Abstract
Wood structure might be altered through the physiological responses to atmospheric carbon dioxide concentration ([CO2]) and nitrogen (N) deposition. We investigated growth, water relations and wood structure of 1-year-old seedlings of two deciduous broad-leaved tree species, Quercus mongolica (oak, a ring-porous species) and Alnus hirsuta (alder, a diffuse-porous species and N2–fixer), grown under a factorial combination of two levels of [CO2] (36 and 72 Pa) and nitrogen supply (N; low and high) for 141 days in phytotron chambers. In oak, there was no significant effect of [CO2] on wood structure, although elevated [CO2] tended to decrease stomatal conductance (g s) and increased water use efficiency regardless of the N treatment. However, high N supply increased root biomass and induced wider earlywood and larger vessels in the secondary xylem in stems, leading to increased hydraulic conductance. In alder, there was significant interactive effect of [CO2] and N on vessel density, and high N supply increased the mean vessel area. Our results suggest that wood structures related to water transport were not markedly altered, although elevated [CO2] induced changes in physiological parameters such as g s and biomass allocation, and that N fertilization had more pronounced effects on non-N2-fixing oak than on N2-fixing alder.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because of increasing atmospheric carbon dioxide concentrations ([CO2]) caused by combustion of fossil fuels and enhanced nitrogen (N) deposition by human activities, it is predicted that forest dynamics will change, especially in the regions of middle and high latitudes in northern hemisphere (e.g., Bucher et al. 1998; Oren et al. 2001). It is urgent to evaluate not only forest dynamics but also wood properties, because forested trees are expected to be sinking organs for CO2. However, there is little information on changes in wood properties induced by elevated [CO2] and N deposition (Kostiainen et al. 2004).
Wood structure might be altered through the physiological responses to elevated [CO2]. In general, it has been reported that elevated [CO2] reduced stomatal conductance (g s) and transpiration (T r) and enhanced water use efficiency (WUE) in trees grown in phytotron chambers and under field conditions (e.g., Ceulemans and Mousseau 1994; Saxe et al. 1998; Urban 2003; Ainsworth and Rogers 2007). Elevated [CO2] also enhanced total biomass and root:shoot ratio in many tree species (Saxe et al. 1998). These physiological and morphological changes will induce change not only in the internal water balance, but also in the anatomical features of conduit cells such as tracheids and vessel elements in secondary xylem.
A few studies have addressed changes in wood structure caused by elevated [CO2] (Telewski et al. 1999; Yazaki et al. 2005). Atkinson and Taylor (1996) showed that elevated [CO2] increased vessel diameters in the ring-porous wood of Quercus robur seedlings. On the other hand, no obvious effect was found in diffuse-porous woods including that of Populus tremuloides (Kaakinen et al. 2004) and Betula pendula (Kostiainen et al. 2006), whereas the vessel lumen area increased significantly in seedlings of Quercus ilex under high [CO2] (Gartner et al. 2003).
Increasing N deposition would also affect the physiological characteristics of forest trees and stimulate wood production (e.g., Ericsson et al. 1996; Wallace et al. 2007). Some investigations showed that combination of high [CO2] and high N fertilization stimulated biomass allocation such as increased aboveground biomass (e.g., Tognetti and Johnson 1999) and enhanced root biomass (e.g., Oh and Choung 2005). Furthermore, it has been reported that N2-fixing species respond differently from non-N2-fixing species under elevated [CO2], because they have N2-fixing microorganisms Frankia in their root system (e.g., Tobita et al. 2005). For N-limited boreal forest, N2-fixing species are important because of their N2 fixation ability, and elevated [CO2] can increase their rate of N2 fixation (Hibbs et al. 1995; Vogel et al. 1997). However, increasing N deposition would have strong influences on the activity of N2-fixing species, and a combination of elevated [CO2] and N deposition will influence physiological and morphological traits of N2-fixing species.
The effects of [CO2] on the wood of forest trees and with nitrogen acting in combination have been investigated (e.g., Hättenschwiler et al. 1996; Yazaki et al. 2001; Kostiainen et al. 2004). Elevated [CO2] and N-fertilization altered the dimensions of xylem vessels and wood fibers in a genotype-specific manner in three species of Populus (Luo et al. 2005). However, little information is available regarding the effects of the changing environment on wood formation (Kirschbaum 2004; Yazaki et al. 2004, 2005). Therefore, we studied combined effects of [CO2] and nitrogen on wood formation and water relations in tree species in northern Japan.
We studied oak and alder seedlings under strictly controlled conditions in a phytotron, because young seedlings are most responsive to [CO2] (Saxe et al. 1998; Medlyn et al. 2001) and may be indicators of wood quality in mature trees (Yazaki et al. 2001). Oak, a ring-porous tree lacking the ability for N2 fixation, was expected to be more sensitive to nutrient limitations and show more severe changes in wood structure than alder, a diffuse-porous tree and N2 fixer. We tested this prediction in oak and alder seedlings raised under factorial combinations of elevated [CO2] and nitrogen.
Material and methods
Plant materials
One-year-old seedlings of oak [Quercus mongolica Fisch. ex Ledeb. var. crispula (Blume) Ohashi] and alder (Alnus hirsuta Turcz.) obtained from Oji Forestry and Landscaping, Sapporo, Japan, were cultured in 5-l pots filled with 1:1 (v/v) Kanuma pumice and clay loam, and grown in a natural daylight phytotron in spring 2003. The initial height of seedlings was 14–18 cm in both species.
Both species are representative deciduous broad-leaved trees in boreal forests of northeast Asia; the sensitivity to environmental stressors is similar between seedlings and adult trees (Koike 1988). Alnus hirsuta is an actinorhizal N2-fixer that may contribute significant amounts of N to N-limited temperate forest ecosystems (Dawson 1983; Tobita et al. 2005). The patterns of water transport in the stem are different in oak and alder, which produce ring-porous wood and diffuse-porous wood, respectively (Chaney and Kozlowski 1977; Utsumi et al. 1998, 1999).
Treatments
Two natural daylight phytotron chambers (Koito Industries, Yokohama, Japan) equipped with [CO2] controllers (DAIWA Air Co. Ltd, Sapporo, Japan) were used as described previously (e.g., Koike 1995; Tobita et al. 2005). Batches of six seedlings (48 seedlings per species) were grown for up to 141 days at 36 Pa (ambient) or 72 Pa (elevated) CO2 from mid-May. Seedlings were supplied with N at 52.5 mg N pot−1 week−1 (high-N) or 5.25 mg N pot−1 week−1 (low-N) in 0.5 × Hoagland solution (Asher and Edwards 1983; 3 mM KNO3, 2 mM Ca(NO3)2/4 H2O, 0.5 mM NH4H2PO4, 1 mM MgSO4/7 H2O, 25 μM EDTA-Fe, 4.5 μM MnCl2/4 H2O, 23 μM H3BO3, 0.4 μM ZnSO4/7 H2O, 0.15 μM CuSO4/5 H2O, 0.007 μM (NH4)6Mo7O24/4 H2O). In the low-N treatment, KCl, CaCl2 and KH2PO4 were added to provide the same concentrations of K+ and Ca2+ as in high-N tests. Air temperature was maintained at 26/16°C (day/night). Pots were kept in trays with water to avoid desiccation.
Gas-exchange
Leaf gas exchange measurements were made on six mature leaves of each treatment on days 112–114 (Q. mongolica) and on days 93–100 (A. hirsuta). The age of the leaves was about 60 days (Q. mongolica) and 21 days (A. hirsuta). In alder, the sixth and the eighth leaves, counted from the tip of leader shoots, were used, whereas in oak, secondary flushed leaves produced during the experimental period were examined.
Light-saturated net photosynthetic rates per leaf area (A sat), stomatal conductance (g s), and transpiration rate (T r) of mature leaves were determined using an open gas exchange system (LI-6400; Li-Cor Inc., Lincoln, NE, USA) at both 36 and 72 Pa CO2. Saturating photon flux density (PFD) at the upper leaf surface was 1,200 μmol m−2 s−1, as determined based on light response curves of photosynthetic rates (data not shown). Leaf temperature was maintained at 25°C and the relative humidity within the measuring chamber was kept at 70%. Instantaneous WUE (A sat per T r) and leaf mass per area (LMA) of leaves used for gas-exchange measurements were determined.
Growth and biomass allocation
After 141 days, 6 seedlings of each treatment were harvested, and dry masses of organs (leaf, shoot, stem and root) as well as total leaf area (LA) were determined. The LA ratio (LAR; LA per total biomass) and top-to-root ratio (T/R ratio; aboveground biomass per root biomass) of each seedling were calculated.
Wood anatomy
We took wood samples from stem bases after harvesting and fixed them in 4% FAA (formaldehyde, acetic acid and 70% ethanol) immediately. Thick transverse sections (15 μm thickness) were cut with a sliding microtome, stained with safranin (1%, w/v) for 10 min and mounted permanently.
The sections were photographed using a light microscope (Axioskop 2 plus, Zeiss, Oberkochen, Germany) with a 10× objective equipped with a digital camera (Nikon digital sight, Nikon, Tokyo, Japan). Five micrographs (the resolution of a micrograph: 1,280 × 960 pixel) were obtained from the outermost (=the most recently formed) annual ring of secondary xylem in each section. We measured vessel lumen area using the Image J software (National Institute of Health, Bethesda, MD) and calculated the total vessel area, proportion of vessel area per total micrograph area (about 2.8 mm2), mean vessel area for one vessel and number of vessels per square millimeter (mm2) on the micrograph. To distinguish vessels from other cell types in the automated analysis, we determined the minimum vessel lumen area and used it as a threshold value; cells with smaller lumen areas were not included in our analysis (oak, 1,256 μm2 lumen area; alder, 314 μm2 lumen area). This decision was made according to the frequency distribution of porous areas in all annual rings of one sample (data not shown).
In oak, vessel elements were selected from earlywood formed in the same year. In alder, vessel elements were selected from the central part of the annual ring, because average vessel sizes in diffuse-porous woods decrease from earlywood to latewood. Frequencies of vessel sizes were determined in all samples. To evaluate the hydraulic conductance of the wood sampled, a “hydraulic mean” vessel diameter, calculated using the assumption that hydraulic conductance was proportional to diameter raised to fourth power as predicted by the Hagen–Poiseuille law, was computed from data of vessel lumen area (Sperry and Ikeda 1997).
Statistical analysis
Two-way analysis of variance (ANOVA) for split-plot designs was used to evaluate the effects of [CO2] and N-treatments on physiological properties (A sat, g s, T r, WUE), growth properties (total biomass, LA, LAR, T/R ratio, stem basal diameter, LMA) and wood properties (total vessel area, mean vessel area, vessel proportion, vessel density, vessel size frequency, “hydraulic mean” vessel diameter) at the probability level P < 0.05, using JMP (SAS Institute 2003). The probability level P < 0.15 was considered to indicate a trend.
Results
Effects of elevated [CO2] and N supply on photosynthesis and water relations
In oak, stomatal conductance (g s) and transpiration (T r) were slightly, but not significantly, decreased under elevated [CO2] as compared to ambient [CO2] (Table 1). Elevated [CO2] enhanced light-saturated net photosynthetic rates per LA, in particular in high-N plants (Table 1). There was a significant [CO2]-dependent promotion of the instantaneous WUE (Table 1). g s and T r in high-N plants were significantly higher than in low-N plants, but WUE was not significantly affected by N supply (Table 1).
Alder showed similar statistically insignificant tendencies for g s in response to elevated [CO2] (Table 2). Elevated [CO2] enhanced A sat, whereas there were no significant [CO2] effects on T r and WUE. No significant N effects were found on g s, T r and WUE in alder (Table 2). No significant interactive effects of [CO2] and N on g s, T r and WUE were detected in oak (Table 1) and alder (Table 2).
Effects of elevated [CO2] and N supply on biomass allocation
In oak, there were no significant effects of [CO2] on total LA and T/R ratio, whereas LAR tended to decline under elevated [CO2]. There were significant effects of N on LA, LAR and the T/R ratio (Table 1), and also significant interactive effects of [CO2] and N on stem basal diameter and total biomass (Table 1). High N treatment enhanced total biomass in both [CO2] treatments after 141 days, whereas high-N plants showed significantly increased total biomass under elevated [CO2]. LA, T/R ratio, basal stem diameter and total biomass increased with increased N supply. There was a significant CO2-induced increase of LMA in low-N plants (Table 1); a similar trend occurred in high-N plants.
In alder, high-N plants showed high LA at elevated [CO2] (Table 2). No significant [CO2] effects on LAR and T/R ratio were found in any N treatment. Stem basal diameter tended to be larger under elevated [CO2], in particular in high-N plants (Table 2). Total biomass accumulation under elevated [CO2] was stimulated (Table 2). Stem diameter and total biomass under elevated [CO2] and low-N tended to be larger than that at ambient [CO2] in alder but not in oak. Elevated [CO2] tended to increase LMA in both N treatments. Higher N supply stimulated total biomass, but no significant interactive effects of [CO2] and N on biomass became evident.
Effects of elevated [CO2] and N supply on wood anatomy
Transverse sections of secondary xylem of oak seedlings were analyzed (Fig. 1). Oak produces ring-porous wood, but young seedlings showed semi-ring-porous traits. Under high-N treatment (Fig. 1a, b), earlywood regions were wider than under low-N seedlings (Fig. 1c, d) and larger vessels appeared.
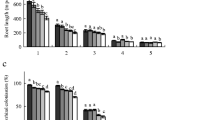
In oak, total vessel area and the mean vessel area were not significantly influenced by [CO2] in any N treatment, while N increased both the total vessel area and the mean vessel area (Fig. 2). No interactive effects of [CO2] and N were detected.
In alder, there was significant interactive effect of [CO2] and N on vessel density, and there were also trends of interaction between [CO2] and N on total vessel area and vessel proportion (Fig. 3). Total vessel area and vessel proportion tended to be larger under elevated [CO2] at high N. There was a significant positive effect of N on mean vessel area (Fig. 3).
Total vessel area, mean vessel area, vessel proportion and vessel density (per mm2) of Alnus hirsuta seedlings. Values shown are means + SE (n = 5–6). H, high-N; L, low-N; 36, ambient-[CO2]; 72, elevated-[CO2]. Results of two-way ANOVA are also shown (n.s. not significant). Actual P values are shown when P < 0.15
We analyzed vessel size frequency to clarify effects of [CO2] and N (Fig. 4). No effect of elevated [CO2] was shown in vessel size frequency in both species, except class A (<1,000 μm2) in alder. In oak, the size frequency was higher in high-N than in low-N plants in class D (3,000–4,000 μm2), E (4,000–5,000 μm2) and F (>5,000 μm2). In alder, N supply increased vessel size frequency in class C (2,000–3,000 μm2).
Vessel frequency in Quercus mongolica and Alnus hirsuta seedlings. Values shown are means ± SE (n = 5–6). H, high-N; L, low-N; 36, ambient-[CO2]; 72, elevated-[CO2]. Results of two-way ANOVA are also shown. Actual P values are shown when P < 0.15. No significant interactive effects of [CO2] and N were found in any of the classes (at P < 0.15)
“Hydraulic mean” vessel diameters in oak showed significant effects of N supply but not of [CO2] (Fig. 5). In alder, high-N plants had a tendency to increase the “hydraulic mean” vessel diameter, but none of the differences observed were significant (Fig. 5).
Discussion
Reductions in g s and T r, and enhanced WUE induced by elevated [CO2] will ameliorate the internal water balance in plants (e.g., Tyree and Alexander 1993). Furthermore, elevated [CO2] enhances whole-plant biomass, in particular root biomass and LA (Ceulemans and Mousseau 1994; Saxe et al. 1998). In this study, we examined whether CO2-dependent changes in water balance and water allocation patterns correlated with modified wood structure in the stem of two hardwood species under doubled [CO2], 72 Pa, which is predicted to be reached by the end of this century as an effect of fossil fuel burning and land cover change (Houghton et al. 2001).
The physiological properties except A sat (g s, T r and WUE) of oak seedling were affected by elevated [CO2] similarly in both N treatments, and the seedlings grown under elevated [CO2] tended to reduce T r and enhance WUE (Table 1). These results agree with those for Quercus alba (e.g., Norby et al. 1995), Q. robur (e.g., Heath and Kerstiens 1997) and Q. mongolica (Oh and Choung 2005). Though Norby et al. (1995) pointed out that increased LA under high [CO2] results in a decrease of g s, which helps to balance water supply and demand in Q. alba, LA of oak seedlings was unaffected by elevated [CO2] in this study (Table 1). The [CO2]-induced physiological effects we observed in Q. mongolica were not matched by any significant changes of wood structure related to water transport (Fig. 2). In contrast, Atkinson and Taylor (1996) found that, in Q. robur seedlings, total stem vessel area, mean vessel area and vessel number increased significantly under elevated [CO2]. Because we measured vessel lumen area in earlywood formed at about 2 weeks after CO2 exposure, it is possible that physiological changes had not affected wood structure yet.
The effects of elevated [CO2] on the morphological properties except LA of oak seedlings were different between N treatments (Table 1). With high N supply, elevated [CO2] enhanced total biomass, in particular root biomass indicated by lower T/R ratio compared to ambient [CO2] (Table 1), which is in accord with a previous study (Oh and Choung 2005). Similarly, seedlings of Q. virginiana treated with high [CO2] combined with high N levels enhanced total biomass, in particular height and stem diameter (Tognetti and Johnson 1999). On the other hand, an imbalance of other nutrient elements to N could be a reason for the observed T/R ratios (Ericsson et al. 1996).
Increased biomass allocation to roots in high-N seedlings grown under elevated [CO2] might result in increased water and nutrient uptake. Increased N supply had stronger effects on vessel formation than elevated [CO2] and induced larger vessels in wider earlywood region in oak (Figs. 1, 2, 4). Consequently, the “hydraulic mean” vessel diameter increased, suggesting increased hydraulic conductance in the stem (Fig. 5). In oak, combined effects of elevated [CO2] and nitrogen supply might enhance water uptake and transport, but larger vessels also affect wood quality in oak (Savill and Mather 1990).
In alder, there was no interactive effect of [CO2] and N on the physiological properties, and the seedlings grown under elevated [CO2] enhanced A sat and tended to reduce g s (Table 2). In contrast, Hibbs et al. (1995) showed that elevated [CO2] enhanced g s and WUE in well-watered Alnus rubra seedlings. In our study, responses to elevated [CO2] in terms of biomass allocation, LA, stem basal diameter and total biomass differed between N treatments (Table 2). The enhanced total biomass and stem basal diameter with unchanged LA observed at low N appears to indicate changes in the internal water balance. At high N, elevated [CO2] enhanced both LA and total biomass and stem basal diameter.
In alder, interactive effects of [CO2] and N causes changes in wood properties, such as total vessel area, vessel proportion and vessel density. Low-N plants tended to decrease total vessel area, because vessel density tended to decrease without change in mean vessel area under elevated [CO2] (Fig. 3). The observed imbalance between increased stem diameter (water supply) and unchanged LA (water demand) is in line with the decreased vessel density.
On the other hand, high-N plants of alder tended to increase total vessel area due to increased vessel density at elevated [CO2], and increased N supply induced larger mean vessel area (Fig. 3). These results imply an increased hydraulic conductance under high-N (Fig. 5). In alder, we measured vessel lumen area in the central parts of annual rings formed in the same year. Therefore, changes in biomass allocation caused by interaction between elevated [CO2] and N supply, i.e., increased stem diameter and unchanged LA at low N and increased stem diameter and LA at high N, cause an inner water balance, resulting in the altered wood structure.
Because earlywood vessels in ring-porous woods are the main determinants of water transport capacity (Chaney and Kozlowski 1977; Utsumi et al. 1999), they may show responses to elevated [CO2] in the long term. In our study, however, oak seedlings grown under elevated [CO2] showed no short-term effects. CO2 exposure might induce changes in wood structure following alterations in the internal water balance in oak. However, because vessels formed in diffuse-porous wood remain functional for several years (Chaney and Kozlowski 1977; Utsumi et al. 1998), structural changes of wood tissue may not occur immediately after the onset of CO2 exposure. In alder (A. hirsuta), there were interactive effects of [CO2] and N on wood structure related to water transport (Fig. 3). No significant changes in mean vessel size and total vessel area were found in seedlings of Prunus avicum × pseudocerasus (Atkinson and Taylor 1996) and Betula pendula (Kostiainen et al. 2006) grown under elevated [CO2]. On the other hand, Gartner et al. (2003) showed that Q. ilex grown under elevated [CO2] developed a higher proportion of large-diameter vessels and a greater mean vessel area than plants grown under ambient [CO2]. Enrichment of [CO2] led to wider vessels in Populus nigra and Populus × euramericana but not in Populus alba (Luo et al. 2005). These discrepancies might be due to different durations of CO2 exposure or specific sensitivities to [CO2] stimulation in different species. Our study suggests that diffuse-porous woods might regulate vessel size and/or numbers in response to changes in the internal water balance induced by elevated [CO2] and N supply.
Both oak and alder seedlings showed an increase in mean vessel area in response to increased N supply (Figs. 2, 3). Cooke et al. (2003) indicated that N-dependent changes in gene expression will alter the partitioning of C and N and subsequently influence wood quality and the quantity of materials provided for the biosynthesis of cell wall components. Therefore, changes in wood structure such as wider earlywood and an increased number of large vessels in oak, as well as an increase in mean vessel area in alder, may be due to altered gene expression patterns under enhanced N supply. Quercus mongolica is a gap-dependent species and probably responds to nutrient conditions. On the other hand, alder is an N2-fixing species. At low N supplies, A. hirsuta forms large amount of root nodules; photosynthates may be allocated to nodulated roots to support the activity of the Frankia symbionts in the nodules (Tjepkema 1985). At high-N, plants may preferentially take up N provided as fertilizer, reducing the demand for photosynthates. It is likely that these different allocation patterns affect cambial activity, leading to altered wood structure.
This study conducted short-term CO2 exposure in controlled environments using phytotron chambers with 1-year-old seedlings in pots. Since the exposure techniques used for CO2 enrichments are likely to alter the environment surrounding the seedlings (e.g., Arp 1991; Ainsworth and Rogers 2007), it will be impossible to scale the seedling responses of our study to whole trees and forest stands under natural conditions as previously suggested (Körner 1995; Nowak et al. 2004). However, the controlled environments should be useful to clear the interactive effects of elevated [CO2] and N supply on the physiological properties, such as stomatal conductance and biomass allocation, and wood properties, because the other environmental factors will be removed during experiments. In addition, it is important to determine the characteristic response of each species to elevated [CO2] and N deposition, because the responses to global climate changes depend on species (Nowak et al. 2004). Therefore, our results might contribute an indicator on wood anatomical features of trees in the near future.
Conclusion
Nitrogen deposition affects wood structure more strongly than elevated [CO2]. Combinatory effects of [CO2] and N enhance total biomass, but the increase in the proportion of larger vessels decreases wood quality in oak and alder, irrespective of the ability to fix N2 of the latter. Changes in wood properties would affect CO2 sink ability of forest trees.
References
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30:258–270
Arp WJ (1991) Effects of source–sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ 14:869–875
Asher CJ, Edwards DG (1983) Modern solution culture techniques. In: Läuchli A, Bieleski RL (eds) Inorganic plant nutrition. Encyclopedia of plant physiology, NS. vol 15A. Springer, Berlin, pp 94–119
Atkinson CJ, Taylor JM (1996) Effects of elevated CO2 on stem growth, vessel area and hydraulic conductivity of oak and cherry seedlings. New Phytol 133:617–626
Bucher JB, Tarjan DP, Siegwolf RTW, Saurer M, Blum H, Hendrey GR (1998) Growth of a deciduous tree seedling community in response to elevated CO2 and nutrient supply. Chemosphere 36:777–782
Ceulemans R, Mousseau M (1994) Effects of elevated atmospheric CO2 on woody plants. New Phytol 127:425–446
Chaney WR, Kozlowski TT (1977) Patterns of water movement in intact and excised stems of Fraxinus americana and Acer saccharum seedlings. Ann Bot 41:1093–1100
Cooke JEK, Brown KA, Wu R, Davis JM (2003) Gene expression associated with N-induced shifts in resource allocation in poplar. Plant Cell Environ 26:757–770
Dawson JO (1983) Dinitrogen fixation in forest ecosystems. Can J Microbiol 29:979–992
Ericsson T, Rytter L, Vapaavuori E (1996) Physiology of carbon allocation in trees. Biomass Bioenergy 11:115–127
Gartner BL, Roy J, Huc R (2003) Effects of tension wood on specific conductivity and vulnerability to embolism of Quercus ilex seedlings grown at two atmospheric CO2 concentrations. Tree Physiol 23:387–395
Hättenschwiler S, Schweingruber FH, Körner Ch (1996) Tree ring responses to elevated CO2 and increased N deposition in Picea abies. Plant Cell Environ 19:1369–1378
Heath J, Kerstiens G (1997) Effects of elevated CO2 on leaf gas exchange in beech and oak at two levels of nutrient supply: consequences for sensitivity to drought in beech. Plant Cell Environ 20:57–67
Hibbs DE, Chan SS, Castellano M, Niu CH (1995) Response of red alder seedlings to CO2 enrichment and water stress. New Phytol 129:569–577
Houghton JT, Ding Y, Griggs DJ, Noguer M, van des Linden PJ, Dai X, Maskell K, Johnson CA (2001) Climate change 2001: the scientific basis: contribution of working group I to the third assessment report of the intergovernmental panel on climate change. Cambridge University Press, New York
Kaakinen S, Kostiainen K, Ek F, Saranpää P, Kubiske ME, Sober J, Karnosky DF, Vapaavuori E (2004) Stem wood properties of Populus tremuloides, Betula papyrifera and Acer saccharum saplings after 3 years of treatments to elevated carbon dioxide and ozone. Global Change Biol 10:1513–1525
Kirschbaum MUF (2004) Direct and indirect climate change effects on photosynthesis and transpiration. Plant Biol 6:242–253
Koike T (1988) Leaf structure and photosynthetic performance as related to the forest succession of deciduous broad-leaved trees. Plant Species Biol 3:77–87
Koike T (1995) Effects of CO2 in interaction with temperature and soil fertility on the foliar phenology of alder, birch, and maple seedlings. Can J Bot 73:149–157
Körner Ch (1995) Towards a better experimental basis for upscaling plant responses to elevated CO2 and climate warming. Plant Cell Environ 18:1101–1110
Kostiainen K, Kaakinen S, Saranpää P, Sigurdsson BD, Linder S, Vapaavuori E (2004) Effect of elevated [CO2] on stem wood properties of mature Norway spruce grown at different soil nutrient availability. Global Change Biol 10:1526–1538
Kostiainen K, Jalkanen H, Kaakinen S, Saranpää P, Vapaavuori E (2006) Wood properties of two silver birch clones exposed to elevated CO2 and O3. Global Change Biol 12:1230–1240
Luo ZB, Langenfeld-Heyser R, Calfapietra C, Polle A (2005) Influence of free air CO2 enrichment (EUROFACE) and nitrogen fertilisation on the anatomy of juvenile wood of three poplar species after coppicing. Trees 19:109–118
Medlyn BE, Barton CVM, Broadmeadow MSJ, Ceulemans R, De Angelis P, Forstreuter M, Freeman M, Jackson SB, Kellomäki S, Laitat E, Rey A, Roberntz P, Sigurdsson BD, Strassemeyer J, Wang K, Curtis PS, Jarvis PG (2001) Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytol 149:247–264
Norby RJ, Wullschleger SD, Gunderson CA, Nietch CT (1995) Increased growth efficiency of Quercus alba trees in a CO2-enriched atmosphere. New Phytol 131:91–97
Nowak RS, Ellsworth DS, Smith SD (2004) Functional responses of plants to elevated atmospheric CO2—do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol 162:253–280
Oh HK, Choung Y (2005) Does elevated CO2 affect the physiology and growth of Quercus mongolica under different nitrogen conditions? Key Eng Mat 277–279:528–535
Oren R, Ellsworth DS, Johnsen KH, Phillips N, Ewers BE, Maier C, Schäfer KVR, McCarthy H, Hendrey G, McNulty SG, Katul GG (2001) Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enrichment atmosphere. Nature 411:469–472
SAS Institute (2003) JMP: statistics and graphics guide, version 5.1. SAS Institute, Cary
Savill PS, Mather RA (1990) A possible indicator of shake in oak: relationship between flushing dates and vessel sizes. Forestry 63:355–362
Saxe H, Ellsworth DS, Heath J (1998) Tree and forest functioning in an enriched CO2 atmosphere. New Phytol 139:395–436
Sperry JS, Ikeda T (1997) Xylem cavitation in roots and stems of Douglas-fir and white fir. Tree Physiol 17:275–280
Telewski FW, Swanson RT, Strain BR, Burns JM (1999) Wood properties and ring width responses to long-term atmospheric CO2 enrichment in field-grown loblolly pine (Pinus taeda L.). Plant Cell Environ 22:213–219
Tjepkema JD (1985) Utilization of photosynthate for nitrogen fixation in seedlings of Myrica gale and Alnus rubra. In: Ludden PW, Burris JE (eds) Nitrogen fixation and CO2 metabolism. Elsevier, New York, pp 183–192
Tobita H, Kitao M, Koike T, Maruyama Y (2005) Effects of elevated CO2 and nitrogen availability on nodulation of Alnus hirsuta Turcz. Phyton 45:125–131
Tognetti R, Johnson JD (1999) Responses of growth, nitrogen and carbon partitioning to elevated atmospheric CO2 concentration in live oak (Quercus virginiana Mill.) seedlings in relation to nutrient supply. Ann For Sci 56:91–105
Tyree MT, Alexander JD (1993) Plant water relations and the effects of elevated CO2: a review and suggestions for future research. Vegetatio 104/105:47–62
Urban O (2003) Physiological impacts of elevated CO2 concentration ranging from molecular to whole plant responses. Photosynthetica 41:9–20
Utsumi Y, Sano Y, Fujikawa S, Funada R, Ohtani J (1998) Visualization of cavitated vessels in winter and refilled vessels in spring in diffuse-porous trees by cryo-scanning electron microscopy. Plant Physiol 117:1463–1471
Utsumi Y, Sano Y, Funada R, Fujikawa S, Ohtani J (1999) The progression of cavitation in earlywood vessels of Fraxinus mandshurica var japonica during freezing and thawing. Plant Physiol 121:897–904
Vogel CS, Curtis PS, Thomas RB (1997) Growth and nitrogen accretion of dinitrogen-fixing Alnus glutinosa (L.) Gaertn. under elevated carbon dioxide. Plant Ecol 130:63–70
Wallace ZP, Lovett GM, Hart JE, Machona B (2007) Effects of nitrogen saturation on tree growth and death in mixed-oak forest. For Ecol Manage 243:210–218
Yazaki K, Funada R, Mori S, Maruyama Y, Abaimov AP, Kayama M, Koike T (2001) Growth and annual ring structure of Larix sibirica grown at different carbon dioxide concentrations and nutrient supply rates. Tree Physiol 21:1223–1229
Yazaki K, Ishida S, Kawagishi T, Fukatsu E, Maruyama Y, Kitao M, Tobita H, Koike T, Funada R (2004) Effects of elevated CO2 concentration on growth, annual ring structure and photosynthesis in Larix kaempferi seedlings. Tree Physiol 24:941–949
Yazaki K, Maruyama Y, Mori S, Koike T, Funada R (2005) Effects of elevated carbon dioxide concentration on wood structure and formation in trees. In: Omasa K, Nouchi I, De Kok LJ (eds) Plant responses to air pollution and global change. Springer, Tokyo, pp 89–97
Acknowledgments
We thank Dr. S. Kitaoka and Ms. H. Taoka for their help in sampling and Mr. M. Tanaka, Mr. K. Mima and Ms. N. Morii for technical assistance. This study was supported in part by a Grant-in-Aid for Research Revolution 2002 (RR2002) Project from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and a Grant-in-Aid for Scientific Research (A) from Japan Society for the Promotion of Science (17,208,013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Linder.
Rights and permissions
About this article
Cite this article
Watanabe, Y., Tobita, H., Kitao, M. et al. Effects of elevated CO2 and nitrogen on wood structure related to water transport in seedlings of two deciduous broad-leaved tree species. Trees 22, 403–411 (2008). https://doi.org/10.1007/s00468-007-0201-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-007-0201-8