Abstract
Background
Anatomical hepatectomy is an ideal curative treatment for hepatocellular carcinoma (HCC). We have standardized our laparoscopic anatomical hepatectomy (LAH) procedure, gradually extending its indications. In the present study, we describe our experience and the perioperative and oncological outcomes of LAH for HCC compared to those of open anatomical hepatectomy (OAH) during the gradual introduction of LAH.
Methods
Seventy patients with primary HCC underwent anatomical hepatectomy in our institution from November 2008 to April 2014. As we gained experience with LAH, our indications for choosing LAH over OAH gradually expanded. Ultimately, 40 and 30 patients underwent LAH and OAH, respectively. Perioperative and oncological outcomes were compared between the two groups.
Results
There were no significant differences in age, sex, background of liver disease, liver function, tumor size, tumor number, or type of liver resection between the two groups. Major complications and mortality rates were similar between the LAH and OAH groups (12.5% vs. 20%; p = 0.582, and 0% vs. 3.3%; p = 0.429, respectively). The median follow-up time after surgery was 40.5 months in the LAH group and 32.9 months in the OAH group (p = 0.835). The 1-, 3-, and 5-year overall survival rates were 89.9, 84.7, and 70.9%, in the LAH group, and 89.8, 68.0, and 63.1% in the OAH group, respectively (p = 0.255). The 1-, 3-, and 5-year disease-free survival rates were 79.5, 58.0, and 42.5%, in the LAH group, and 72.4, 56.1, and 50.4% in the OAH group, respectively (p = 0.980).
Conclusions
Through gradual introduction of LAH, we obtained comparable results to those achieved with OAH. LAH can be a feasible surgical treatment for primary HCC, with good oncological outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third most common cause of cancer-related death [1]. Potential curative treatments for HCC include hepatectomy, local ablation, and liver transplantation. Among these, hepatectomy, categorized as either anatomical or nonanatomical, is the standard treatment for resectable HCC [2], and anatomical hepatectomy is associated with more favorable oncological outcomes than nonanatomical hepatectomy [3,4,5,6,7].
Laparoscopic hepatectomy has recently undergone rapid evolution, and is often used for the treatment of HCC [8,9,10]. Several studies, including meta-analyses and case–control studies with propensity score matching, have demonstrated that the results of laparoscopic hepatectomy for HCC are comparable to those achieved with open surgery [11,12,13,14,15,16]. However, the most frequent type of laparoscopic hepatectomy performed in these studies was partial hepatectomy, and the anatomical resection rate ranged from 34.5 to 63.0% [10,11,12,13]. Although open anatomical hepatectomy (OAH) is generally accepted as a curative treatment for HCC, laparoscopic anatomical hepatectomy (LAH) is not widely performed, mainly because of the high skill level required for the performance of this procedure, such as the ability to effectively expose the Glissonean pedicles and hepatic veins in the cutting plane. Moreover, the development and standardization of LAH procedures continue to progress. Therefore, comparative studies regarding LAH for HCC continue to have their own limitations, although some well-planned comparative studies have already been reported.
In this situation, since the initiation of laparoscopic hepatectomy at our institution, we have developed a standardized LAH procedure and gradually extended its indications, based on the maturation of our skills in the performance of OAH [17,18,19,20,21,22]. We describe herein our experience with LAH for primary HCC, including our experiences during the introductory period, and report that our introduction of LAH was safe and feasible, in terms of perioperative and oncological outcomes in comparison with OAH.
Materials and methods
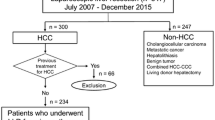
A total of 130 patients with primary HCC underwent hepatectomy at Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, from January 2008 to April 2014. Among these patients, 70 underwent anatomical hepatectomy; 40 of these 70 patients underwent LAH, while the other 30 patients underwent OAH. We generally evaluated the indications for surgery and selected the operative procedure based on the tumor extent and the hepatic reserve as assessed by the Child–Pugh score and the criteria of Makuuchi et al. [23]. Left lateral sectionectomy was performed as the first LAH procedure for primary HCC in November 2008. Our criteria for laparoscopic hepatectomy have been gradually extended from those for partial hepatectomy and are now almost identical to the general criteria for open hepatectomy, even for hemi-hepatectomy and trisegmentectomy, although we have excluded patients requiring reconstruction of the blood vessels or bile ducts, those with an estimated operating time >8 h, and those with cardiopulmonary disorders that might render it impossible to maintain central venous pressure (CVP) at a low level. Over the entire study period, we employed no criterion with regard to tumor size.
The present study was approved by the institutional ethics committee of Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, and conformed to the ethical guidelines of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Preoperative management
The preoperative evaluation included determination of age, sex, blood chemistry parameters, indocyanine green retention rate at 15 min, Child–Pugh classification, hepatitis virus markers (hepatitis B virus surface antigen and hepatitis C virus antibody), the presence or absence of cirrhosis, serum alpha fetoprotein (AFP) level, and plasma des-gamma carboxyprothrombin (DCP) level. Tumor characteristics (morphology, size, number, and location) were evaluated with both contrast-enhanced computed tomography (CT) and ethoxybenzyl-enhanced magnetic resonance imaging (MRI). The hepatic vascular anatomy was reviewed using three-dimensional reconstructed CT images, and the liver segment volumes were calculated, after which the data were used for treatment planning.
Surgical technique
Anatomical resection procedures were defined based on the Brisbane 2000 classification [24]. All LAH procedures were intended to be entirely laparoscopic and were performed based on our standardized methods [18,19,20,21,22]. A tourniquet was always prepared for the Pringle maneuver or hemihepatic vascular occlusion and was used intermittently to keep the operative field dry [21]. Low CVP was maintained through reduction of the infusion volume from the start of the surgery until completion of the liver resection. Pneumoperitoneum pressure was usually set at 10 mmHg [25]. The liver parenchyma was divided with the CUSA EXcel® system (Integra LifeSciences Corporation, Plainsboro, NJ, USA) to clearly expose the Glissonean branches and hepatic veins in the cutting plane without precoagulation (Fig. 1). To keep the operating field dry, oozing from the divided parenchyma was often controlled with thermal coagulation using the low-voltage electrical cautery mode of VIO® (Erbe Elektromedizin GmbH, Tübingen, Germany) at the tip of the CUSA EXcel®. Small vessels left by the CUSA EXcel® were simply cut, whereas larger vessels were cut with a HARMONIC® device (Ethicon Endo-Surgery, Inc., Cincinnati, OH, USA) after being clipped on the remnant side. The roots of the Glissonean pedicle and main trunk of the hepatic vein were usually divided using a stapler. The resected specimen was removed through the extended trocar incision at the umbilicus after being placed in a plastic bag, and surgery was completed after routinely placing a closed suction drain near the cutting plane. In OAH, right subcostal or upper midline or inverted T-shaped incisions were used according to tumor location. Liver parenchymal resection was performed mainly with the CUSA EXcel®, as in LAH, but larger vessels were cut with scissors following suture ligation. In both LAH and OAH, we resected the liver parenchyma according to the demarcation line after occlusion of the inflow. Intraoperative ultrasonography was performed routinely to confirm the spatial relationship between the tumor and vascular structures.
Postoperative management and follow-up
In both patients who underwent LAH and those who underwent OAH, the drain was usually removed on postoperative day 3 after confirming the absence of hemorrhage and bile leakage. Both medical and surgical complications were closely monitored. Morbidities were stratified by severity based on the Clavien–Dindo classification [26]. Pathological findings were defined based on the General Rules for the Clinical and Pathological Study of Primary Liver Cancer developed by the Liver Cancer Study Group of Japan [27]. Postoperative follow-up was performed once a month for clinical and laboratory parameters, including tumor markers (AFP and DCP), whereas radiological assessment (contrast-enhanced CT or dynamic MRI) was performed every 3–4 months or if elevation of tumor markers was detected. Recurrence was defined as the appearance of new lesions with typical radiologic features of HCC, which was confirmed by imaging modalities. Early and late recurrences were defined as recurrence within or more than 2 years after surgery, respectively. Local recurrence was defined as a tumor within 2 cm of the surgical margin or in the same segment as the initial tumor [28].
Statistical analysis
Continuous variables were compared using unpaired t-tests, and categorical variables were compared using Fisher’s exact test or the χ2 test. Overall survival was defined as the interval between the initial surgery and death or the date of the last or most recent follow-up visit. Disease-free survival was defined as the interval between the initial surgery and the date when recurrence was detected by radiological examination. Survival curves were calculated by the Kaplan–Meier method and compared using the log-rank test. A Cox proportional hazards model was used for multivariate analyses of factors related to survival and recurrence. In performing Cox proportional hazards regression analysis, continuous variables were converted into binary variables. All analyses were performed using the JMP 11 software package (SAS Institute Inc., Cary, NC, USA). p values of < 0.05 were considered to indicate statistical significance.
Results
Patient characteristics
The comparison of clinicopathological characteristics between the two groups is shown in Table 1. The study group comprised 54 men and 16 women, with a median age of 69 years. Hepatitis C was the background liver disease in 31 patients (44%). Three (7.5%) patients in the LAH group and 6 (20.0%) in the OAH group had a history of upper abdominal surgery. There were no patients that had repeat hepatectomy. The median tumor size was 4.0 cm and the median number of tumors was one. All patients enrolled in this study had Child–Pugh class A liver function. The two groups were well matched in terms of clinicopathological data, with the exception of DCP.
Surgical procedures and outcomes
Table 2 summarizes the perioperative data of the two groups. Although there were more cases with trisegmentectomy and hemi-hepatectomy and fewer with sectionectomy in the OAH group, there were no significant differences in the types of liver resection between the two groups. The median operating time was longer in the LAH group than in the OAH group (382 min vs. 328 min; p = 0.037). However, the median blood loss was significantly lower in the LAH group than in the OAH group (250 g vs. 795 g; p < 0.001), and no patients in the LAH group required intraoperative blood transfusion. Conversion to open surgery was required in one patient (conversion rate: 2.5%) in the LAH group. Conversion to open hepatectomy in the patient who underwent segment 8 segmentectomy was performed midway through parenchymal dissection because we estimated that more than 10 h would be required for the laparoscopic approach owing to insufficient procedure standardization.
The median time until drain removal and the median postoperative hospital stay were significantly shorter in the LAH group than in the OAH group (3 days vs. 4 days; p < 0.001, and 8 days vs. 12 days; p = 0.005, respectively). Postoperative morbidity did not significantly differ between the two groups. No postoperative bleeding was noted in either group. The overall major morbidity rates (Clavien grade 3) were similar (12.5% in the LAH group and 20.0% in the OAH group; p = 0.582). Clavien grade 4 or 5 complications did not occur (no in-hospital mortality) in the LAH group, while 1 patient experienced in-hospital mortality in the OAH group. This patient died on postoperative day 51 for postoperative liver failure after left trisectionectomy.
Pathological findings
Pathological findings in the two groups are shown in Table 3. The variables of macroscopic type, histological grade, pathological portal invasion, pathological venous invasion, and histological liver cirrhosis were similar in the two groups. The microscopically negative surgical margin rates were also similar in the LAH and OAH groups (95% vs. 83%; p = 0.107). In the LAH group, a microscopically positive surgical margin was found in 2 patients (5%). Of these 2 patients, 1 underwent segmentectomy (segments 5 and 6) for a single tumor that was 5 cm in diameter, and a tumor thrombus that was not detected before surgery was found at the stump of the portal vein. The other patient underwent segmentectomy (segment 1) for a single tumor that was 4 cm in diameter, which was found to have no obvious capsule and was exposed at the surgical margin.
Long-term oncological outcomes
The median follow-up time after surgery was 40.5 (1.4–93.5) months in the LAH group and 32.9 (1.7–97.0) months in the OAH group (p = 0.835). The 1-, 3-, and 5-year overall survival rates were 89.9, 84.7, and 70.9%, in the LAH group, and 89.8, 68.0, and 63.1% in the OAH group, respectively (Fig. 2). The 1-, 3-, and 5-year disease-free survival rates were 79.5, 58.0, and 42.5%, in the LAH group, and 72.4, 56.1, and 50.4% in the OAH group, respectively (Fig. 3). There were no significant differences in the overall survival and disease-free survival rates between the LAH and OAH groups (p = 0.255 and p = 0.980, respectively).
During the follow-up period, recurrence was observed in 30 of the 70 patients in both groups. The remnant liver was the first site of recurrence in 29 of these patients, although lung, lymph node, or brain metastasis occurred simultaneously or later in some, and 1 patient experienced only bone metastasis. Among the 29 patients with intrahepatic recurrence, 20 experienced early recurrence and 9 had late recurrence. Among 20 patients with early recurrence, 12 were in the LAH group and 8 were in the OAH group. The early recurrence rate after surgery did not significantly differ between the LAH and OAH groups (30.0% vs. 26.7%; p = 0.597). In addition, no patient in the LAH group experienced local recurrence or port site recurrence.
The factors associated with overall survival rates were evaluated with univariate and multivariate analyses (Table 4). Multivariate analysis showed that pathologic findings of a simple nodule with extranodular growth (SN with EG) or confluent multinodular (CM) (relative risk [RR] 3.70, 95% confidence interval [95% CI], 1.13–16.5; p = 0.029), and microscopically positive surgical margin (RR 3.69, 95% CI 1.20–10.6; p = 0.025) were independent risk factors for lower overall survival. The factors associated with disease-free survival rates were also evaluated by univariate and multivariate analyses (Table 5). Multivariate analysis showed that multiple tumors (RR 3.58, 95% CI 1.22–9.94; p = 0.021), SN with EG or CM (RR 2.19, 95% CI 1.01–4.90; p = 0.046), microvascular (pathological portal and/or venous) invasion (RR 3.79, 95% CI 1.67–8.75; p = 0.001), and microscopically positive surgical margin (RR 3.46, 95% CI 1.06–9.73; p = 0.040) were independent risk factors for lower disease-free survival. Accordingly, LAH was not confirmed as an independent risk factor for lower overall survival or disease-free survival.
Discussion
Laparoscopic hepatectomy should be performed in the same manner as open hepatectomy because the oncological outcome should not be jeopardized in exchange for the postoperative benefits of laparoscopic surgery. We performed OAH defined by strict standards [17]; we also defined and performed LAH according to standards equally as strict as those applied to OAH (Fig. 1). We defined hepatectomy, in which required the major hepatic vein was not exposed on the cutting plane, as partial hepatectomy. Therefore, the present study can demonstrate the data for true LAH procedures, which were compared with those of true OAH procedures for HCC.
Although laparoscopic hepatectomy is usually associated with decreased blood loss and decreased requirement for blood transfusion compared to open hepatectomy [10, 15], one of the main reasons for conversion to open surgery is major hemorrhage during laparoscopic hepatectomy [29]. Especially in LAH, because the major hepatic veins are exposed on the cutting surface, bleeding from these veins is expected to become a reason for conversion. However, in our series, the median blood loss with LAH was 250 mL, significantly lower than that of OAH, and there were no instances of conversion performed because of hemorrhage. We believe that this was because of continuous maintenance of low CVP not only by reduction of the infusion volume, but also by meticulous control of the pneumoperitoneum and airway pressures [25]. We also noted that specific skills to avoid split injury of the major hepatic veins, such as moving the CUSA from the root side toward the peripheral side, are important to prevent uncontrollable bleeding [18, 20, 22]. Eventually, morbidity and mortality rate did not increase with the use of LAH. There was also no significant difference in pathologically negative surgical margin rates (95% in the LAH group vs. 83% in the OAH group; p = 0.107). These findings suggest that our cautious shift from OAH to LAH was technically feasible and safe.
Anatomical hepatectomy for HCC achieves better oncological outcomes and improved overall and disease-free survival rates compared with nonanatomical hepatectomy, as previously reported [3,4,5,6,7]. In the present study, the 5-year overall survival and disease-free survival rates for LAH versus OAH were 70.9% versus 63.1% (p = 0.255), and 42.5% versus 50.4% (p = 0.980), respectively. There were no significant differences in overall survival and disease-free survival between the two groups. The reported 5-year overall and disease-free survival rates after OAH for HCC in previous studies were 66–71% and 34–46%, respectively [3, 6, 7]. The overall survival and disease-free survival in our series were comparable with those reported previously for OAH. Furthermore, the early recurrence rate of 30% for LAH was comparable with the rate of 26.7% for OAH in the present study (p = 0.597). Regarding recurrence type, intrahepatic recurrence after hepatectomy is generally divided into two types, intrahepatic metastasis from the primary tumor and multicentric occurrence of new tumors [30]. In previous studies, intrahepatic metastasis from the primary tumor, which may involve intrahepatic dissemination due to the manipulation during hepatectomy, was shown to mainly occur as early recurrence within 2 years after hepatectomy [30, 31]. The present results suggest that the manipulation occurring during laparoscopic procedures did not significantly increase intrahepatic metastasis. In multivariate analyses, LAH was not confirmed as an independent risk factor for overall survival and disease-free survival (RR 0.58, 95% CI 0.22–1.49; p = 0.259, and RR 0.99, 95% CI 0.49–2.03; p = 0.980, respectively). Our results suggest the oncological feasibility of LAH for HCC.
The present study had some limitations. We found no statistically significant differences between the characteristics of the two groups of this study. However, there were some selection biases in the first half of the period of this study, but the indication criteria for LAH were almost identical to the general criteria for OAH in the latter half, because our criteria for laparoscopic hepatectomy have been gradually extended. Additionally, this study was conducted using retrospective analysis of a single-center prospective database, and it lacked statistical power owing to the small sample size. Further studies, such as well-designed, large-scale, case-matched studies with propensity score matching or randomized clinical trials comparing LAH with OAH in HCC, which are designed by nominating the cases operated with standardized LAH procedures, are needed to precisely evaluate the relative outcomes of LAH for HCC. However, we believe that selection biases are required in the introductory period of a novel difficult laparoscopic technique and it is socially desirable to report the results of a careful introduction, giving priority to patients’ interests.
In conclusion, we obtained comparable results to those of OAH with LAH through gradual introduction of the procedure. LAH can be a feasible procedure for primary HCC, with good oncological outcomes.
References
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150
Takayama T, Makuuchi M, Hirohashi S, Sakamoto M (1998) Early hepatocellular carcinoma as an entity with a high cure rate of surgical cure. Hepatology 28:1241–1246
Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M (2005) Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg 242:252–259
Kobayashi A, Miyagawa S, Miwa S, Nakata T (2008) Prognostic impact of anatomical resection on early and late intrahepatic recurrence in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 15:515–521
Eguchi S, Kanematsu T, Arii S, Okazaki M, Okita K, Omata M, Ikai I, Kudo M, Kojiro M, Makuuchi M, Monden M, Matsuyama Y, Nakanuma Y, Takayasu K, Liver Cancer Study Group of Japan (2008) Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery 143:469–475
Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Matsushita M, Todo S (2010) The impact of anatomical resection for hepatocellular carcinoma that meets Milan criteria. J Surg Oncol 101:54–60
Kudo A, Tanaka S, Ban D, Matsumura S, Irie T, Nakamura N, Arii S (2014) Anatomical resection reduces the recurrence of solitary hepatocellular carcinoma ≤5 cm without macrovascular invasion. Am J Surg 207:863–869
Kaneko H, Takagi S, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, Maeda T, Shiba T (2005) Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg 189:190–194
Nguyen KT, Gamblin TC, Geller DA (2009) World review of laparoscopic liver resection—2804 patients. Ann Surg 250:931–941
Soubrane O, Goumard C, Laurent A, Tranchart H, Truant S, Gayet B, Salloum C, Luc G, Dokmak S, Piardi T, Cherqui D, Dagher I, Boleslawski E, Vibert E, Cunha AS, Belghiti J, Pessaux P, Boelle PY, Scatton O (2014) Laparoscopic resection of hepatocellular carcinoma: a French survey in 351 patients. HPB 16:357–365
Lee KF, Chong CN, Wong J, Cheung YS, Wong J, Lai P (2011) Long-term results of laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: a case-matched analysis. World J Surg 35:2268–2274
Truant S, Bouras AF, Hebbar M, Boleslawski E, Fromont G, Dharancy S, Leteutre E, Zerbib P, Pruvot FR (2011) Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: a case-matched study. Surg Endosc 25:3668–3677
Kim H, Suh KS, Lee KW, Yi NJ, Hong G, Suh SW, Yoo T, Park MS, Choi YR, Lee HW (2014) Long-term outcome of laparoscopic versus open liver resection for hepatocellular carcinoma: a case-controlled study with propensity score matching. Surg Endosc 28:950–960
Fancellu A, Rosman AS, Sanna V, Nigri GR, Zorcolo L, Pisano M, Melis M (2011) Meta-analysis of trials comparing minimally-invasive and open liver resections for hepatocellular carcinoma. J Surg Res 171:e33–e45
Li N, Wu YR, Wu B, Lu MQ (2012) Surgical and oncologic outcomes following laparoscopic versus open liver resection for hepatocellular carcinoma: a meta-analysis. Hepatol Res 42:51–59
Yin Z, Fan X, Ye H, Yin D, Wang J (2013) Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol 20:1203–1215
Honda G, Kurata M, Tsuruta K (2008) Approach for systematic resection of the liver antero-superior area: exposing Glissonean pedicles by prior dissection of the major hepatic fissure. J Am Coll Surg 207:e1–e4
Honda G, Kurata M, Okuda Y, Kobayashi S, Tadano S, Yamaguchi T, Matsumoto H, Nakano D, Takahashi K (2013) Totally laparoscopic hepatectomy exposing the major vessels. J Hepatobiliary Pancreat Sci 20:435–440
Honda G, Kurata M, Okuda Y, Kobayashi S, Sakamoto K (2014) Totally laparoscopic hepatectomy exposing the vessels around the tumor intended to secure the surgical margin. Surg Endosc 28:1331–1332
Honda G, Kurata M, Okuda Y, Kobayashi S, Sakamoto K, Takahashi K (2014) Totally laparoscopic anatomical hepatectomy exposing the major hepatic veins from the root side: a case of the right anterior sectorectomy (with video). J Gastrointest Surg 18:1379–1380
Okuda Y, Honda G, Kurata M, Kobayashi S (2013) A useful and convenient procedure for intermittent vascular occlusion in laparoscopic hepatectomy. Asian J Endosc Surg 6:100–103
Okuda Y, Honda G, Kurata M, Kobayashi S, Sakamoto K (2014) Dorsal approach to the middle hepatic vein in laparoscopic left hemihepatectomy. J Am Coll Surg 219:e1–e4
Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S (1993) Surgery for small liver cancers. Semin Surg Oncol 9:298–304
Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, Makuuchi M, Strong RW (2000) The Brisbane 2000 terminology of liver anatomy and resections. HBP 2:333–339
Kobayashi S, Honda G, Kurata M, Tadano S, Sakamoto K, Okuda Y (2016) An experimental study on the relationship among airway pressure, pneumoperitoneum pressure, and central venous pressure in pure laparoscopic hepatectomy. Ann Surg 263:1159–1163
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Liver Cancer Study Group of Japan (2010) General rules for the clinical and pathological study of primary liver cancer, 3rd edn. Kanehara, Tokyo
Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T (2000) Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomized trial. Lancet 356:802–807
Cauchy F, Fuks D, Nomi T, Schwarz L, Barbier L, Dokmak S, Scatton O, Belghiti J, Soubrane O, Gayet B (2015) Risk factors and consequences of conversion in laparoscopic major liver resection. Br J Surg 102:785–795
Takenaka K, Kawahara N, Yamamoto K, Kajiyama K, Maeda T, Itasaka H, Shirabe K, Nishizaki T, Yanaga K, Sugimachi K (1996) Results of 280 liver resections for hepatocellular carcinoma. Arch Surg 131:71–76
Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, Makuuchi M (1996) Recurrence of hepatocellular carcinoma after surgery. Br J Surg 83:1219–1222
Disclosures
Goro Honda has received lecture fees from Johnson & Johnson and Covidien. Tomoki Ryu, Masanao Kurata, Shin Kobayashi, Katsunori Sakamoto, and Masahiko Honjo have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryu, T., Honda, G., Kurata, M. et al. Perioperative and oncological outcomes of laparoscopic anatomical hepatectomy for hepatocellular carcinoma introduced gradually in a single center. Surg Endosc 32, 790–798 (2018). https://doi.org/10.1007/s00464-017-5745-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5745-0