Abstract
Quercus suber L. is an important species producing cork whose wood characteristics have not been investigated a lot. Cork oak wood vessels are a striking feature and the most abundant wood tissue largely influencing density and permeability. Vessel size and distribution were studied in approximately 40 year-old and never debarked cork oaks by continuously measuring along the radial direction in the transverse section of wood discs taken at 1.3 m of height using image analysis techniques. The vessel size increases with age from 7660 ± 2286 to 21136 ± 6119 μm2, the conductive area from 5.4 ± 2.2 to 11.6 ± 3.9%, and the vessel density remains approximately constant between 5.2 ± 1.5 and 7.3 ± 3.5 vessels/mm2. In comparison with ring-porous and some evergreen oaks, cork oaks show a similar conductive area but smaller vessels. Vessel architecture is known to play an important role on oaks tolerance to hydric stress, and these cork oak trees were growing under very harsh edaphoclimatic conditions, not tolerated by other oaks. The well-developed and deep root system allowing access to constant water supply may contribute to the cork oak’s relatively high conductive area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cork oak (Quercus suber L.) is an evergreen oak, native to the maritime countries of Europe and North Africa that surround the western Mediterranean basin (Pereira and Tomé 2004). During the Discoveries Period, in the sixteenth and early seventeenth centuries, cork oak wood was extensively used and very appreciated for boat construction due to its high mechanical resistance and natural durability. Nowadays cork oaks are exclusively oriented towards the production of cork and therefore, research has been mainly focused on issues related to cork characterisation (e.g. Ferreira et al. 2000; Pereira 1988; Pereira and Tomé 2004; Pereira et al. 1996) and production (e.g. Vázquez and Pereira 2005), with only a few recent studies on tree growth and the factors influencing it (e.g. Costa et al. 2001; 2002; Leal and Pereira 2006). Little value is given to the wood and not much has been investigated on the characteristics of the secondary xylem of cork oak. A strong impeditive reason for the study of cork oak wood is that tree harvesting is forbidden by law and sampling has to be done by taking advantage of authorised fellings, which do not always provide material with appropriate quality for research purposes.
Like other oak woods, cork oak wood has a strong aesthetical character due to its particular anatomical features, namely frequent large rays and vessels. These, together with the strength and durability characteristics, make this wood potentially adequate for high value sawn wood products. When the production of high quality products is envisaged, it is essential to study the wood properties, especially the anatomical characteristics which may have a large influence on the processing and product performance (Butterfield 2003).
Anatomical characteristics of wood are also involved in tree growth and its environmental framework. Cork oak trees frequently face harsh environmental conditions of drought and low soil fertility which other oak species would be unable to tolerate (Natividade 1950). They have a well-developed root system in extension and depth that is considered to be one of the main survival strategies during summer drought (Abrams 1990; Costa et al. 2002), as well as physiological mechanisms of protection against the high radiation and temperature occurring during the dry season (Faria et al. 1996; Pereira et al. 1998). Cork oak’s architecture of the wood vessel system in terms of the variations in vessel size and distribution, besides of its technological implications and importance, could also contribute to this species particularly high resistance to drought since it is known that these characteristics are related to oak’s tolerance to water stress (Abrams 1990).
Cork oak wood anatomical descriptions date from the nineteenth century (Coutinho 1886; Mathieu 1887; Pláy Rave 1880) and more recently cell biometric data have been reported in a few studies (Carvalho 1953; 1997; Gaio 1951; García-Esteban and Guíndeo-Casasús 1989; Natividade 1950; Nunes 1998; Wagenführ and Scheiber 1985) but the results are sometimes unclear and inconsistent, and give only average values for the species. The same authors of the present study have reported on the radial variability of wood cells biometry in cork oak focusing on fibres, and uni- and multiseriate rays (Leal et al. 2006). The presence of large multiseriate rays is the most conspicuous characteristic of cork oak wood. Using the same material of young trees growing under especially harsh conditions, we now characterise cork oak wood in relation to the radial variation in dimensions and architecture of vessels, the second most striking feature in cork oak wood and usually the most abundant tissue in hardwoods.
Materials and methods
The study area is situated in the state forest area Perímetro Florestal da Contenda (PFC), located in Beja, southeastern Portugal, with edaphoclimatic conditions that are considered marginal for the growth of cork oaks because of very dry summers, and low soil fertility and water retention capacity (Natividade 1950). The climate is typically Mediterranean, with Atlantic influence, with very dry and hot summers extending from June to September.
The forest area included planted cork oak stands with approximately 40 year-old trees that had not yet entered the cork production cycle, i.e., cork had never been harvested. The surrounding areas were forested with holm oak (Quercus rotundifolia) with the occasional presence of cork oak. Nine healthy cork oak trees were harvested in 2002, taking advantage of authorised fellings. Total tree height varied between 7 and 11 m, and tree diameter at 1.30 m of height between 28 and 38 cm. A more detailed description of site and stands is given in Leal et al. (2006).
One disc per tree was collected at 1.30 m of tree height, from which three radial strips running from pith to bark and free of defects were randomly selected. The transversal surfaces of the radial strips were sanded manually, through three grades of grit up to P400, and rubbed with a common white wax crayon to impregnate the vessels in order to distinguish them from the rest of the tissues under a low power microscope.
Sequential images were taken from the wood cross-sections, using a low power microscope (Olympus SZH10) connected to a video camera (JVC model TK-C1380E), and stored in JPEG graphic format using the image analysis software LEICA Qwin. With a total magnification of 30×, each image covered 2176 μm on the radial direction and 2911 μm on the tangential direction. Images were converted to binary format and, after applying threshold and minimum size settings, vessels were clearly identified as separate objects. Whenever necessary, manual corrections were applied to the image in order to eliminate image elements that could be mistaken for vessels. In each measuring frame (covering the whole image radial length and 1327 μm in the tangential direction) the number of vessels, individual vessel area and vessel location coordinates within the image were automatically recorded.
In ring-porous oaks, high peaks in vessel size correspond to ring boundaries, i.e., a change from small latewood vessels, formed during one growth season, to large early wood vessels, formed at the beginning of the next growth season. However, in cork oak these peaks do not always correspond to ring boundaries and it is in general not possible to accurately identify tree rings (Gourlay and Pereira 1998; Leal and Pereira 2006). Several attempts were made in order to identify rings on the wood (without and with magnification) as well as on graphical representations of the radial variation in vessel size but without reaching sufficient confidence of accuracy. Therefore, another method was used in order to link vessel measurements to tree age. A growth and yield model for cork oak growth at 1.30 m of tree height (Tomé et al. 1999) was used to estimate diameter for each of the sampled trees, at each successive five years of cambial age and a cork oak height and diameter growth model (González et al. 2005) was used to calculate the number of years necessary for each tree to attain 1.30 m of height, assuming that the trees were 40 years-old when sampling was performed. According to the model, cambial age at 1.30 m of tree height varied between 30 and 34 years when the trees were sampled.
Vessels were classified by size into two groups, “large vessels” and “small vessels”, if respectively above or below an average trend line calculated by the linear fitting to each radial profile of vessel area variation. Average values of vessel area (in μm2) both for small and large vessels, density (number of vessels per square millimetre of cross sectional area), and total conductive area (percentage of cross sectional area occupied by vessels) were calculated for each period of 5 years of cambial age.
Descriptive anatomical terminology followed the IAWA List of Microscopic Features for Hardwood Identification (IAWA Committee 1989).
Statistical treatment of data included analysis of variance (ANOVA) and t-tests for comparison of measurements between groups of samples using commercial software.
Results
Cork oak wood shows a semi-ring-porosity. Vessels are solitary, in general with a circular cross-section, and arranged in a radial pattern (Fig. 1). The occlusion of the cork oak vessels with tyloses was frequent. Profiles of radial variation in vessel size show an increasing tendency from pith to bark (Fig. 2). Additionally to this long-term variability trend, there is a large amount of high-frequency variability, shown by the several up and down swings on the graph.
Average vessel area increased from 7660 ± 2286, near the pith, until 21136 ± 6119 μm2, near the bark (Fig. 3a); small vessels showed a linear increasing pattern in the area from pith to bark, changing from 4664 ± 1013 to 12257 ± 3130 μm2 (Fig. 3b); large vessels area varied from 14721 ± 3168 to 32813 ± 8333 μm2 with age (Fig. 3c). Analysis of variance shows that most of the variability in vessel size is explained by cambial age (70% for average vessel area, 74% for small vessels area, and 64% for large vessels area), and a smaller percentage is due to the tree effect (28% for average vessel area, 24% for small vessels area, and 34% for large vessels area); the interaction between both effects and the error component have a minor contribution to the variability.
Average vessel area and average large vessels area began to stabilize from 20 years of cambial age onwards, although this effect is not visible in small vessels. T-tests confirm that the differences of average vessel area and average large vessels area between measurements taken at 20 and 25, 25 and 30, and 30 and > 30 years of cambial age are not statistically significant. Age differences in average small vessels are not statistically significant only between the ages of 30 and > 30 years of cambial age.
Vessel density remained relatively constant with cambial age, ranging between 5.2 ± 1.5 and 7.3 ± 3.5 vessels/mm2 (Fig. 4). The factor tree explains 72% of variability in vessel density, tree age explains 16% of the variability, and the error component 10%, interaction between the two factors have a minor effect. Results from t-tests show no significant differences in average vessel density between the several ages.
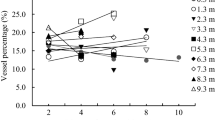
Total conductive area presents a slightly increasing tendency with cambial age from 5.4 ± 2.2, near pith, to 11.6 ± 3.9 %, near bark, flattening out around 20 years of age (Fig. 5). Most of the variability in conductive area (59%) is due to the differences between trees, and cambial age also explains a considerable amount of variability (36%); the interactions between the two effects and the error component contribute little to the variability. The apparent increase observed on the graph is however not statistically significant, and the difference in conductive area is significant only between 5 and 10 years of cambial age.
Discussion
The results show an overall increasing trend in vessel size (Fig. 3), constancy in vessel density (Fig. 4), and a slight tendency for increasing conductive area with cambial age (Fig. 5). The linear tendency of vessels to become larger with cambial age found in this study has already been reported for several species by other authors (Gasson 1987; Voulgaridis 1990). In comparison with other cork oak wood anatomy studies, the present results for large vessels are according to what was reported by Natividade (1950) for early wood but are much smaller than the values pointed out by Garcia-Ésteban and Guíndeo-Casasús (1989). It should be noted that the value given by the last authors refers to the maximum size early wood vessels may achieve and corresponding therefore, to exceptionally large vessels. None of the authors has mentioned whether their values refer to juvenile or mature wood. In the present study the small vessels are larger than what was pointed out by Natividade (1950) for latewood, probably corresponding to exceptionally small vessels and not to the usual latewood vessels size. Average vessel area values are in agreement with the results from Nunes (1998).
In comparison with other evergreen oak species studied by Voulgaridis (1990), the vessel size in cork oak is close to the values reported for Q. coccifera and smaller than those for Q. ilex. In relation to some deciduous oaks, cork oak large vessels are smaller than early wood vessels in Q. conferta (Voulgaridis 1990) and Q. robur (Wagenführ and Scheiber 1985). On the other hand, when comparing our results with the ones from Huber (1993), who distinguishes between juvenile and mature vessels in Q. robur and Q. petraea, cork oak vessels only reach sizes similar to those in juvenile wood. Our results are in agreement with an on-going cork oak wood anatomy study (Barij et al. 2004) and do not show as well the usual age-related decreasing pattern in vessel density that is common for other species (Helińska-Raczkowska 1994).
The combination of vessel area and density allows the calculation of the area covered by vessels or the conductive area of the stem cross-section, which has been cited as significant in determining rates of conduction (Carlquist 1984; Salleo et al. 1985). Comparative studies of deciduous and evergreen Quercus species show a higher sap velocity for deciduous oaks (Borghetti et al. 1992). The values for conductive area are in agreement with the results from Nunes (1998) The comparison with other oaks shows that the conductive area of the cork oak is within the values found in the latewood of Q. robur and Q. castaneifolia (Parsa Pajouh 1990; Wagenführ and Scheiber 1985) as well as in Q. alba juvenile wood, when considering an average for latewood and early wood (Phelps and Workman 1994).
Technological implications of vessels size and distribution
Wood density is the most studied wood property and it is pointed out by some authors as the most determinant property for wood quality. Assuming a constant cell wall density, the wood density will be mainly determined by the voids in the wood mass, i.e., by the size of the wood cells (Saranpää 2003). The size and density of vessels have therefore a major effect on wood density and tend to be inversely proportional to this property (Savidge 2003). The lack of radial variation of vessels size and the comparatively small vessel areas are in relation with the particular high density of cork oak wood and its reduced variation within the tree (Knapič et al. 2006).
Vessel size is also related to permeability because it is the principal flow path for fluids in hardwoods. Wood permeability is an especially important property for the impregnation with, for example, preservatives for protection against decay. It also affects the ease of diffusion of water to or from the wood during the drying process or, while in use, in response to changes in air relative humidity (Siau 1995). Cork oak wood vessels are often occluded with tyloses that can reduce permeability.
Biological importance of cork oak vessel architecture
Cork oak wood has conductive areas similar to other oaks but provided by smaller vessels, in comparison with deciduous oaks and some evergreen oaks that do not decrease in density with age. The cork oak trees used for this study experienced rather extreme growth conditions while growing in a site that is considered marginal for the species, even if in general cork oaks are very well adapted to water stress, and are able to grow in poor soils and under very dry conditions (Natividade 1950). We therefore hypothesise that cork oak vessel architecture may be a response of these trees to water stress. The xylem anatomy of several oaks is adapted to xeric environments because their small latewood vessels allow a reduced but sustained water movement during drought periods (Abrams 1990). In large vessels water moves with a minimum of hydraulic resistance (Zimmermann 1977) allowing rapid rates of sap movement, while small vessels are less sensitive to cavitation than large ones (Tyree and Dixon 1986). Unfavourable conditions for tree growth may result in a decrease in vessel size and an increase in their density (Baas 1982; Carlquist 1988; Over van den et al. 1981). It has been reported that vessel size in oaks is influenced by precipitation (Eckstein and Frisse 1979; Woodcock 1989; García-González and Eckstein 2003). The same trees used in this study have already been reported to show constancy in wood ray’s size with cambial age, which was interpreted as a way to increase water storage (Leal et al. 2006). However, this hypothesis is speculative and the results need to be complemented with further studies.
For the present study no physiological data on water transport were collected but these will be considered in further studies. Such data are very important to understand the way cork oak trees use the water resources (Oliveira et al. 1992; David et al. 2006) and could complement the results presented in this study by bringing new insight on the trees mechanisms to survive in conditions of limited water availability.
Conclusions
Our results show an overall increasing trend in vessel size with cambial age, common in other species; constancy in vessel density, not usual in other species; and a slight increase in conductive area with age. The conductive area is similar to other oaks but it is provided by smaller and denser vessels, in comparison with ring-porous and some evergreen oaks. The size and density of vessels have a major effect on wood density. The comparatively small vessel areas in cork oak together with the absence of radial variation in vessel distribution explain the particular high wood density and its reduced variation within the tree, reported by other authors. The well developed and deep rooting system, existing in mature trees, allows access to constant water supply and may contribute for the cork oak’s relatively high conductive area.
References
Abrams MD (1990) Adaptations and responses to drought in Quercus species of North America. Tree Physiol 7:227–238
Baas P (1982) Systematic, phylogenetic, and ecological wood anatomy – history and perspectives. In: Baas P (ed) New perspectives in wood anatomy. Martinus Nijhoff/Dr. W. Junk Publ, Hague, pp 23–58
Barij N, Stokes A, Cermak J (2004) Influence of xylem anatomy on water efficiency through stems: xylem structure in stems of Quercus suber. In: International symposium on wood sciences, 24–29 Oct. 2004, Montpellier, pp 5
Borghetti M, De Angelis P, Raschi A, Scarascia Mugnozza G, Valentini R (1992) Relations between sap velocity and cavitation in broadleaved trees. In: Borghetti M, Raschi A, Grace J (eds) Global changes and plant water relations. Cambridge University Press, Cambridge, pp 114–128
Butterfield BG (2003) Wood anatomy in relation to wood quality. In: Barnett JR, Jeronimidis G (eds) Wood quality and its biological basis. Blackwell, Oxford, pp 30–49
Carlquist S (1984) Wood and stem anatomy of Lardizabalaceae, with comments on the vinning habitat, ecology and systematics. Bot J Linn Soc 88:257–277
Carlquist S (1988) Comparative Wood Anatomy. Springer, Berlin Heidelberg New York
Carvalho A (1953) Madeiras de Folhosas – Contribuição para o seu Estudo e Identificação. Graduation Thesis, Instituto Superior de Agronomia, Lisboa
Carvalho A (1997) Madeiras Portuguesas – Estrutura Anatómica, Propriedades e Utilizações. Vol II, Direcção-Geral das Florestas, Lisboa
Costa A, Pereira H, Oliveira A (2001) Dendroclimatological approach to diameter growth in cork oak adult trees under cork production. Trees 15:438–443
Costa A, Pereira H, Oliveira A (2002) Influence of climate on the seasonality of radial growth of cork oak during a cork production cycle. Ann For Sci 59:429–437
Coutinho AXP (1886) Curso de Silvicultura – 1 Botânica Florestal, Typografia da Academia Real das Sciencias de Lisboa, Lisboa
David TS, Henriques MO, Kurz-Besson C, Nunes J, Valente F, Vaz M, Pereira JS, Siegwolf R, Chaves MM, Gazarini LC, David JS (2006) Water use strategies in two co-ocurring Mediterranean evergreen oaks: surviving the summer drought. Tree Physiol (in press)
Eckstein D, Frisse E (1979) Environmental influences on the vessel size of beech and oak. IAWA Bull 2/3:36–37
Faria T, García-Plazaola JI, Abadía A, Cerasoli S, Pereira JS, Chaves MM (1996) Diurnal changes in photoprotective mechanisms in leaves of cork oak (Quercus suber L.) during summer. Tree Physiol 16: 115–125
Ferreira A, Lopes F, Pereira H (2000) Caractérisation de la croissance et de la qualité du liège dans une région de production. Ann For Sci 57:187–193
Gaio FVL (1951) A Madeira de Sobreiro na Produção da Celulose Industrial, Graduation Thesis, Instituto Superior de Agronomia, Lisboa
García-Esteban L, Guíndeo-Casasús A (1989) Anatomía de las Maderas Afrondosas Españolas, AITIM, Madrid
García-González I, Eckstein D (2003) Climatic signal of earlywood vessels of oak on a maritime site. Tree Physiol 23:497–504
Gasson P (1987) Some implications of anatomical variations in wood of pedunculate oak (Quercus robur L.), including comparison with common beech (Fagus sylvatica L.). IAWA Bull 8:149–166
González MS, Tomé M, Montero G (2005) Modelling height and diameter growth of dominant cork oak trees in Spain. Ann For Sci 62:633–644
Gourlay ID, Pereira H (1998) The effect of bark stripping on wood production in cork oak (Quercus suber L.) and problems of growth ring definition. In: Pereira H (ed) Cork oak and cork, Centro de Estudos Florestais, Lisboa, pp 99–107
Helińska-Raczkowska L (1994) Variation of vessel lumen diameter in radial direction as an indication of the juvenile wood growth in oak (Quercus petraea Liebl). Ann Sci For 51:283–290
Huber F (1993) Déterminisme de la surface des vaisseaux du bois des chênes indigènes (Quercus robur L., Quercus petraea Liebl) – effect individuel, effect de l’appareil foliaire, des conditions climatiques et de l’âge de l’arbre. Ann Sci For 50:509–524
IAWA Committee (1989) IAWA list of microscopic features for hardwood identification, with an appendix on non-anatomical information. IAWA Bull 10:219–332
Knapič S, Louzada JL, Leal S, Pereira H (2006) Radial variation of wood density components and ring width in cork oak trees. Ann For Sci (in press)
Leal S, Pereira H (2006) Influence of cork removal and precipitation on ring width and vessel characteristics of Quercus suber L. Eur J For Res (submitted)
Leal S, Sousa VB, Pereira H (2006) Variability of cell biometry in the wood of cork oak (Quercus suber L.). Wood Sci Technol 40(7):585–597
Mathieu A (1887) Flore Forestière. Berger-Levrault, Paris
Natividade JV (1950) Subericultura. Direcção-Geral dos Serviços Florestais e Aquícolas, Lisboa
Nunes EMC (1998) Estudo da influência da precipitação e temperatura no crescimento juvenil de Quercus suber L. através da análise dos anéis anuais de crescimento. Ms D Thesis, Instituto Superior de Agronomia, Lisboa
Oliveira G, Correia OA, Martins-Loução MA, Catarino FM (1992) Water relations of cork-oak (Quercus suber L.) under natural conditions. Vegetatio 99–100:199–208
Over van den L, Baas P, Zandee M (1981) Comparative wood anatomy of Symplocos and latitude of provenance. IAWA Bull 2:3–24
Parsa Pajouh D (1990) Wood anatomy of three broadleaved species native to the Caspian forest of Iran. IAWA Bull 11:134
Pereira H (1988) Chemical composition and variability of cork from Quercus suber L. Wood Sci Technol 22:211–218
Pereira H, Tomé M (2004) Cork Oak. In: Burley J, Evans J, Youngquist JA (eds) Encyclopedia of forest sciences. Academic Press, pp 613–620
Pereira H, Lopes F, Graça J (1996) The evaluation of the quality of cork planks by image analysis. Holzforschung 50:111–115
Pereira JS, Faria T, Chaves MM (1998) Impacts of climate change and elevated CO2 on the physiology and survival of cork-oak (Quercus suber L.). In: Pereira H (ed) Cork oak and cork Centro de Estudos Florestais, Lisboa, pp 182–191
Phelps JE, Workman ECJr (1994) Vessel area studies in white oak (Quercus alba L.). Wood Fiber Sci 26:315–322
Pláy Rave E (1880) Tratado de las Maderas de Construcción Civil y Naval. Imprenta Estereotipica y Galvanoplastia de Aribau y Ca (Sucesores de Rivadereyra), Madrid
Salleo S, Lo MA, Gullo F, Oliveri F (1985) Hydraulic parameters measured in 1-year-old twigs of some Mediterranean species with diffuse-porous word: changes in hydraulic conductivity and their possible functional significance. J Exp Bot 36:1–11
Saranpää P (2003) Wood density and growth. In: Barnett JR, Jeronimidis G (eds) Wood quality and its biological basis. Blackwell, Oxford pp 87–117
Savidge RA (2003) Tree growth and wood quality. In: Barnett, JR, Jeronimidis G (eds) Wood quality and its biological Basis. Blackwell, Oxford, pp 1–29
Siau JF (1995) Wood: Influence of Moisture on Physical Properties. Department of Wood Science and Forest Products, Virginia Polytechnic Institute and State University
Tomé M, Coelho MB, Pereira H, Lopes F (1999) A management oriented growth and yield model for cork oak and cork oak stands in Portugal. In: Amaro A, Tomé M (eds), Empirical and process-based models for forest tree and stand growth simulation. Edições Salamandra, Lisboa, pp 189–271
Tyree MT, Dixon MA (1986) Water stress induced cavitation and embolism in some woody plants. Physiol Plant 66:397–405
Vázquez J, Pereira H (2005) Distance dependent and distance independent models to estimate tree cork weight in Portugal. For Ecol Manag 213:117–132
Voulgaridis E (1990) Wood cell morphology characteristics of some oak species and Mediterranean schrubs. Holz Roh- Werkst 48:261–267
Wagenführ R, Scheiber C (1985) Holzatlas. VEB Fachbuchverlag. Leipzig
Woodcock DW (1989) Climate sensitivity of wood-anatomical features in a ring-porous oak (Quercus macrocarpa). Can J For Res 19:639–644
Zimmermann MH, Brown CL (1977) Trees Structure and Function. Springer, Berlin Heidelberg New York
Acknowledgments
This study is part of the European project Suberwood (QLRT 2000-0701). The first author acknowledges additional funding granted by the Portuguese Science Foundation (FCT) under the programme POCI – 2010 Formação Avançada para a Ciência – Medida IV.3. We wish to thank Sofia Knapič and Joana Paulo for providing help during the fieldwork.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leal, S., Sousa, V.B. & Pereira, H. Radial variation of vessel size and distribution in cork oak wood (Quercus suber L.). Wood Sci Technol 41, 339–350 (2007). https://doi.org/10.1007/s00226-006-0112-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-006-0112-7