Abstract
Background
There is debate surrounding the use of laparoscopic resection for advanced gastric cancer in the Western population. Here we aim to assess the feasibility and short-term outcomes of laparoscopic gastrectomy in consecutive patients in a Western population.
Methods
From 2012 to 2014, retrospective review of 28 patients with clinically staged advanced gastric cancer (≥T3 or ≥N1) treated with laparoscopic resection.
Results
Sixty-one percentage of patients were male. Median age was 67 years (range 35–86). Median BMI was 26.5 (range 19.4–46.1). Resection types were proximal (n = 2), distal (n = 14), and total (n = 12). Twenty-six (93 %) patients underwent D2 lymphadenectomy. Four patients underwent conversion to open. Median blood loss was 125 mL (range 30–300). Median LOS was 7 days (range 4–16). Of postoperative complications, five were minor: arrhythmia (n = 1), surgical site infection (n = 3), in-hospital fall (n = 1); and four were major (intra-abdominal abscess, stricture, PE, and anastomotic bleed). T stages were Tx (n = 1), T2 (n = 3), T3 (n = 18), and T4 (n = 6). N stages were N0 (n = 4), N1 (n = 8), N2 (n = 1), and N3 (n = 15). Median tumor size was 5.8 cm (range 0–9.5). Median lymph node yield was 22 (range 6–53). All margins were negative. Median follow-up was 12.8 months (range 2–27). Six patients have died of progressive disease.
Conclusion
Following total laparoscopic resection for advanced gastric cancer, oncologic endpoints, postoperative course, and early cancer-specific follow-up are excellent. The results demonstrated here support the routine use of these techniques in the Western patient population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
There are approximately 22,220 new cases of gastric cancer diagnosed per year with an estimated 5-year survival of 28.3 % [1]. Patients commonly present with advanced disease as identified by CT scanning or endoscopic ultrasound (EUS). Advanced gastric cancer (AGC) patients (≥T2 or ≥N1) are frequently recommended for neoadjuvant chemotherapy or adjuvant chemoradiotherapy due to improvement in survival outcomes [2–4].
While there has been previous debate as to the survival benefit of a D2 lymphadenectomy in these patients, currently many experienced centers perform a spleen and pancreas preserving D2 lymphadenectomy due to a trend toward improved survival [5–8]. There has been hesitancy to accept minimally invasive techniques for the definitive management of gastric cancer given concern for the adequacy of oncologic resection including lymphadenectomy using laparoscopic approaches. Laparoscopy is widely accepted for gastric cancer staging and demonstrates metastatic disease in approximately one-third of patients with a sensitivity of 84 % [9]. With the increased incidence of gastric malignancy in Asian countries, there is more experience with both open and minimally invasive surgical approaches in this region. There are numerous reports supporting the use of laparoscopy or laparoscopic-assisted procedures in the management of early gastric cancer [10–17]. Initial studies into the use of a laparoscopic approach for AGC suggested a “laparoscopic-assisted” approach with conversion to an open procedure after initial exploration, mobilization, and lymphadenectomy for resection and subsequent reconstruction [12, 16–22]. There have been several combined trials with both early and AGC comparing laparoscopic to open approaches and some reports on the use of laparoscopic approaches for AGC [23–33]. The majority of these studies are from the Eastern world with a few more recently from Italy and the USA [23, 24, 27–30, 32, 33]. Nevertheless, there remains a paucity of data from North America, and the oncologic feasibility and safety of laparoscopic gastrectomy with D2 lymphadenectomy for AGC in this patient population is unknown. Here we present one of the largest experiences with total laparoscopic proximal, distal, and total gastrectomy for AGC in consecutive patients from a North American population with the aim to assess feasibility and short-term outcomes over the 2-year time period.
Materials and methods
Patients
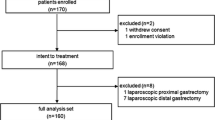
Institutional review board approval was obtained prior to study initiation. Retrospective review was completed on 28 patients with clinically staged AGC (≥T3 or ≥N1) who underwent a total laparoscopic resection from 2012 to 2014. During this time frame, all consecutive patients with gastric cancer involving the gastric cardia, body, or antrum, regardless of the clinical stage, were offered a minimally invasive resection with the exception of one patient who had a previous margin positive distal gastrectomy for presumed ulcer disease at a referring hospital 4 months prior to repeat resection for AGC. Those patients clinically staged as ≤T2, N0 disease were excluded from analysis. Procedures were performed at Roswell Park Cancer Institute (Buffalo, NY) and University of Florida (Gainesville, FL) by two independent, experienced laparoscopic surgeons. Ethnicity was collected and assigned by the clinical team at the time of intake history and physical examination.
Preoperative evaluation and management
The standard recommendations for the evaluation, staging, and management of gastric cancer were utilized including EUS and CT scans [34]. Diagnostic laparoscopy and peritoneal washings were used for the evaluation of loco-regionally advanced disease in patients being considered for neoadjuvant therapy as a separate procedure. All patients were discussed at a multidisciplinary tumor conference and considered for neoadjuvant or adjuvant therapy. Patients received therapy according to two different treatment strategies determined by patient and physician preferences. Patients were offered either neoadjuvant chemotherapy followed by surgery with the possibility of additional adjuvant chemotherapy or surgery followed by adjuvant chemoradiotherapy [3, 4]. During the later time frame of this report, more patients were directed toward neoadjuvant and adjuvant chemotherapy to avoid the use of postoperative radiotherapy.
Surgery
Participating surgeons had fellowship training in minimally invasive surgery or surgical oncology; both routinely perform more than 150 advanced, laparoscopic operations per year. All operations began with the intent for a completely laparoscopic approach with intracorporeal anastomosis. Laparoscopic proximal, distal, and total gastrectomies were included. Proximal gastrectomy was not frequently offered secondary to less optimal outcomes; however, in older patients with proximal lesions who were felt less likely to tolerate total gastrectomy, a proximal gastrectomy was felt to be a better alternative. When appropriate, laparoscopic distal gastrectomy with D2 lymphadenectomy was completed as previously described [35]. For total gastrectomy, the technique for creation of the anastomosis was completed with either the use of an Orvil stapling device or a laparoscopic side-to-side stapling technique. D2 lymphadenectomy involved resection of nodal tissue based on tumor location according to the Japanese Gastric Cancer Association [36]. Conversion to an open procedure was performed when necessary to complete a R0 oncologic resection.
Results
Patient characteristics
Median patient age was 67 years (range 35–86). Sixty-one percent of patients were male. Median BMI was 26.5 (range 19.4–46.1). The majority of patients, 71 %, were Caucasian. Sixty-one percent of patients had previous abdominal surgery. Forty-three percent of patients had a smoking history, and 39 % of patients had a history of clinically significant alcohol use. Ninety-six percent of patients had at least one medical comorbidity; 61 % of patients had three or more medical comorbidities. Fourteen percent of patients received neoadjuvant therapy only, 39 % of patients received adjuvant therapy only, 29 % of patients received both neoadjuvant and adjuvant therapy, and 18 % of patients received neither neoadjuvant or adjuvant therapy (Table 1). In five patients, reasons for not receiving either neoadjuvant or adjuvant therapy included advanced age (≥80 years) and/or comorbidities.
Details of surgery
Seven percent of patients underwent a proximal gastrectomy, 50 % of patients underwent a distal gastrectomy, and 43 % of patients underwent a total gastrectomy. Ninety-three percent of patients underwent a D2 lymphadenectomy. Two patients did not undergo a D2 lymphadenectomy due to advanced age and/or comorbidities. Twenty-four of 28 patients underwent successful laparoscopic resection with laparoscopic intracorporeal anastomosis. Four operations were converted to an open procedure. Reasons for conversion were: tumor adherent to pancreas, inability to identify an appropriate point for margin negative transection of the stomach, to obtain additional esophageal margin after intraoperative proximal margin was positive during minimally invasive approach, and to assist with anvil placement into the esophagus during total gastrectomy. Median operative time was 328.5 min (range 232–481). Median estimated blood loss was 125 mL (range 30–300) (Table 2).
Pathology
All patients had adenocarcinoma. The types of adenocarcinoma were: mucinous adenocarcinoma with a signet ring cell component (1), well differentiated (1), moderately differentiated (8), poorly differentiated without signet ring cell features (2), and poorly differentiated with signet ring cell features (15). One patient had no residual tumor following neoadjuvant therapy. Median tumor size was 5.8 cm (range 0.0–9.5). T stages were Tx(n = 1), T1(0), T2(3), T3(18), and T4a(6). N stages were N0(n = 4), N1(8), N2(1), and N3(15). Two patients were found to have metastatic disease on final pathology; one patient had periaortic lymph node involvement, and the other had a positive peritoneal biopsy recognized on final pathology only. Final pathologic stages were x(1), IB(1), IIA(3), IIIA(9), IIIB(9), IIIC(3), and IV(2). All patients had negative proximal and distal margins. Median number of lymph nodes harvested was 22 (range 6–53); median number of positive nodes was 9 (range 0–39, Table 3).
Postoperative outcomes
There were no immediate intraoperative complications, and no patients required reoperation. Median total hospital length of stay was 7 days (range 4–16). One patient required readmission within 30 days of discharge. There were nine complications in eight patients. There were five minor complications: arrhythmia (1), surgical site infection (3), and in-hospital fall (1). There were four major complications: intra-abdominal abscess (1), pulmonary embolism (1), anastomotic stricture requiring dilation × 3 (1), and bleeding from anastomosis following initiation of anti-coagulation requiring cessation of anticoagulation and placement of an inferior vena cava filter (1). Clavien-Dindo classifications were I (3), II (2), and III (4). Median follow-up was 12.8 months (range 2–27). Six patients have died of progressive disease, one is alive with disease, and the remainder of patients is currently without evidence of disease (Table 4).
Discussion
Laparoscopy for staging and resection of early gastric cancer is widely accepted. Previous studies have demonstrated reduced blood loss, shorter hospital stay, and earlier return to oral intake with laparoscopic compared to open gastrectomy [15, 18, 19, 21, 23]. Kim et al. [37] reported improved quality of life outcomes following laparoscopic compared to open gastrectomy for early gastric cancer with regard to patients’ physical, emotional, and social function. Specifically, patients reported improvement in pain scores, and body image. Still the majority of the available literature supports a “combined” or “laparoscopic-assisted” approach to gastrectomy for advanced gastric cancer. This is a popular approach in Asia, where the gastric cancer incidence is much higher and experience with minimally invasive approaches is far greater for this disease [12, 18–21].
This combined procedure involves the completion of the lymphadenectomy laparoscopically through a 5-port technique. The procedure is converted to an open approach near completion of the lymphadenectomy for the remaining gastrectomy and anastomosis [18, 19, 21]. This approach has been shown to result in significantly longer operative times when compared to an open approach; but similar to previous reports in early gastric cancer, a laparoscopic-assisted approach also resulted in lower blood loss, analgesic use, and shortened recovery time [18, 19, 21]. The popularity of the combined approach results from the concern over the technical difficulty of an adequate lymphadenectomy when completed through a completely laparoscopic technique [12]. Huscher et al. reported on a totally laparoscopic approach to the management of early and advanced gastric cancer. In their series of 100 patients, they reported acceptable outcomes in regards to oncologic resection, morbidity, and mortality.
There have been only a few studies from North America focusing on laparoscopic resection for gastric cancer [27, 29, 30, 32, 33] In 2003 and 2006, Weber et al. [33] and Varela et al. [32], respectively, reported on their combined series of patients undergoing laparoscopic resection for gastric cancer. In total, nine patients presented with advanced disease (≥Stage II) were treated with laparoscopic resection with acceptable outcomes. In 2009, Strong et al. [29] reported on their institutional series of subtotal gastrectomies for gastric cancer. Of the 30 patients treated with laparoscopic resection, 12 had AGC (≥Stage II). Similar to other studies, laparoscopic resection was associated with longer operative time, decreased analgesic use, and shorter length of stay. Kachikwu et al. [27] also reported on their pilot series of 16 patients undergoing laparoscopic total gastrectomy, nine of whom had AGC (≥Stage II). There were no conversions in their series with two patients requiring en bloc additional visceral resections. Both studies concluded that the minimally invasive approach was safe and adequate for gastric cancer patients.
Most recently, Kelly et al. [30] published their retrospective series of patients undergoing laparoscopic gastrectomy compared with patients treated with an open resection over the same time period. In this series, 87 patients underwent laparoscopic gastric cancer resection, with a minority (n = 32) of those patients treated for advanced gastric cancer (≥Stage II). Overall, laparoscopic resection was associated with longer operative time, decreased blood loss, decreased analgesic use, shorter length of stay, and fewer minor complications. There was one 30-day mortality in the laparoscopic group and none in the open group. Of note, eight of the patients treated with laparoscopic resection had microscopically positive margins. The present report compares favorably to this series as there was a similar number of patients undergoing laparoscopic resection for AGC with a zero percent incidence of microscopically positive margins. Perhaps the zero margin positivity rate is related to appropriate conversion to an open procedure when concerned for adequate oncologic resection, evidenced by the 14 % conversion rate reported in this study.
Complete laparoscopic gastrectomy for AGC including proximal, distal, and total gastrectomies is feasible in the majority of patients and safe. Here we present a 28-patient series with eight patients experiencing a morbidity (28.6 %), four of which were minor. There were no patients experiencing a postoperative mortality. The median length of stay in our patient population was 7 days (range 4–16). This compares favorably to previous reports from larger database analyses (NSQIP), which included primarily open approaches, where the median length of stay was 12 days [38]. Reflecting the predominant patient population in North America, BMI was elevated in our patient population which was predominantly Caucasian. Nevertheless, we feel the immediate and short-term oncologic outcomes of these techniques are comparable to open approaches with low morbidity rates. We believe laparoscopic approaches should be routinely considered and offered in North America and the Western population.
References
National Cancer Institute Surveillance, Epidemiology, and End Results Program Stomach Cancer Stat Fact Sheet (2011). Available: http://seer.cancer.gov/statfacts/html/stomach.html. Accessed 2 Feb 2015
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines(R)): Gastric Cancer. (2015). Available: http://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Accessed 2 Feb 2015
Cunningham D, Allum WH, Stenning SP et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. New Engl J Med 355:11–20
Macdonald JS, Smalley SR, Benedetti J et al (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. New Engl J Med 345:725–730
Bonenkamp JJ, Hermans J, Sasako M, Van De Velde CJH (1999) Extended lymph-node dissection for gastric cancer. New Engl J Med 340:908–914
Seevaratnam R, Bocicariu A, Cardoso R et al (2013) A meta-analysis of D1 versus D2 lymph node dissection. Gastric Cancer 15:S60–S69
Griffith JP, Sue-Ling HM, Martin I, Dixon MF, McMahon MJ, Axon ATR, Johnston D (1995) Preservation of the spleen improves survival after radical surgery for gastric cancer. Gut 36:684–690
Wanebo HJ, Kennedy BJ, Winchester DP, Stewart AK, Fremgen AM (1997) Role of splenectomy in gastric cancer surgery: adverse effect of elective splenectomy on longterm survival. J Am Coll Surg 185:177–184
Burke EC, Karpeh MS Jr, Conlon KC, Brennan MF (1997) Laparoscopy in the management of gastric adenocarcinoma. Ann Surg 225:262–267
Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y (2002) A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 131:S306–S311
Lee J-H, Han H-S, Lee J-H (2005) A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc Interv Tech 19:168–173
Azagra JS, Goergen M, De Simone P, Ibañez-Aguirre J (1999) Minimally invasive surgery for gastric cancer. Surg Endosc 13:351–357
Kim H-H, Hyung WJ, Cho GS et al (2010) Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report-a phase III multicenter, prospective, randomized trial (KLASS trial). Ann Surg 251:417–420
Mochiki E, Kamiyama Y, Aihara R, Nakabayashi T, Asao T, Kuwano H (2005) Laparoscopic assisted distal gastrectomy for early gastric cancer: five years’ experience. Surgery 137:317–322
Tanimura S, Higashino M, Fukunaga Y, Kishida S, Nishikawa M, Ogata A, Osugi H (2005) Laparoscopic distal gastrectomy with regional lymph node dissection for gastric cancer. Surg Endosc Interv Tech 19:1177–1181
Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T (2011) A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg 28:331–337
Fang C, Hua J, Li J et al (2014) Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymphadenectomy for advanced gastric cancer. Am J Surg 208:391–396
Ziqiang W, Feng Q, Zhimin C, Miao W, Lian Q, Huaxing L, Peiwu Y (2006) Comparison of laparoscopically assisted and open radical distal gastrectomy with extended lymphadenectomy for gastric cancer management. Surg Endosc Interv Tech 20:1738–1743
Hwang SI, Kim HO, Yoo CH, Shin JH, Son BH (2009) Laparoscopic-assisted distal gastrectomy versus open distal gastrectomy for advanced gastric cancer. Surg Endosc Interv Tech 23:1252–1258
Park DJ, Han S-, Hyung WJ et al (2012) Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc Interv Tech 26:1548–1553
Shuang J, Qi S, Zheng J et al (2011) A case–control study of laparoscopy-assisted and open distal gastrectomy for advanced gastric cancer. J Gastrointest Surg 15:57–62
Zhao Y, Yu P, Hao Y et al (2011) Comparison of outcomes for laparoscopically assisted and open radical distal gastrectomy with lymphadenectomy for advanced gastric cancer. Surg Endosc Interv Tech 25:2960–2966
Huscher CGS, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C (2005) Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 241:232–237
Huscher CGS, Mingoli A, Sgarzini G, Brachini G, Binda B, Di Paola M, Ponzano C (2007) Totally laparoscopic total and subtotal gastrectomy with extended lymph node dissection for early and advanced gastric cancer: early and long-term results of a 100-patient series. Am J Surg 194:839–844
Goh PMY, Khan AZ, So JBY, Lomanto D, Cheah W-K, Muthiah R, Gandhi A (2001) Early experience with laparoscopic radical gastrectomy for advanced gastric cancer. Surg Laparosc Endosc Percutaneous Tech 11:83–87
Uyama I, Sugioka A, Fujita J, Komori Y, Matsui H, Hasumi A (1999) Laparoscopic total gastrectomy with distal pancreatosplenectomy and D2 lymphadenectomy for advanced gastric cancer. Gastric Cancer 2:230–234
Kachikwu EL, Trisal V, Kim J, Pigazzi A, Ellenhorn JDI (2011) Minimally invasive total gastrectomy for gastric cancer: a pilot series. J Gastrointest Surg 15:81–86
Scatizzi M, Kröning KC, Lenzi E, Moraldi L, Cantafio S, Feroci F (2011) Laparoscopic versus open distal gastrectomy for locally advanced gastric cancer: a case-control study. Updates Surg 63:17–23
Strong VE, Devaud N, Allen PJ, Gonen M, Brennan MF, Coit D (2009) Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: a case–control study. Ann Surg Oncol 16:1507–1513
Kelly KJ, Selby L, Chou JF et al. (2015) Laparoscopic versus open gastrectomy for gastric adenocarcinoma in the west: a case–control study. Ann Surg Oncol 22:3590–3596
Pugliese R, Maggioni D, Sansonna F et al (2007) Total and subtotal laparoscopic gastrectomy for adenocarcinoma. Surg Endosc Interv Tech 21:21–27
Varela JE, Hiyashi M, Nguyen T, Sabio A, Wilson SE, Nguyen NT (2006) Comparison of laparoscopic and open gastrectomy for gastric cancer. Am J Surg 192:837–842
Weber KJ, Reyes CD, Gagner M, Divino CM (2003) Comparison of laparoscopic and open gastrectomy for malignant disease. Surg Endosc Interv Tech 17:968–971
Layke JC, Lopez PP (2004) Gastric cancer: diagnosis and treatment options. Am Fam Phys 69:1133–1140
Ben-David K, Tuttle R, Kukar M, Oxenberg J, Hochwald SN (2014) Laparoscopic distal, subtotal gastrectomy for advanced gastric cancer. J Gastrointest Surg 19:369–374
Sano T, Kodera Y (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123
Kim Y-W, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, Bae J-M (2008) Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: Results of a prospective randomized clinical trial. Ann Surg 248:721–727
Papenfuss WA, Kukar M, Oxenberg J, Attwood K, Nurkin S, Malhotra U, Wilkinson NW (2014) Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol 21:3008–3014
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Rebecca Tuttle and Moshim Kukar have nothing to disclose. Drs. Steven Hochwald and Kfir Ben-David are consultants for Ethicon EndoSurgery.
Rights and permissions
About this article
Cite this article
Tuttle, R., Hochwald, S.N., Kukar, M. et al. Total laparoscopic resection for advanced gastric cancer is safe and feasible in the Western population. Surg Endosc 30, 3552–3558 (2016). https://doi.org/10.1007/s00464-015-4652-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4652-5