annu Rev Plant Physiol Plant Mol Biol 48:609–639
Duan H, Amthor JS, Duursma RA, O’Grady AP, Choat B, Tissue DT (2013) Carbon dynamics of eucalypt seedlings exposed to progressive drought in elevated [CO2] and elevated temperature. Tree Physiol 33:779–792. https://doi.org/10.1093/treephys/tpt061
Dutta SK, Patel VB, Viswanathan C, Singh SK, Singh AK (2015) Physiological and biochemical adaptation of arbuscular mycorhizal fungi (AMF) inoculated Citrus jambhiri (Jatti khatti) seedlings under water deficit stress conditions. Progress Hortic 47:229–236. https://doi.org/10.5958/2249-5258.2015.00041.X
Fahey TJ, Tierney GL, Fitzhugh RD, Wilson GF, Siccama TG (2005) Soil respiration and soil carbon balance in a northern hardwood forest ecosystem. Can J for Res 35:244–253
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:185–212. https://doi.org/10.1051/agro:2008021
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537. https://doi.org/10.1146/annurev.pp.40.060189.002443
Gharibi S, Tabatabaei BES, Saeidi G, Goli SAH (2016) Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl Biochem Biotechnol 178:796–809. https://doi.org/10.1007/s12010-015-1909-3
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Guo J, Yang Y, Wang G, Yang L, Sun X (2010) Ecophysiological responses of Abies fabri seedlings to drought stress and nitrogen supply. Physiol Plant 139:335–347. https://doi.org/10.1111/j.1399-3054.2010.01370.x
Hamzepour M, Bordbar SK, Joukar L, Abbasi AR (2006) The potential of rehabilitation of wild pistacio forests through straight seed sowing and seedling planting. Iran J For Poplar Res 14(3):207–220
Handa IT, Hagedorn F, Hättenschwiler S (2008) No stimulation in root production in response to 4 years of in situ CO2 enrichment at the Swiss treeline. Funct Ecol 22:348–358
Heydari M, Naderi S, Karamshahi A, Mezbani A (2016) Autecology and phenology of Pistacia atlantica in relation to edaphic and physiographic factors in Kabirkoh forests of Darreh Shahr county, Ilam Province. J Plant Res 29(1):80–95
Hosseini A, Hosseini SM, Linares JC (2017) Site factors and stand conditions associated with Persian oak decline in Zagros mountain forests. For Syst 26:3. https://doi.org/10.5424/fs/2017263-112
IPCC (2013) Climate change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Contribution of working group I to the fifth assessment report of the intergovermental panel on climate change. Cambridge University Press, Cambridge
IPCC (2018) Summary for policymakers. In: Masson-Delmotte V, Zhai P, Portner HO, Roberts D, Skea J, Shukla PR et al (Eds) Global warming of 1.5 °C an IPCC special report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty, 32 pp. World Meteorological Organization, Geneva
Jafarnia S, Akbarinia M, Hosseinpour B, Modarres Sanavi SAM, Salami SA (2017) Effect of drought stress on some growth, morphological, physiological, and biochemical parameters of two different populations of Quercus brantii. iForest 11:212–220. https://doi.org/10.3832/ifor2496-010
Jahanpour FA, Fatahi M, Karamian R (2010) Studying the influence of light on surviving of pistachio saplings in Lorestan province. Iran J for 3(2):91–98
Jentsch A, Beierkuhnlein C (2008) Research frontiers in climate change: effects of extreme meteorological events on ecosystems. C R Geosci 340:621–628. https://doi.org/10.1016/j.crte.2008.07.002
Jeong J, Bolan NS, Kim C (2021) Allocation of photoassimilated carbon of radiata pine (Pinus radiata) seedlings as affected by soil water stress. Aust For 84:4–12. https://doi.org/10.1080/00049158.2020.1864944
Jiang M, Kelly JWG, Atwell BJ, Tissue DT, Medlyn BE (2021) Drought by CO2 interactions in trees: a test of the water savings mechanism. New Phytol 230:1421–1434. https://doi.org/10.1111/nph.17233
Kobe RK, Iyer M, Walters MB (2010) Optimal partitioning theory revisited: nonstructural carbohydrates dominate root mass responses to nitrogen. Ecology 91:166–179. https://doi.org/10.1890/09-0027.1
Kocheva K, Lambrev P, Georgiev G, Goltsev V, Karabaliev M (2004) Evaluation of chlorophyll fluorescence and membrane injury in the leaves of barley cultivars under osmotic stress. Bioelectrochemistry 63:121–124. https://doi.org/10.1016/j.bioelechem.2003.09.020
Lauriks F, Salomon RL, Steppe K (2021) Temporal variability in tree responses to elevated atmospheric CO2. Plant Cell Environ 44:1292–1310. https://doi.org/10.1111/pce.13986
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Lotfi N, Soleimani A, Vahdati K, Cakmakci R (2019) Comprehensive biochemical insights into the seed germination of walnut under drought stress. Sci Hort 250:329–343. https://doi.org/10.1016/j.scienta.2019.02.060
Luo Y, Hui D, Zhang D (2006) Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: a meta-analysis. Ecology 87:53–63. https://doi.org/10.1890/04-1724
MacAdam JW, Nelson CJ, Sharp RE (1992) Peroxidase activity in the leaf elongation zone of tall fescue: I. Spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol 99:872–878. https://doi.org/10.1104/pp.99.3.872
Mahmoudian M, Rahemi M, Karimi S, Yazdani N, Tajdini Z, Sarikahni S, Vahdati K (2021) Role of kaolin on drought tolerance and nut quality of Persian walnut. J Saudi Soc Agric Sci 20:409–416. https://doi.org/10.1016/j.jssas.2021.05.002
Medlyn BE, Barton CVM, Broadmeadow MSJ, Ceulemans R, De Angelis P, Forstreuter M, Freeman M, Jackson SB, Kellomaki S, Laitat E, Rey A, Roberntz P, Sigurdsson BD, Strassemeyer J, Wang K, Curtis PS, Jarvis PG (2001) Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytol 149:247–264. https://doi.org/10.1046/j.1469-8137.2001.00028.x
Miranda-Apodaca J, Perez-Lopez U, Lacuesta M, Mena-Petite A, Muñoz-Rueda A (2015) The type of competition modulates the ecophysiological response of grassland species to elevated CO2 and drought. Plant Biol 17:298–310. https://doi.org/10.1111/plb.12249
Miranda-Apodaca J, Perez-Lopez U, Lacuesta M, Mena-Petite A, Munoz-Rueda A (2018) The interaction between drought and elevated CO2 in water relations in two grassland species is species-specific. J Plant Physiol 220:193–202. https://doi.org/10.1016/j.jplph.2017.11.006
Mirzaei J, Karamshahi A (2015) Effects of drought stress on growth and physiological characteristics of Pistacia atlantica seedlings. J Wood For Sci Technol 22:31–43
Monda K, Araki H, Kuhara S, Ishigaki G, Akashi R, Negi J et al (2016) Enhanced stomatal conductance by a spontaneous Arabidopsis tetraploid, Me-0, results from increased stomatal size and greater stomatal aperture. Plant Physiol 170:1435–1444. https://doi.org/10.1104/pp.15.01450
Moutinho-Pereira J, Goncalves B, Bacelar E, Boaventura Cunha J, Cotinho J, Correal CM (2009) Effects of elevated CO2 on grapevine (Vitis vinifera L.): physiological and yield attributes. Vitis 48:159–165. https://doi.org/10.5073/vitis.2009.48.159-165
Mozdzer TJ, Caplan JS (2018) Complementary responses of morphology and physiology enhance the stand-scale production of a model invasive species under elevated CO2 and nitrogen. Funct Ecol 32:1784–1796. https://doi.org/10.1111/1365-2435.13106
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nayyar H (2003) Accumulation of osmolytes and osmotic adjustment in water-stressed wheat (Triticum aestivum) and maize (Zea mays) as affected by calcium and its antagonists. Environ Exp Bot 50:253–264. https://doi.org/10.1016/S0098-8472(03)00038-8
Negahdarsaber MR, Abbasi AR (2010) Impacts of ground cover vegetation on natural regeneration of Beneh (Pistacia atlantica) (Case study: Beneh experimental forest, Fars province). Iran J For Poplar Res 18(4):630–638
Nie M, Lu M, Bell J, Raut S, Pendall E (2013) Altered root traits due to elevated CO2: a meta-analysis. Glob Ecol Biogeogr 22:1095–1105. https://doi.org/10.1111/geb.12062
Norby RJ, Ledford J, Reilly CD, Miller NE, O’Neill EG (2004) Fine-root production dominates response of a deciduous forest to atmospheric CO2 enrichment. Proc Natl Acad Sci USA 101:9689–9693. https://doi.org/10.1073/pnas.0403491101
Norouzi Haroni N, Badehian Z, Zarafshar M, Bazot S (2019) The effect of oil sludge contamination on morphological and physiological characteristics of some tree species. Ecotoxicology 28:507–519. https://doi.org/10.1007/s10646-019-02034-0
Owensby C, Ham J, Knapp A, Bremer D, Auen L (1997) Water vapour fluxes and their impact under elevated CO2 in a C4-tallgrass prairie. Glob Change Biol 3:189–195. https://doi.org/10.1046/j.1365-2486.1997.00084.x
Patel PK, Hemantaranjan A (2012) Antioxidant defense system in chickpea (Cicer arietinum L.): influence by drought stress implemented at pre- and post-anthesis stage. Am J Plant Physiol 7:164–173. https://doi.org/10.3923/ajpp.2012.164.173
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202. https://doi.org/10.1016/j.jplph.2004.01.013
Ritchie SW, Nguyen HT, Holaday AS (1990) Leaf water content and gas-exchange parameters of two wheat genotypes differing in drought resistance. Crop Sci 30:105–111. https://doi.org/10.2135/cropsci1990.0011183X003000010025x
Sadeghzadeh Hallaj MH, Azadfar D, Mirzaei Nodoushan H, Eskandari S, Tiefenbacher JP (2022) Shade moderates the drought stress on saplings of Beneh (Pistacia atlantica Desf. Subsp. mutica) in semiarid areas of Iran. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-19635-8
Sanchez-Blanco MJ, Ferrandez T, Morales MA, Morte A, Alarcon JJ (2004) Variations in water status, gas exchange and growth in Rosmarinus officinalis plant infected with Glomus deserticola under drought condition. J Plant Physiol 161:675–682
Santos M, Barros V, Lima L, Frosi G, Santos MG (2021) Whole plant water status and non-structural carbohydrates under progressive drought in a Caatinga deciduous woody species. Trees 35:1257–1266. https://doi.org/10.1007/s00468-021-02113-y
Schall P, Lodige C, Beck M, Ammer C (2012) Biomass allocation to roots and shoots is more sensitive to shade and drought in European beech than in Norway spruce seedlings. For Ecol Manag 266:246–253. https://doi.org/10.1016/j.foreco.2011.11.017
Schwanz P, Picon C, Vivin P, Dreyer E, Guehl JM, Polle A (1996) Responses of antioxidative systems to drought stress in pendunculate oak and maritime pine as modulated by elevated CO2. Plant Physiol 110:393–402. https://doi.org/10.1104/pp.110.2.393
Sippel S, Reichstein M, Ma X, Mahecha MD, Lange H, Flach M, Frank D (2018) Drought, heat and the carbon cycle: a review. Curr Clim Change Rep 4:266–286. https://doi.org/10.1007/s40641-018-0103-4
Song WK, Byeon SY, Lee HT, Lee MS, Ryu D, Kang JW, Han SH, Oh CY, Kim HS (2020) Species-specific morphological and physiological characteristics and progressive nitrogen limitation under elevated CO2 concentration. iForest 13:270–278. https://doi.org/10.3832/ifor3288-013
Sreeharsha RV, Sekhar KM, Reddy AR (2015) Delayed flowering is associated with lack of photosynthetic acclimation in Pigeon pea (Cajanus cajan L.) grown under elevated CO2. Plant Sci 231:82–93. https://doi.org/10.1016/j.plantsci.2014.11.012
Valentovic P, Luxova M, Kolarovic L, Gasparikova O (2006) Effect of osmotic stress on compatible solutes content, membrane stability and water relations in two maize cultivars. Plant Soil Environ 52:186–191. https://doi.org/10.17221/3364-PSE
Vaz M, Cochard H, Gazarini L, Graca J, Chaves MM, Pereira JS (2012) Cork oak (Quercus suber L.) seedlings acclimate to elevated CO2 and water stress: photosynthesis, growth, wood anatomy and hydraulic conductivity. Trees 26:1145–1157. https://doi.org/10.1007/s00468-012-0691-x
Wang LF (2014) Physiological and molecular responses to drought stress in rubber tree (Hevea brasiliensis Muell. Arg.). Plant Physiol Biochem 83:243–249. https://doi.org/10.1016/j.plaphy.2014.08.012
Watanabe Y, Satomura T, Sasa K, Funada R, Koike T (2010) Differential anatomical responses to elevated CO2 in saplings of four hardwood species. Plant Cell Environ 33:1101–1111. https://doi.org/10.1111/j.1365-3040.2010.02132.x
Wilcox DB, Dove D, Mcdavid D (2002) Greer image tool. University of Texas Health Science Center, San Antonio, San Antonio
Wullschleger SD, Tschaplinski TJ, Norby RJ (2002) Plant water relations at elevated CO2—implications for water-limited environments. Plant Cell Environ 25:319–331. https://doi.org/10.1046/j.1365-3040.2002.00796.x
Xu X, Peng G, Wu C, Korpelainen H, Li C (2008) Drought inhibits photosynthetic capacity more in females than in males of Populus cathayana. Tree Physiol 28:1751–1759. https://doi.org/10.1093/treephys/28.11.1751
Xu Z, Shimizu H, Yagasaki Y, Ito S, Zheng Y, Zhou G (2013) Interactive effects of elevated CO2, drought, and warming on plants. J Plant Growth Regul 32:692–707. https://doi.org/10.1007/s00344-013-9337-5
Xu Z, Shimizu H, Ito S, Yagasaki Y, Zou C, Zhou G, Zheng Y (2014) Effects of elevated CO2, warming and precipitation change on plant growth, photosynthesis and peroxidation in dominant species from North Chinagrassland. Planta 239:421–435. https://doi.org/10.1007/s00425-013-1987-9
Xu Z, Jiang Y, Jia B, Zhou G (2016) Elevated-CO2 response of stomata and its dependence on environmental factors. Front Plant Sci 7:657. https://doi.org/10.3389/fpls.2016.00657
Yan K, Chen W, Zhang G, Xu S, Liu Z, He X, Wang L (2010) Elevated CO2 ameliorated oxidative stress induced by elevated O3 in Quercus mongolica. Acta Physiol Plant 32:375–385. https://doi.org/10.1007/s11738-009-0415-z
Zeppel MJB, Lewis JD, Chaszar B, Smith RA, Medlyn BE, Huxman TE, Tissue DT (2012) Nocturnal stomatal conductance responses to rising [CO2], temperature and drought. New Phytol 193:929–938. https://doi.org/10.1111/j.1469-8137.2011.03993.x
Zhang T, Hu Y, Zhang K, Tian C, Guo J (2018) Arbuscular mycorrhizal fungi improve plant growth of Ricinus communis by altering photosynthetic properties and increasing pigments under drought and salt stress. Ind Crop Prod 117:13–19. https://doi.org/10.1016/j.indcrop.2018.02.087
Zhang Z, Zhang J, Xu G, Zhou L, Li Y (2019) Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Zenia insignis seedlings under drought stress. New For 50:593–604. https://doi.org/10.1007/s11056-018-9681-1
Zhou S, Duursma RA, Medlyn BE, Kelly JWG, Prentice IC (2013) How should we model plant response to drought? An analysis of stomatal and non-stomatal responses to water stress. Agric For Meteorol 182–183:204–214. https://doi.org/10.1016/j.agrformet.2013.05.009
Zinta G, AbdElgawad H, Domagalska MA, Vergauwen L, Knapen D, Nijs I, Janssens IA, Beemster GT, Asard H (2014) Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob Change Biol 20:3670–3685. https://doi.org/10.1111/gcb.12626
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
PY: Running the experiment, laboratory analysis, measuring the data, statistical analysis, writing the draft of the manuscript. BP: Supervisor, Conceptualization, Methodology, Monitoring data analysis, and Original manuscript editing. AHN: Advisor, Tips for drought stress. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest declared.
Additional information
Communicated by Rüdiger Grote.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yousefvand, P., Pilehvar, B. & Nasrolahi, A.H. Morphological, physiological, and biochemical responses of Pistacia atlantica seedlings to elevated CO2 concentration and drought stress. Eur J Forest Res 142, 657–670 (2023). https://doi.org/10.1007/s10342-023-01548-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-023-01548-x