Abstract
Background
Patients with hepatocellular adenomas are, in selected cases, candidates for liver resection, which can be approached via laparoscopy or laparotomy. The present study aimed to investigate the effects of the surgical approach on the postoperative morbidities of both minor and major liver resections.
Methods
In this multi-institutional study, all patients who underwent open or laparoscopic hepatectomies for hepatocellular adenomas between 1989 and 2013 in 27 European centers were retrospectively reviewed. A multiple imputation model was constructed to manage missing variables. Comparisons of both the overall rate and the types of complications between open and laparoscopic hepatectomy were performed after propensity score adjustment (via the standardized mortality ratio weighting method) on the factors that influenced the choice of the surgical approach.
Results
The laparoscopic approach was selected in 208 (38%) of the 533 included patients. There were 194 (93%) women. The median age was 38.9 years. After the application of multiple imputation, 208 patients who underwent laparoscopic operations were compared with 216 patients who underwent laparotomic operations. After adjustment, there were 20 (9.6%) major liver resections in the laparoscopy group and 17 (7.9%) in the open group. The conversion rate was 6.3%. The two surgical approaches exhibited similar postoperative morbidity rates and severities. Laparoscopic resection was associated with significantly less blood loss (93 vs. 196 ml, p < 0.001), a less frequent need for pedicle clamping (21 vs. 40%, p = 0.002), a reduced need for transfusion (8 vs. 24 red blood cells units, p < 0.001), and a shorter hospital stay (5 vs. 7 days, p < 0.001). The mortality was nil.
Conclusions
Laparoscopy can achieve short-term outcomes similar to those of open surgery for hepatocellular adenomas and has the additional benefits of a reduced blood loss, need for transfusion, and a shorter hospital stay.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Hepatocellular adenomas (HCAs) are rare benign liver tumors with the potential for malignant transformation [1] and a prevalence that ranges between 0.007 and 0.012% in the adult population [2]. The risk of developing HCA increases with the protracted use of oral contraception (OC) and metabolic syndrome [3–5]. The estimated annual incidence is 3/1,000,000, but this value might be 100-fold higher in long-term and high-dose users of OCs [3, 6]. Due to the widespread diffusion of OC use and the increase in obesity in western populations in addition to the frequency of incidental diagnosis upon imaging, the prevalence of HCA is expected to increase in the future [7].
The surgical indications are related to the prevention or treatment of complications that primarily consist of bleeding and cancer transformation, which are estimated to occur in 17 and 4% of cases, respectively [8, 9]. The following four subtypes of HCA have been identified: steatotic, inflammatory, β-catenin-activated, and unclassified [1, 10]. The patient’s background and the size and subtype of the lesion, which may be correlated with radiologic features [11], define the risk of complications and guide surgeons in the decision-making process [2, 12–15].
The increasing diffusion of and expertise in laparoscopic liver resections (LLRs) in recent years has aided the defining of benign liver lesions, such as HCAs, as an excellent indication for minimally invasive surgery [16–18]. Nevertheless, even if minor LLRs are confirmed to be a standard practice in surgery, major LLRs involve an innovative procedure that is still being explored and is associated with incompletely defined risks compared to open liver resections (OLRs) [19, 20]. The few studies in the literature that have focused on the laparoscopic management of HCAs have described very small and heterogeneous populations of patients with low proportions of major liver resections, and this information does not allow for the making of any clear recommendation [21–25].
Thus, the primary objective of the present study was to compare the short-term outcomes and surgical morbidities of elective LLRs and OLRs in a large population of patients with indications for liver resection for HCA.
Materials and methods
Patient selection
Based on our previous retrospective cross-sectional study of 117 HPB European surgical centers [15], we performed a nested and dedicated study of LLRs.
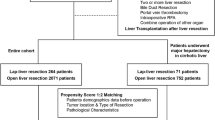
To this end, we excluded the following patients from the previous population study: those who underwent emergency resections for a ruptured tumor, and non-exclusive LLRs (i.e., hand-assisted or hybrid techniques) [19]. The choice of the surgical approach was based on imaging and the patient’s characteristics. All participating centers shared similar criteria regarding the selection of the patients who were eligible for laparoscopy for major liver resection. These criteria included the size of the lesion (<10 cm), a safe distance between the major vessels and the transection lines, and the lack of a need for vascular or biliary reconstruction. The analyses were performed in an intention-to-treat manner; therefore, the patients who underwent LLR and were converted to OLR were considered to be in the laparoscopic group. Liver resections were classified according to the Brisbane classification [26]. Central hepatectomy was defined as resection of segments four, five, and eight [27]. The ethics committees of all hospitals approved the study. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Surgical technique
Regardless of the surgical approach, the central venous pressure was maintained at a low level (<5 mmHg) during the liver transections via the control of intravenous fluid administration. Abdominal drainage was selectively used in cases with concerns about intraoperative bile control regardless of the approach.
All LLRs were performed using 4–6 ports as previously described [18, 23, 28–31]. In cases of major procedures, the operative technique consisted of the isolation and division of the hepatic inflow, the mobilization and transection of the liver, and the division of the hepatic outflow. Liver parenchyma transections were performed in each center using both an ultrasonic dissection device with bipolar cautery and the LigaSure® (Valleylab, Covidien Inc., USA) or the Harmonic® Scalpel (Ethicon EndoSurgery Inc., Cincinnati, OH, USA). The resected specimen was retrieved in a plastic bag through a Pfannestiel incision. The incision was closed, and the absence of bleeding and biliary leakage was then verified by laparoscopy. Conversion was defined as the need for laparotomy at any time during the procedure.
For OLR, either a right subcostal J-shape or midline incision was created. The crush and clamp technique, bipolar cautery, ultrasonic surgical aspirators, and vascular staplers were variably used in the different centers for parenchyma transection. In all patients, prior to transection, intraoperative liver ultrasounds were performed to guide the resection margins. Inflow clamping was performed in cases of bleeding.
Data collection and perioperative outcomes
Epidemiologic data [age, gender, body mass index (BMI), circumstances of the diagnosis, symptoms, and the use of OCs or anabolic steroids], comorbidities, American Society of Anesthesiologists (ASA) score, years of surgery, blood tests (prothrombin time, total bilirubin, hemoglobin, transaminases, and α-fetoprotein serum levels), serological tests (hepatitis B and C), operative variables (operative time, blood loss, transfusion, duration of inflow clamping, and conversion rate), postoperative variables (complications and hospital stay), and pathological variables (fibrosis, steatosis, hepatocellular injury, HCA subtype, and malignant transformation) were recorded.
Postoperative complications were stratified according to the Clavien-Dindo classification (CD) [32]. Liver function tests were measured on postoperative days (PODs) 1, 3, 5, 7, and 10 in cases of major hepatectomy. Liver failure was defined according to the “50–50 criteria” on POD 5 [33]. Ultrasound and/or CT scans were performed in cases of suspicion of complications. Complications were considered as those that occurred within 90 days after surgery. The mid- and long-term follow-ups included clinical, biological, and radiological evaluations 90 days after surgery and every six months thereafter in all centers.
Statistical analysis
The continuous variables are summarized with the median and the first (Q1) and third (Q3) quartiles. The categorical variables are presented as the frequencies and percentages. To improve the validity of the comparisons between groups, two stages were followed.
First, some data were missing from the dataset. The variables with appreciable amounts of missing data were the following: BMI (19.7% missing), prothrombin time (40.7%), bilirubin, hemoglobin, and ALAT and ASAT values, ranging from 22 to 27%. Therefore, multiple imputation (MI) via a chained equation was used to create the 20 multiply imputed datasets: the ALAT, ASAT, and bilirubin serum values were log-transformed before MI due to skewed distributions. Convergence was examined by graphical diagnosis. The incomplete variables were imputed under fully conditional specification because of its flexibility in the specification of the method and the set of predictors to be used for each incomplete variable [34].
Second, a propensity score (PS) method was used to reduce the bias caused by the differences between the two groups. The variables included in the PS estimation were age, gender, BMI, ASA grade, diabetes, hemoglobin, quick time, bilirubin, ALAT, ASAT, F0/F1-F2 fibrosis, steatosis, the number and maximum size of lesions, the type of hepatectomy, the year of surgery, and the surgical team. Because the number of patients included was relatively small, to balance the covariates across treatment groups, PS was implemented by assigning a weight of 1 to each patient in the LLR cohort and weighting each patient in the OLR cohort by the propensity odds [PS/(1 − PS)]. This PS weighting resulted in a pseudo-population of OLR that had the same distribution of measured covariates as that observed in the patients in the LLR group (standardized mortality ratio weighting, SMRW) [35]. With this approach, no LLR individuals were excluded from the analysis. PSs and SMRWs were generated for each imputed dataset.
Comparisons between groups were performed with Chi-square tests for categorical variables and Wilcoxon score tests for continuous variables for each imputed dataset, and the statistics were combined to obtain the final results. All of the analyses were performed with SAS® 9.3 (SAS Institute Inc., Cay, NC, USA). A p value < 0.05 was considered statistically significant.
Results
Demographic and operative characteristics
Among the source population of 573 patients [15], 40 patients were excluded as follows: emergency resections for ruptured HCA (n = 13), hybrid LLR (n = 14), and hand-assisted LLR (n = 13).
The baseline characteristics of the LLR and OLR groups before MI are provided in Table 1. Before MI, there were no differences between the LLR and OLR groups in terms of OC use (51 vs. 57.5%; p = 0.153), bleeding HCAs before surgery (20.2 vs. 16.9%; p = 0.359), patients presenting with abdominal pain (50.5 vs. 54.4%; p = 0.375), prothrombin activity (98 vs. 96.1%; p = 0.205), AFP values > 10 ng/ml (1.4 vs. 3.7%; p = 0.180), or hemoglobin (13 vs. 12.7 g/l; p = 0.185). In contrast, the LLR patients had lower total bilirubin (8 vs. 9 mmol/l; p = 0.005), AST (23 vs. 30UI/l; p = 0.002), and ALT serum level values (26.5 vs. 30UI/l; p < 0.001) than the OLR patients.
The baseline characteristics after MI are provided in the supplementary material (Suppl. Table 1B). After MI, there were higher prevalence of women (93.3 vs. 84.6%; p = 0.008) and patients with diabetes (9.1 vs. 4.3%; p = 0.026) in the LLR than the OLR group. Moreover, LLR patients presented with higher hemoglobin (13.1 vs. 12.7 g/l; p = 0.007) and prothrombin activity (96.9 vs. 90.7%; p < 0.001) values and lower AST (26.3 vs. 30 UI/l; p = 0.021) values than the OLR patients. There were no group differences in the proportions of symptomatic patients, BMI, ASA score, complicated HCA, number of lesions, or the incidence of mild fibrosis or steatosis. The median tumor size was significantly smaller in the LLR group than the OLR group (60 vs. 70 mm, respectively; p = 0.009). Major liver resections were more frequent in the OLR group than the LLR group (34.8 vs. 9.6%; p < 0.001), although the overall bisegmentectomy proportions did not significantly differ between the two groups. Left lateral sectionectomy (LLS) was more prevalent in the LLR than the OLR group (79.7 vs. 49.3%; p < 0.001). Wedge resections were more frequent in the LLR group than the OLR group (50.5 vs. 30.2%; p < 0.001; Supplementary Table).
Perioperative outcomes before adjustment
The perioperative outcomes before propensity score adjustment (PSA) revealed that the patients in the OLR group presented longer operative times (180 vs. 150 min, respectively, p < 0.001), greater blood loss (300 vs. 100 ml, respectively, p < 0.001), a greater transfusion rate (45 vs. 8 RBC units, respectively, p < 0.001), and a greater need for inflow clamping (56.6 vs. 21.2%, respectively, p < 0.001) compared with the LLR group. The conversion rate for the LLR group was 6.3%.
No mortality was registered across the entire study population. The OLR group exhibited a higher rate of postoperative morbidity than the LLR group before adjustment (41.2 vs. 24.0%, respectively, p < 0.001), and there were no group differences in the proportion of complications. Postoperative bleeding was the most frequently observed complication in both groups (5.8 vs. 2.9%; p = 0.143). Overall, the severity of postoperative complications based on CD classification revealed greater proportions of severe complications (≥grade III) and mild complications (CD I/II) in the OLR group than the LLR group (10.5 vs. 4.8%; p = 0.033 and 31.7 vs. 19.7%; p = 0.003, respectively). The median duration of hospital stay was significantly shorter for the LLR group than the OLR group (5 vs. 8 days; p < 0.001).
Propensity score adjusted model
After the construction of the SMRW-PSA model, 208 LLR patients were compared with 216 OLR patients. The balance of covariates was assessed for the accuracy of the model. The baseline characteristics of the matched study population are summarized in Table 2. There were no significant differences in the demographic or surgical characteristics between the groups with the exception of preoperative arterial embolization, which occurred in one patient in the OLR group and nine patients in the LLR groups (0.5 vs. 4.3%; p < 0.001).
After PSA analysis, the patients in the OLR group presented with greater blood loss (196 vs. 93 ml, respectively; p < 0.001), transfusion rates (24 vs. 8 RBC units, respectively, p < 0.001), and the need for inflow clamping (40.3 vs. 21.2%, respectively, p = 0.002) compared with the LLR group (Table 3). In contrast, no difference was observed between the LLR and OLR groups in the overall postoperative morbidity rate (24 vs. 22.7%, respectively, p = 0.778). The proportion of specific complications, which was non-significant in the pre-weighted analysis, exhibited the same behavior in the post-PSA analysis between the LLR and OLR groups. Overall, the post-PSA severity of postoperative complications based on CD classification did not differ between the groups. After adjustment, the difference in favor of the laparoscopic approach compared with the OLR group in the duration of hospital stay was maintained (5 vs. 7 days, respectively, p < 0.001).
Discussion
The present study compared the short-term outcomes of pure LLR and OLR in patients with HCA. The relevance of this study is derived from the large sample of patients, which reflects the synthesis of the international experiences of a rare pathology of twenty-seven tertiary hepatobiliary centers in Europe. To date, this study represents the largest series in this setting. Overall, the present study revealed a benefit of the laparoscopic approach over the open approach for liver resection in patients with HCA.
The present study has strengths related to its relatively large sample of patients who were operated on via laparoscopy for the same indication. To make it possible, a dedicated statistical approach using MI, PSs, and SMRWs was performed. This method, via the inclusion of the year of the surgery and the center, was able to adequately handle time effects and center effects.
During the Second International Consensus Conference in Morioka about LLR, evidence was evaluated using GRADE and recommendations were made according to the Zurich-Danish consensus conference model [20]. To do so, the jury, based on their relative importance, retained 14 comparators. Among these comparators, the following five were relevant for benign tumors: mortality, complications, blood loss, length of stay, and recovery. The present study was able to report the relevant results and their comparisons for all but one comparator, i.e., mortality, which was nil. LLRs were associated with an equivalent rate of complications relative to OLRs, but the LLRs exhibited less blood loss and a shorter length of stay. Recovery, which was considered as a major comparator by the jury in Morioka, was not evaluated because of the retrospective design of the study. In addition to those major comparators, LLR group was associated with reduced blood loss and reduced need for clamping, with a related effect of reduced red blood cell transfusion rate.
After PSA analysis, there are still differences regarding preoperative arterial embolization rate that was more frequent in the LLR group in a significant way (4.3% vs. 0.5%, p < 0.001; Table 2). Some authors can argue that percutaneous embolization prior to resection could influence blood loss, especially for bigger lesions that may receive important arterial blood supply. However, arterial embolization procedure appears to have a little impact on blood loss in both groups. Indeed, although the median arterial embolization rate was significantly higher in the LLR group than in the OLR group, an embolization proportion inferior to 5% in the LLR group did not influence the blood loss amount in a relevant way, as represented by a median value that was more than double in OLR group compared with the LLR group (196 vs. 93 ml, respectively).
The inflow clamping was used less often in the laparoscopic group than open group, but there were no differences in clamping time when it was applied. The hemostatic effect of the pneumoperitoneum as well as the use of ultrasonic dissection devices were likely responsible for the reduced blood loss in the LLR group. As a consequence of this, a reduced rate of Pringle maneuver was necessary in the laparoscopic group. A similar outcome was reported in previous studies [36–38], which observed a reduced use of pedicle clamping in LLR associated with a reduced blood loss compared with OLR. Nevertheless, these outcomes must be interpreted with caution because of the inherent risk of bias related to retrospective nature of the studies. A prospective randomized study of LLR vs. OLR could help clarifying this issue.
Aside from these additional two comparators, the conversion rate was 6.3%, which is a proportion that is comparable to series that have combined minor and major liver resections [16]. These results are in agreement with previous studies on LLRs [36, 39–41]. These excellent results may reflect the fact that all of the resections were performed in tertiary HPB referral centers, some of which were pioneers in this particular field. However, because we included all cases regardless of the year of the surgery and we matched patients based on the year of surgery, these results may be interpreted to indicate that there is almost no learning curve for minor pure LLR once the surgeons already have major expertise in both the liver and minimally invasive surgeries.
In the present series, after 2008, LLR became the approach of choice in AFC-HCA-2013 Study Group centers [15]. This result is not surprising because all AFC-HCA-2013 Study Group centers are tertiary HBP surgery reference centers and minor resections are now performed laparoscopically by many teams worldwide [16]. Some detractors might argue that the surgical need was influenced by the introduction of laparoscopy [7, 42]. This argument was rejected for the first time by Bryant and coworkers in 2009 [18] and more recently in a nationwide US study that included 2633 patients who underwent surgery for benign hepatic tumors in which only 5.5% of patients underwent laparoscopic surgeries. Notably, an increase in the rate from 1.9% in 2000 to 7.4% in 2011 was observed [7].
However, the present study has some limitations that should be considered. First, regarding the external validity of the study findings, it is important to highlight that experienced surgeons performed all of the operations. Thus, the present results must not be generalized to centers with limited experience in hepatobiliary and minimally invasive surgeries. Second, despite the use of the MI method, to control bias due to missing data, there is the possibility of statistical confounding effects in the generation of the PS analysis. Third, the proportions of major resections were less than 10% in each study group after PSA; therefore, this study cannot be generalized to this subset of challenging hepatectomies.
In conclusion, based on the comparators that were retained by the jury during the Second International Consensus Conference in Morioka and the results of comparisons of the short-term outcomes of LLR and OLR with adjustments for the effects of the year of resection, the center, and the background factors, LLR is confirmed as the standard approach for minor liver resections.
References
Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S, Wendum D, Chiche L, Fabre M, Mellottee L, Laurent C, Partensky C, Castaing D, Zafrani ES, Laurent-Puig P, Balabaud C, Bioulac-Sage P (2006) Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology 43:515–524
Marrero JA, Ahn J, Rajender Reddy K, Americal College of Gastroenterologists (2014) ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol 109:1328–1347
Rooks JB, Ory HW, Ishak KG, Strauss LT, Greenspan JR, Hill AP, Tyler CW Jr (1979) Epidemiology of hepatocellular adenoma. The role of oral contraceptive use. JAMA 242:644–648
Bioulac-Sage P, Taouji S, Possenti L, Balabaud C (2012) Hepatocellular adenoma subtypes: the impact of overweight and obesity. Liver Int 32:1217–1221
Paradis V, Champault A, Ronot M, Deschamps L, Valla DC, Vidaud D, Vilgrain V, Belghiti J, Bedossa P (2007) Telangiectatic adenoma: an entity associated with increased body mass index and inflammation. Hepatology 46:140–146
Barthelmes L, Tait IS (2005) Liver cell adenoma and liver cell adenomatosis. HPB 7:186–196
Kim Y, Amini N, He J, Margonis GA, Weiss M, Wolfgang CL, Makary M, Hirose K, Spolverato G, Pawlik TM (2015) National trends in the use of surgery for benign hepatic tumors in the United States. Surgery 157:1055–1064
van Aalten SM, de Man RA, JN IJ, Terkivatan T (2012) Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg 99:911–916
Stoot JH, Coelen RJ, De Jong MC, Dejong CH (2010) Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB 12:509–522
Bioulac-Sage P, Laumonier H, Couchy G, Le Bail B, Sa Cunha A, Rullier A, Laurent C, Blanc JF, Cubel G, Trillaud H, Zucman-Rossi J, Balabaud C, Saric J (2009) Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology 50:481–489
Ronot M, Bahrami S, Calderaro J, Valla DC, Bedossa P, Belghiti J, Vilgrain V, Paradis V (2011) Hepatocellular adenomas: accuracy of magnetic resonance imaging and liver biopsy in subtype classification. Hepatology 53:1182–1191
Dokmak S, Paradis V, Vilgrain V, Sauvanet A, Farges O, Valla D, Bedossa P, Belghiti J (2009) A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology 137:1698–1705
Karkar AM, Tang LH, Kashikar ND, Gonen M, Solomon SB, Dematteo RP, MI DA, Correa-Gallego C, Jarnagin WR, Fong Y, Getrajdman GI, Allen P, Kingham TP (2013) Management of hepatocellular adenoma: comparison of resection, embolization and observation. HPB 15:235–243
Bieze M, Phoa SS, Verheij J, van Lienden KP, van Gulik TM (2014) Risk factors for bleeding in hepatocellular adenoma. Br J Surg 101:847–855
Laurent A, Dokmak S, Nault JC, Pruvot FR, Fabre JM, Letoublon C, Bachellier P, Capussotti L, Farges O, Mabrut JY, Le Treut YP, Ayav A, Suc B, Soubrane O, Mentha G, Popescu I, Montorsi M, Demartines N, Belghiti J, Torzilli G, Cherqui D, Hardwigsen J (2016) European experience of 573 liver resections for hepatocellular adenoma: a cross-sectional study by the AFC-HCA-2013 study group. HPB 18:748–755
Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G (2016) Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 263:761–777
Nguyen KT, Gamblin TC, Geller DA (2009) World review of laparoscopic liver resection-2804 patients. Ann Surg 250:831–841
Bryant R, Laurent A, Tayar C, Cherqui D (2009) Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg 250:103–111
Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker C-G, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D’Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey J-N, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS (2009) The International Position on Laparoscopic Liver Surgery. Ann Surg 250:825–830
Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, O’Rourke N, Tanabe M, Koffron AJ, Tsung A, Soubrane O, Machado MA, Gayet B, Troisi RI, Pessaux P, Van Dam RM, Scatton O, Abu Hilal M, Belli G, Kwon CH, Edwin B, Choi GH, Aldrighetti LA, Cai X, Cleary S, Chen KH, Schon MR, Sugioka A, Tang CN, Herman P, Pekolj J, Chen XP, Dagher I, Jarnagin W, Yamamoto M, Strong R, Jagannath P, Lo CM, Clavien PA, Kokudo N, Barkun J, Strasberg SM (2015) Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261:619–629
Bieze M, Busch OR, Tanis PJ, Verheij J, Phoa SS, Gouma DJ, van Gulik TM (2014) Outcomes of liver resection in hepatocellular adenoma and focal nodular hyperplasia. HPB (Oxford) 16:140–149
Abu Hilal M, Di Fabio F, Wiltshire RD, Hamdan M, Layfield DM, Pearce NW (2011) Laparoscopic liver resection for hepatocellular adenoma. World. J Gastrointest Surg 3:101–105
Cho SW, Marsh JW, Steel J, Holloway SE, Heckman JT, Ochoa ER, Geller DA, Gamblin TC (2008) Surgical management of hepatocellular adenoma: take it or leave it? Ann Surg Oncol 15:2795–2803
de’Angelis N, Memeo R, Calderaro J, Felli E, Salloum C, Compagnon P, Luciani A, Laurent A, Cherqui D, Azoulay D (2014) Open and laparoscopic resection of hepatocellular adenoma: trends over 23 years at a specialist hepatobiliary unit. HPB (Oxford) 16:783–788
Herman P, Coelho FF, Perini MV, Lupinacci RM, D’Albuquerque LA, Cecconello I (2012) Hepatocellular adenoma: an excellent indication for laparoscopic liver resection. HPB (Oxford) 14:390–395
Belghiti J, Clavien PA, Gadzijev E, Garden OJ, Lau WY, Makuuchi M, Strong RW (2000) The Brisbane 2000 Terminology of Liver Anatomy and Resections. HPB (Oxford) 2:333–339
de’Angelis N, Pascal G, Salloum C, Lahat E, Ichai P, Saliba F, Adam R, Castaing D, Azoulay D (2016) Central hepatectomy versus extended hepatectomy for malignant tumors: a propensity score analysis of postoperative complications. World J Surg 40:2745–2757
Goumard C, Farges O, Laurent A, Cherqui D, Soubrane O, Gayet B, Pessaux P, Pruvot FR, Scatton O (2015) An update on laparoscopic liver resection: The French Hepato-Bilio-Pancreatic Surgery Association statement. J Visc Surg 152:107–112
Cherqui D, Husson E, Hammoud R, Malassagne B, Stephan F, Bensaid S, Rotman N, Fagniez PL (2000) Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg 232:753–762
Chang S, Laurent A, Tayar C, Karoui M, Cherqui D (2007) Laparoscopy as a routine approach for left lateral sectionectomy. Br J Surg 94:58–63
Dokmak S, Raut V, Aussilhou B, Fteriche FS, Farges O, Sauvanet A, Belghiti J (2014) Laparoscopic left lateral resection is the gold standard for benign liver lesions: a case-control study. HPB 16:183–187
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F (2005) The “50–50 Criteria” on Postoperative Day 5. Ann Surg 242:824–829
van Buuren S (2007) Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 16:219–242
Sato T, Matsuyama Y (2003) Marginal structural models as a tool for standardization. Epidemiology 14:680–686
de’Angelis N, Eshkenazy R, Brunetti F, Valente R, Costa M, Disabato M, Salloum C, Compagnon P, Laurent A, Azoulay D (2015) Laparoscopic versus open resection for colorectal liver metastases: a single-center study with propensity score analysis. J Laparoendosc Adv Surg Tech 25:12–20
Morino M, Morra I, Rosso E, Miglietta C, Garrone C (2003) Laparoscopic vs open hepatic resection: a comparative study. Surg Endosc 17:1914–1918
Polignano FM, Quyn AJ, de Figueiredo RS, Henderson NA, Kulli C, Tait IS (2008) Laparoscopic versus open liver segmentectomy: prospective, case-matched, intention-to-treat analysis of clinical outcomes and cost effectiveness. Surg Endosc 22:2564–2570
Koffron AJ, Auffenberg G, Kung R, Abecassis M (2007) Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg 246:385–394
Topal B, Fieuws S, Aerts R, Vandeweyer H, Penninckx F (2008) Laparoscopic versus open liver resection of hepatic neoplasms: comparative analysis of short-term results. Surg Endosc 22:2208–2213
Meguro M, Mizuguchi T, Kawamoto M, Ota S, Ishii M, Nishidate T, Okita K, Kimura Y, Hirata K (2015) Clinical comparison of laparoscopic and open liver resection after propensity matching selection. Surgery 158:573–587
Toro A, Gagner M, Di Carlo I (2013) Has laparoscopy increased surgical indications for benign tumors of the liver? Langenbecks Arch Surg 398:195–210
Collaborators of the AFC-HCA-2013 Study Group
François-René Pruvot Department of Digestive Surgery and Transplantation, Lille University Hospital, Nord de France University, Lille, France. Jean-Michel Fabre Department of Digestive Surgery and Transplantation, St Eloi Hospital, Montpellier, France. Christian Letoublon Clinique d’ Hépatogastroentérologie, pôle DigiDune, Grenoble University Hospital, France. Philippe Bachellier Department of Surgery, University Hospital of Hautepierre, Strasbourg, France. Olivier Farges, Department of Hepatopancreatobiliary Surgery and Liver Transplantation, Beaujon Hospital, Clichy, France. Jean-Yves Mabrut Department of Surgery, Croix Rousse Hospital, Lyon. Yves-Patrice Le Treut Department of Surgery, la Timone Hospital, Marseille, France. Bertrand Suc Department of Digestive Surgery and Transplantation, Rangueil University Hospital, Toulouse, France. Irinel Popescu Center of Gastrointestinal Disease and Liver Transplantation, Fundeni Clinical Institute, Bucharest, Romania. Marco Montorsi Department of General Surgery, Humanitas University and Research Hospital, Milano, Italy. Jacques Belghiti, Department of Hepatopancreatobiliary Surgery and Liver Transplantation, Beaujon Hospital, Clichy, France. Jean-Marc Régimbaud – CHU Amiens-Picardie, France. Romain Riboud – CHU Grenoble, France. Alexandra Dili – La Louvière, Hôpital de Jolimont, Belgium. Pierre Allemann – (CHUV), Lausanne, Switzerland. Emmanuel Boleslawski - CHRU Lille, France. Benjamin Darnis – CHU Lyon, France. Mustapha Adham, CHU Lyon, France. Emilie Bollon – CHU Marseille, France. Bernard Pol – Hôpital Saint-Joseph, Marseille, France. Jean-Robert Delpero – Institut Paoli-Calmettes, Marseille, France. Olivier Turrini – Institut Paoli-Calmettes, Marseille, France. Frédéric Borie – CHU-Nimes, France. Mathieu Gonot-Gachard – CHU-Nimes, France. Laura Ornella Perotto – CHU Pitié-Salpétrière, Paris, France. Riccardo Gauzolino – CHU-Poitier, France. Marie Castagnet – CHU-Poitier, France. Reza Kianmanesh – CHU-Reims, France. Daniele Sommacale – CHU-Reims, France. Mikael Chetboun – CHU-Reims, France. Jean-Luc Porcheron – CHU-St-Etienne, France. Alexandre Filippello – CHU-St-Etienne, France. Patrick Pessaux – CHU-Strasbourg, France. Pietro Addeo – CHU-Strasbourg, France. Manuela Cesaretti – CHU-Strasbourg, France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Filippo Landi, Nicola de’Angelis, Olivier Scatton, Xavier Vidal, Ahmet Ayav, Fabrice Muscari, Safi Dokmak, Guido Torzilli, Nicolas Demartines, Olivier Soubrane, Daniel Cherqui, Jean Hardwigsen, and Alexis Laurent have no conflicts of interest or financial ties to disclose in relation to the results of the present study.
Additional information
Filippo Landi and Nicola de Angelis have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Landi, F., de’ Angelis, N., Scatton, O. et al. Short-term outcomes of laparoscopic vs. open liver resection for hepatocellular adenoma: a multicenter propensity score adjustment analysis by the AFC-HCA-2013 study group. Surg Endosc 31, 4136–4144 (2017). https://doi.org/10.1007/s00464-017-5466-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5466-4