Abstract
Background
Laparoscopic gastrectomies are currently performed in many centers, but compliance with oncologic requirements still represents a subject open to debate. The aim of this work was to compare the short-term and oncologic outcomes after laparoscopic and open surgery in gastric adenocarcinoma.
Methods
From June 2000 through June 2005, 147 patients in our institution underwent gastrectomy by open or mininvasive approach for adenocarcinoma. The laparoscopy group included 48 patients, 29 with early gastric cancer (EGC) and 19 with antral advanced gastric cancer (AGC). The short-term results and oncologic data were compared to those obtained in 99 patients who underwent open surgery. Survival in the laparoscopy group was analyzed.
Results
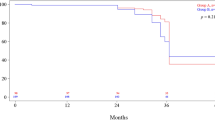
In the laparoscopy group no intraoperative complications were observed, and conversion was needed in only one patient with a large advanced tumor. Overall, 32 lymph nodes were collected by D2 dissection, 30 for EGC, 34 for advanced cancers. The resection margin was 6.7 cm (range: 4–8 cm). The mean operating time was 240 min (range: 150–360 min), with a blood loss of 150 ml on average (range: 70–250 ml). Morbidity included two duodenal leaks that healed without reoperation; after enclosing or reinforcing the staple line, no further leaking was noted. There was one death from massive bleeding in a cirrhotic patient. Ambulation and oral feeding started significantly earlier than in open surgery. The mean hospital stay was 10 days (range: 7–24 days), significantly shorter than the stay of 18 days after open surgery (p < 0.05). All patients treated laparoscopically were alive without recurrence at the end of this study.
Conclusions
Short-term results with laparoscopic gastrectomy were better than with open surgery in this study. Oncologic radicality was a major concern, but in the authors’ experience the extent of lymphadenectomy was the same as in open surgery. This study suggests that laparoscopic gastrectomy in malignancies is a reliable tool and oncologic requirements can be warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopic gastrectomy has gained wide acceptance, but its use in carcinoma still needs further documentation. The first laparoscopic gastrectomy for carcinoma was a distal resection with Billroth I reconstruction reported by Kitano in 1991 [1]. The first laparoscopic distal gastrectomy with Billroth II reconstruction was performed by Goh in 1992 in the treatment of a chronic benign ulcer [10, 11], and the first laparoscopic Billroth II distal gastrectomy for cancer was performed in 1993 by Azagra et al., who also performed the first laparoscopic total gastrectomy [5, 6]. Since then, many authors have reported their experience with minimally invasive surgery of the stomach, reporting adequacy of resection margin and nodal clearance [3, 8, 13–16, 19, 23, 24, 33, 37].
A Japanese randomized trial on distal gastrectomy with Billroth I reconstruction for early gastric cancer (EGC) demonstrated the short-term assets of the laparoscopic approach over open techniques: less need for analgesic drugs, less impairment of pulmonary function, shorter times to ambulation, earlier return to bowel function and oral intake of food, shorter length of hospital stay (LOS) [22]. The good results of laparoscopic surgery with EGC have broadened the indications to include advanced gastric carcinoma (AGC), and with acceptable outcomes [12, 16, 17, 24]. This study examined short-term outcomes and oncologic data of laparoscopic gastrectomy for carcinoma. Morbidity, mortality, length of specimen, extent of resection margin, and number of nodes removed are compared with the same data for open surgery, based on the current authors’ experience.
Patients and methods
Between June 2000 and June 2005, 147 patients in our hospital underwent gastrectomy for nonmetastatic carcinoma. There were 115 men and 32 women (mean age: 70 years; range: 40–85 years). Data collected included age, gender, American Society of Anesthesiologists (ASA) status, and indications for surgery. The incidence of EGC in our series has remained 17% since 1993 [29]. Open surgery was carried out in 99 patients with AGC and included 35 total gastrectomies and 64 subtotal gastrectomies, with D2 or D1 modified dissection (D3 in 26 cases). Laparoscopic gastrectomy was proposed to 48 patients without nodal involvement in the preoperative staging. In selecting patients for laparoscopic gastrectomy, our policy has been to consider, first, patients with early cancers located in any region of the stomach; a second group of patients are those with antral advanced cancers (Table 1). At the outset of this study, both groups included selected patients >75 years of age in whom D2 dissection was not required [9]. According to the latest Japanese Classification of Gastric Carcinoma (JGCA) classification, extended D2 dissection [20] is deemed feasible by laparoscopy only for advanced cancer of the antrum, and not for advanced cancer of other regions of the stomach.
Study population
The surgical risk was calculated from the ASA score. Seventy-eight patients (53%) were ASA II, sixty-three ASA III (43%), and six ASA I (4%). Demographics and preoperative data of patients undergoing laparoscopic gastrectomy are shown in Table 1: associated illnesses were cardiovascular disease (11 patients) and chronic disease of the respiratory tract (4 patients).
Preoperative ultrasound endoscopy demonstrated 27 T1m and 2 T1sm with no lymphatic involvement, confirmed by histological examination. One patient was affected both by early gastric cancer and right colonic cancer, so that laparoscopy-assisted right hemicolectomy was performed, in addition to laparoscopic subtotal gastrectomy (LSG). Laparoscopic total gastrectomy (LTG) with D1 modified dissection was the procedure chosen for early cancers of the upper two-thirds of the stomach. Laparoscopic subtotal gastrectomy with extended nodal clearance according to JGCA criteria [20] was the preferred procedure, both for early and advanced cancers of the lower third of the stomach. In all of the 19 patients with advanced cancers selected for laparoscopic excision, the tumors were located in the lower third of the stomach. This group consisted of 12 men and 7 women (mean age: 78 years; range: 71–85 years). Endoscopy diagnosed 17 advanced cancers, and two others were preoperatively underdiagnosed as early cancers, excised by subtotal gastrectomy with D2 dissection, and staged as advanced cancers after pathologic examination. No patient received neoadjuvant chemotherapy. The mean duration of follow-up in the laparoscopy group was 35.5 months (range: 4–60 months) for patients with early cancer, and 11.4 months (range: 1–24 months) for patients with advanced cancer.
Preoperative work-up
Preoperative staging included endoscopy, biopsy and vital staining, percutaneous echography, and helical-computed tomography (CT) scan. The EGC were marked with Indian ink for reference in laparoscopy and staged by echoendoscopy using a 15 MHz probe. The advanced cancers were staged by endoscopy and CT scan.
Preparation consisted of a diet without starches, 30 carbon tablets, and 4 liters of polyethyleneglycol (PEG) 2 days before surgery. Prophylaxis against thromboembolism with calcic nadroparine 0.3 ml was given subcutaneously the night before surgery, and antibiotic prophylaxis with cephazoline 6 g was administered i.v. the day of surgery.
Study design
This study was designed to evaluate retrospectively the outcome of laparoscopic gastrectomy with D2 dissection and to compare the short-term results of analysis with those of open gastrectomy with D2 dissection in our experience. The main concern was to perform the same operation with nodal dissection as in open surgery. The analysis regarded conversion rate, operating time, blood loss, times to mobilization and ambulation, analgesic medication, return to bowel function, removal of nasogastric tube (NG), resumption of soft diet, morbidity, mortality, length of hospital stay (LOS), length of resection margin, and number of nodes harvested. Patients eligible for minimally invasive surgery were divided into EGC and AGC groups. Data collected in each group were derived from the clinical charts and were compared by analysis of variance (ANOVA) and chi-square test. All statistical calculations were done by SPSS package (version 11.0). Significant values were established at p < 0.05 with a confidence interval of 95%.
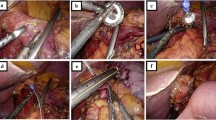
Surgical Technique
The supine patient is positioned with head-up tilt (20°), legs parted, and the left arm abducted with the intravenous line. The surgeon works between the legs of the patient, and the optic assistant stands at the surgeon’s left side. Four surgical ports are used. The carbon dioxide pneumoperitoneum is instituted through a 12-mm umbilical cannula for open laparoscopy (Hasson trocar) and set at 14 mmHg. Three more 10-mm trocars are then inserted in the upper abdomen, below the xyphoid process, in the right, and in the left quadrant.
Gastric dissection, D2 extended lymphadenectomy, and closure of the duodenum
Coloepiploic detachment with omentectomy is the first maneuver, followed by dissection of the gastrocolic ligament. The left gastroepiploic vessels are divided. All these maneuvers are performed with a Harmonic Scalpel (Ethicon Endo-Surgery Inc, Cincinnati, OH).
The dissection next moves to the pylorus, and the right gastroepiploic vein is divided between absorbable clips flush with the Henle venous trunk and ends up in the Fredet area, where tier 14v is removed. The right gastroepiploic artery is cut between absorbable clips at its origin from the gastroduodenal artery, just above the pancreatic head. The pyloric vessels are sectioned, the pylorus is freed, and infrapyloric tiers 4 and 6 are resected together. Incision of the lesser omentum and dissection of the lesser curvature allows lymphatic dissection of groups 1 (right paracardial), 3 (lesser curve), and 5 (suprapyloric), as well as dissection of group 12a in the hepatoduodenal ligament. The duodenum is transected and closed with a 45-mm cartridge linear stapler (blue reloads, with triple-staggered rows of staples). The staple line is enclosed by separated stitches or reinforced by Seamguard bioabsorbale polycarbonate material (W. L. Gore & Associates, Inc., Flagstaff, AZ). Subsequently ablation of tiers 8 (common hepatic artery), 9 (celiac axis), and 11p (proximal splenic artery) is achieved, and the left gastric artery is sectioned by linear endostapler with removal of group 7 (left gastric artery). Lifting of the gastric remnant permits excision of posterior right paracardial nodes in the esophagogastric region (tier 1) and achievement of lymphadenectomy. Entering the lesser sac allows sectioning of adhesions between the pancreas and the posterior wall of the stomach, as well as bursectomy.
Technique for laparoscopic subtotal gastrectomy (LSG)
The stomach is transected with a 45-mm (blue reload) cartridge linear stapler, collected into an endo bag, and left in the right side of the abdominal cavity. The second jejunal loop is chosen to prepare a Roux-en-Y transmesocolic loop. The gastric remnant is set close to the jejunal loop with two stitches, and two openings are created through which to insert the mechanical device. The 45-mm cartridge stapler is then fired so as to fashion a side-to–side gastroenteric anastomosis.
Suture of the openings by interrupted extracorporeal slipknots closes the access to the gastric stump and jejunal limb. The side-to-side jejunojejunal anastomosis at the foot of the Roux-en-Y loop is constructed with the 45-mm endostapler. The endobag containing 4/5 of the stomach resected with the D2 lymphatic stations is retrieved through the umbilical port. Minimal enlargement of the incision (up to 3–4 cm) may be necessary in some instances. A drain is placed near the duodenal stump. Closure of port incisions ends the procedure.
Technique for laparoscopic total gastrectomy (LTG)
The first steps of gastric dissection and closure of the duodenal stump are as described for LSG. The extent of lymphadenectomy is also the same, and that for proximal tumors corresponds to a D1 beta clearance. Dissection of the gastrocolic ligament is conducted as far as the gastrosplenic ligament. Farther on, the shorter vessels are divided with the ultrasound scalpel, and dissection proceeds to the diaphragmatic crus, dividing the phrenoeosphageal membrane and vagal nerves with the harmonic scalpel.
Group 2 (left paracardial) is resected. The esophagus is transected with the 45-mm cartridge linear stapler, and the whole stomach is collected in an endobag and left in the peritoneal cavity. The second jejunal loop and the esophageal stump are prepared by suspending the jejunal loop with stitches at the posterior wall of the esophagus. Two access openings are created, and the mechanical device is inserted to construct a side-to-side esophagojejunal anastomosis according to Orringer’s model [28]. The openings are closed by separated extracorporeal slipknots. The jejunojejunal side-to-side stapled anastomosis at the foot of the Roux-en-Y loop accomplishes restoration of the digestive tract. Minimal enlargement of the umbilical incision is needed to withdraw the bag containing the whole stomach with nodal stations. A drain is placed in Morrison’s pouch near the duodenal stump. Closure of the port incisions ends the operation.
Results
Between 2000 and 2005, 48 patients in our hospital underwent laparoscopic operations for adenocarcinoma of the stomach. Forty-seven laparoscopic gastrectomies (43 LSG and 4 LTG) were performed by means of intracorporeal operations (Table 2). No intraoperative complication occurred. For EGC there was no conversion, whereas one conversion was needed for a large advanced cancer (conversion rate 2%). In the laparoscopic group the short-term outcomes were significantly better than in the open group (Table 3). The mean weight loss after 15 days accounted for 3% of body weight with laparoscopic gastrectomies and for 3.5% with open procedures. Subtotal 4/5 gastrectomy was the procedure of choice also for antral EGC so as to ensure a 6 cm. safety margin. The estimated blood loss was 150 + 85 ml on average (range: 70–250 ml), significantly less than in open surgery (394 + 125 ml). The estimated blood loss for subtotal gastrectomy was 147 + 60 ml, and for total gastrectomy it was 180 + 94 ml (p > 0.05). The operating time was 240 + 23 min on average (range: 150–360 min), not significantly shorter than in open surgery (220 + 31 min). The operating time for LSG was 237 + 18 min, and for LTG it was 275 + 20 min (differences NS = nonsignificant). The postoperative results of laparoscopic surgery for EGC and AGC were statistically compared, but differences between these two groups were NS (p < 0.05). Among the laparoscopic procedures, the average LOS for subtotal gastrectomy was 9.8 + 2.5 days, and for total gastrectomy it was 11.6 + 2 days (p < 0.05). The morbidity rate was lower than in open surgery, although the difference was NS (p > 0.05) (Tables 2 and 3). Leaking of the duodenal stump was observed in the laparoscopic group in 2 patients (4%) treated, respectively, for early and advanced gastric cancer. The leaks occurred when the duodenal stump had just been stapled, and both healed conservatively. The staple line was then routinely enclosed by separated stitches or reinforced by Seamguard polycarbonate material, so that further stump leaks were not observed in this study. No anastomotic leaks were observed in the laparoscopic group. One duodenal leak (1%) and two anastomotic leaks (2%) were observed in 99 open gastrectomies. One death occurred in the laparoscopic group after 13 days: a patient with EGC was affected by alcoholic cirrhosis owing to massive bleeding from gastrojejunal anastomosis and acute hepatic failure with jaundice, ascites, and encephalopathy, and this was the only patient requiring transfusions in the postoperative period. Mortality rate (2%) was lower than in the open group (3%), without significant difference. The average distance of the resection margin from the tumor showed no significant difference with open surgery (Table 3), and the resection margins were always clear of tumor on histologic examination. D2 for antral tumours consisted in ablation of tiers 1, 3–9, 11p, 12a, and 14v according to the latest JGCA classification [20]. The number of nodes resected has grown with the training, and a mean number of 32 + 9 lymph nodes was harvested in laparoscopic D2, 30 + 7.5 for early gastric cancers and 34 + 10 for advanced cancers (Table 2) The mean number of nodes resected with LSG was 31.5 + 9.5, and that with LTG was 35 + 4. The mean number of nodes removed in open gastrectomy was 36 + 14 with D2 dissection and 39 + 2 with D3 dissection. The difference between the number of lymph nodes resected with D2 in laparoscopy and in open surgery was not relevant (p < 0.05). Sensitivity of echoendoscopy for EGC was 100%, whereas specificity was 93% because two advanced cancers were misdiagnosed preoperatively as early cancers (29/31).The extent of lymphadenectomy covered the reach of D2 also for antral EGC, so that adequate nodal clearance was achieved in cases underestimated by echoendoscopy. In the first three cases of laparoscopic subtotal gastrectomy for advanced cancer, after the laparoscopic procedure was complete a median laparotomy was performed just to assess the extent of D2, which actually corresponded to the extent of D2 performed in open surgery: of course, these patients were excluded from analysis of ambulation, analgesic medication, oral intake, and LOS. The one converted patient (pT3N1) had local recurrence after 3 months. Two thirds of patients in this series were affected by EGC (29/48), and they were all alive and disease free. A more detailed comparison of results in antral EGC was possible by matching 24 patients with pT1N0 cured by LSG in this study (excluding operative mortality) with 25 pT1N0 cured by open subtotal gastrectomy (OSG) before June 2000: the short-term results were better with LSG (p < 0.05), in particular mean LOS was 9.5 versus 15 days with OSG. Distance of resection margin (6.5 + 0.7 versus 6 + 0.9) and number of nodes removed (30 + 5 versus 33 + 4) did not show significant differences (p > 0.05). In the AGC group all patients were alive and disease free. Five patients affected by advanced cancer (pT2N1-2) underwent adjuvant chemotherapy. One patient complained of dumping syndrome and symptoms eased off after fragmentation of meals. For detailed comparison of antral pT2 tumors, 17 patients (12 pN0) cured by LSG were matched to 26 patients (11 pN0) cured by OSG in this study, having similar health conditions. Three patients who underwent laparotomy after the end of LSG were excluded from analysis of short-term results, which were better than those of open surgery (p < 0.05), with a mean LOS of 10.8 versus 16.7 days. As to oncologic parameters, distance of resection margin (6.8 + 0.6 versus 6.4 + 0.7) and number of nodes removed (34 + 8 versus 35 + 6) were not significantly different (p > 0.05).
Discussion
Many authors have reported on efficacy and effectiveness of laparoscopic gastrectomies, but these procedures in carcinoma are still under debate. Adachi et al. demonstrated no significant difference in operating time, distance from the margin of resection, number of nodes harvested and morbidity in laparoscopic and open surgery [1]. This study confirmed the short-term assets of laparoscopic over open surgery (Table 3). The average operating time for laparoscopic gastrectomy reported by other authors ranges between 209 and 280 min [1, 3, 5, 11, 12, 26, 37], and in this study it was 240 min, not significantly different from the mean operating time (220 min) needed in open surgery (Table 3). Mean blood loss reported in the literature ranges between 60 and 300 ml [1, 3, 5, 8, 26, 37], and it was 150 ml in our study, significantly less than the mean blood loss (394 ml) in open surgery (Table 3). Many reports show that LOS after laparoscopic gastrectomy (6–17 days) is shorter than after open gastrectomy (9–25 days) [3, 8, 13, 16–19, 23, 24, 26, 27, 30, 31, 33, 34, 36, 37]. In this study mean LOS after laparoscopic gastrectomy was 10 days, significantly shorter than the 18 days registered with open surgery (Table 3), and quite comparable to mean LOS reported in other series. The difference in LOS between total and subtotal gastrectomies was not significant in the present study. Operative morbidity and mortality were lower than in open surgery in our series, although without statistically significant differences (Table 3). Pulmonary morbidity (3 cases of pneumonia) was likely to be affected by including patients >75 years at the outset of experience. Azagra et al. published a series of 24 laparoscopic gastrectomies for benign gastric ulcer reporting no stump or anastomotic complications [4]. In contrast, issues on laparoscopic surgery in gastric cancer reported anastomotic or stump complications [7, 16, 33]. Goh et al. published the early international results of patients undergoing laparoscopic surgery for cancer or benign disease, reporting two duodenal leaks with one death, but it was not detailed whether the leaks belonged to patients operated on for benign or malignant illness [11]. In this study the incidence of duodenal stump leakage after laparoscopic gastrectomies was 4%, until the staple line was enclosed or reinforced as we used to do in open gastrectomies. The incidence of duodenal stump leakage in open surgery was 1.5% in a previous study by the authors [29], and 1% in the present study. For early cancer of the lower distal third or subtotal gastrectomy have been advocated [3, 8, 13, 16, 17, 24]. Laparoscopic techniques in gastric cancer should consist of radical operations with equal node dissection, as in open surgery. Shimizu et al. showed no difference in retrieval of nodes between laparoscopic and open surgery [31]. Other authors have reported removal of at least 31 nodes laparoscopically [5, 13, 16, 17, 33], but Mochiki et al. have reported many advantages of laparoscopic gastrectomy over open gastrectomy, except for the number of nodes removed [26].
Lymphadenectomy in EGC is debated: the incidence of lymph node metastasis in mucosal EGC is 0–10%; in submucosal EGC it is 11%–35%. Hence lymphadenectomy may be considered overtreatment in at least 65% of early gastric cancers [15, 16, 33, 35]. For well-differentiated mucosal EGC <4.5 cm, extensive lymphadenectomy is even unnecessary [15]. In mucosal early gastric cancers D1 dissection with removal of 1.3–6 stations according to JGCA would suffice [20].
Regarding advanced gastric cancer, the effectiveness of extended lymphadenectomy on long-term survival remains a subject for debate, because there is no evidence of statistically significant improvement of survival with D2, either in observational studies or in randomized studies [9, 14, 25]. Moreover, according to a nonrandomized study by Eguchi et al. [9], extended D2 nodal clearance seems to have no impact on long-term survival in patients over 75 years of age. Adequacy of lymphadenectomy was a main concern in this study, which included tumors in which D2 dissection had little impact on prognosis, i.e., early cancers <4 cm in patients >75 years old [9, 15]. The progressive selection of patients in our study resulted in performance of adequate laparoscopic lymphadenectomy with retrieval of an increasing mean number of nodes (Table 2). In this study the overall number of nodes collected by laparoscopic D2 in antral tumors was 32 + 9 (Table 3), and D2 dissection complied with JGCA criteria and also with Western criteria of a minimum of 25 nodes harvested [32], both in early and advanced gastric cancers of the lower third (Table 2). The majority of EGC in this series (23/29 = 79%) were <4 cm and were well or moderately differentiated (28/29 = 96%). Subtotal gastrectomy with D2 dissection in early gastric cancers of the lower third was the preferred procedure in this study, and there are some reasons for this choice. Shimizu et al. reported that 29% of early cancers diagnosed preoperatively as mucosal were submucosal after histological examination [31], and the incidence of lymph node metastasis in submucosal early cancers can be high [35]. In our series two gastric cancers were preoperatively underdiagnosed as early, and their advanced stage was ascertained only after surgery through the histopathologic records.
The incidence of multifocal EGC is 7%–21%, especially for the intestinal or differentiated type, and the risk is higher in older patients [21, 35]. In our series 79% of EGC were intestinal type, 75% were well differentiated, and the mean age of the patients was 68 years (Table 1). Thus for antral neoplasms a safety margin of at least 6 cm with extended nodal clearance was ensured by subtotal gastrectomy. As a result, during the follow-up, the patients who had undergone laparoscopic subtotal gastrectomy for EGC were all alive and disease free. In the AGC group, patients were also alive and disease free. A detailed comparison of outcomes in antral pT1 and pT2 confirmed the data of this study, although with statistical limits as far as long-term results were concerned. In fact, in this series no EGC was cured by open surgery, and patients with pT1 who benefited by LSG were matched to a series of patients with pT1 who had undergone OSG before this study. Beyond that, in particular for advanced cancers, the duration of follow-up was too short. The number of patients treated laparoscopically was small, and they were all alive. Completion of follow-up was deemed essential to obtain adequate statistical statements about survival.
Conclusions
It has been highlighted how laparoscopic gastrectomies can be accomplished by thorough intracorporeal technique with no intraoperative complications, a very low conversion rate, and better short-term outcomes than in open surgery. The results of the present study suggest that laparoscopic surgery of the stomach is a safe procedure: oncological requirements were respected, and the same nodal dissection as in open surgery was achieved.
References
Adachi Y, Shirahishi N, Shiromizu A, Bandoh T, Aramaki M, Kitano S (2000) Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg 135: 806–810
Adachi Y, Shirahishi N, Ikebe K, Aramaki M, Bandoh T, Kitano S (2001) Evaluation of the cost of laparoscopic-assisted Billroth I gastrectomy. Surg Endosc 15: 932–936
Asao T, Hosouchi Y, Nakabayashi T, Haga N, Mochiki E, Kuwano H (2001) Laparoscopically assisted total or distal gastrectomy with lymph node dissection for early gastric cancer. Br J Surg 88: 128–132
Azagra JS, Goergen M, De Simone P, Ibanez-Aguirre J. (1999) The current role of laparoscopy surgery in the treatment of benign gastroduodenal disease. Hepatogastroenterology 46: 1522–1526
Azagra JS, Goergen M, De Simone P, Ibanez-Aguirre J (1999) Minimally invasive surgery for gastric cancer. Surg Endosc 13: 351–357
Azagra J, Goergen M, Gilbart E, Alonso J, Ceuterick M (2001) Laparoscopy-assisted total gastrectomy with extended D2 lymphadenectomy for cancer: technical aspects. Le Jour Coelio-chir 40: 79–83
Ballesta Lopez C, Ruggiero R, Poves I, Bettonica C, Procaccini E (2002) The contribution of laparoscopy to the treatment of gastric cancer. Surg Endosc 16: 616–619
Dulucq JL, Wintringer P, Stabilini C, Solinas L, Perissat J, Mahajina A. (2005) Laparoscopic and open gastric resections for malignant lesions: a prospective comparative study. Surg Endosc 19: 933–938
Eguchi T, Takahashi Y, Ikarashi M, Kasahara M, Fuji M (2000) Is extended lymph node dissection necessary for gastric cancer in elderly patients? Eur J Surg 166: 949–953
Goh P, Tekant Y, Isaac J, Kum CK, Ngoi SS (1992) The technique of laparoscopic Billroth II gastrectomy. Surg Laparosc Endosc 2: 258–260
Goh P, Alponat A, Mak K, Kum CK (1997) Early international results of laparoscopic gastrectomies. Surg Endosc 11: 650–652
Goh PMY, Khan AZ, So JBY, Lomanto D, Cheah WK, Muthiah R, Gandhi A (2001) Early experience with laparoscopic radical gastrectomy for advanced gastric cancer. Surg Laparosc Endosc Percutan Tech 11: 83–87
Han HS, Kim YW, Yi NJ, Fleischer G (2003) Laparoscopic-assisted D2 subtotal gastrectomy in early gastric cancer. Surg Laparosc Endosc Percutan Tech 13: 361–365
Hartgrink HH, van de Velde CJH, Putter H, Bonekamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JHJM, Meijer S, Plukker JTM, van Elk PJ, Obertop H, Gouma DJ, van Lanschot JJB, Taat CW, de Graaf PW, von Meyenfeldt MF, Tilanus H, Sasako M (2004) Extended lymph node dissection for gastric cancer: who may benefit? Final Results of the Randomized Dutch Gastric Cancer Group Trial. J Clin Oncol 22: 2069–2077
Hochwald SN, Brennan MF, Klimstra DS, Kim S, Karpeh MS (1999) Is lymphadenectomy necessary for early gastric cancer? Ann Surg Oncol 6: 664–670
Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Lirici MM, Napolitano C, Piro F (2004) Videolaparoscopic total and subtotal gastrectomy with extended lymph node dissection for gastric cancer. Am J Surg 188: 728–735
Huscher CGS, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C (2005) Laparoscopic versus open subtotal gastrectomy for distal gastric cancer. Five-year results of a randomized prospective trial. Ann Surg 241: 232–237
Hyung WJ, Cheong JH, Kim J, Chen J, Choi SH, Noh SH (2004) Application of minimally invasive treatment for early gastric cancer. J Surg Oncol 85: 181–186
Kim YW, Bae JM, Lee JH, Ryu KW, Choi IJ, Kim CG, Lee JS, Rho JY (2005) The role of hand-assisted laparoscopic distal gastrectomy for distal gastric cancer. Surg Endosc 19: 29–33
Japanese Classification of Gastric Carcinoma. 2nd English edition (1998) Gastric Cancer 1: 10–24
Kitamura K, Yoneguchi T, Okanoto K, Otsuji E, Taniguchi H, Hagiwara A, Sawai K, Takabashi T (1997) Clinicopathologic features of synchronous multifocal early gastric cancers. Anticancer Res 17: 643–646
Kitano S, Shirahishi N, Fuji K, Yasuda K, Inomata M, Adachi Y (2002) A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 131: S306–S311
Kitano S, Shirahishi N (2004) Current status of laparoscopic gastrectomy for cancer in Japan. Surg Endosc 18: 182–185
Lee JH, Han HS, Lee JH (2005) A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 19: 168–173
McCulloch P, Nita ME, Kazi H, Gama-Rodrigues J (2004) Extended versus limited lymph node dissection technique for adenocarcinoma of the stomach (Cochrane Review). In The Cochrane Library , Issue 1. Chichester, UK: John Wiley & Sons, Ltd
Mochiki E, Kaniyama Y,Aihara R, Nakabayashi T, Asao T, Kuwano H (2005) Laparoscopic assisted distal gastrectomy for early gastric cancer. Five years’ experience. Surgery 137: 317–322
Ohgami M, Otani Y, Kumai K, Kubota T, Kim YI, Kitajima M (1999) curative laparoscopic surgery for early gastric cancer: five years experience. World J Surg 23: 187–193
Orringer MB, Marshall B, Iannettoni MD (2000) Eliminating the cervical esophagogastric anastomotic leak with a side-to-side stapled anastomosis. J Thorac Cardiovasc Surg 119: 277–288
Pugliese R, Maggioni D, Berardi V, Scandroglio I, Pisani D, Mariani A, Di Lernia S, Valli C, Cocozza E (2000) Extended (D2) lymphadenectomy in gastric cancer: a five year experience. Int Surg 85: 209–215
Reyes CD, Weber KJ, Gagner M, Divino CM (2001) Laparoscopic vs open gastrectomy. Surg Endosc 15: 928–931
Shimizu S, Uchiyama A, Mizumoto K, Morisaki, Nakamura K, Shimura H, Tanaka M (2000) Laparoscopically assisted distal gastrectomy for early gastric cancer: is it superior to open surgery? Surg Endosc 14: 27–31
Siewert JR, Bottcher K, Stein HJ, Roder JD, the German Gastric Carcinoma Study Group (1998) Relevant prognostic factors in gastric cancer. Ann Surg 228: 449–461
Tanimura S, Higashino M, Fukunaga Y, Osugi H (2003) Laparoscopic distal gastrectomy with regional lymph node dissection for gastric cancer. Surg Endosc 17: 758–762
Tanimura S, Higoshina M, Fukunaga Y, Osugi H (2001) Hand-assisted laparoscopic distal gastrectomy with regional lymph node dissection for gastric cancer. Surg Laparosc Endosc Percutan Tech 11: 155–160
Tuech JJ, Cervi C, Pessaux P, Villapadierna F, Bergamaschi R, Roncery J, Arnaud JP (1999) Early gastric cancer: univariate and multivariate analysis for survival. Hepatogastroenterology 46: 3276–3280
Weber KJ, Reyes CD, Gagner M, Divino CM (2003) Comparison of laparoscopic and open gastrectomy for malignant disease. Surg Endosc 17: 968–971
Yasuda K, Shirahishi N, Inomata M, Fuji K, Sonoda K, Kitano S (2003) Learning curve for laparoscopy-assisted distal gastrectomy. Dig Endosc 15: 280–228
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pugliese, R., Maggioni, D., Sansonna, F. et al. Total and subtotal laparoscopic gastrectomy for adenocarcinoma. Surg Endosc 21, 21–27 (2007). https://doi.org/10.1007/s00464-005-0409-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-005-0409-x