Abstract
Background
The two most commonly performed procedures for bariatric surgery include Roux-en-Y gastric bypass (RYGB) and adjustable gastric banding (AGB). While many studies have commented on short-term, postoperative outcomes of these procedures, few have reported long-term data. The purpose of this study was to compare long-term, postoperative outcomes between RYGB and AGB.
Methods
This was a retrospective, cohort comparing all patients undergoing RYGB or AGB at our institution, from 01/1998 to 08/2012. Patients were followed at 1-, 3-, and 5-year intervals. Adjusted, Cox proportional hazard regression and mixed effects repeated measures modeling were performed to generate cure ratios (CR) and 95 % confidence intervals (CI).
Results
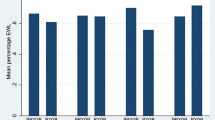
Two thousand four hundred twenty bariatric surgery patients (380 AGB, 2,040 RYGB) were identified by CPT code. Median (range) follow-up for patients was 3 (1–5) years. Preoperatively, RYGB patients were significantly younger, more obese, had higher hemoglobin A1c, and less often suffered from hypertension (HTN), dyslipidemia, and asthma as compared to AGB patients. Postoperatively, RYGB patients experienced significantly longer operating room times, higher incidences of intensive care unit admissions, longer hospital lengths of stay, and increased incidence of small bowel obstruction compared to AGB patients. After adjusting for statistically significant and clinically relevant factors [e.g., age, gender, body mass index, degenerative joint disease (DJD), diabetes, HTN, dyslipidemia, heart disease, apnea, and asthma], RYGB was independently associated with a significantly greater percentage of total body weight loss (p = 0.0065) and greater CR (95 % CI) regarding gastroesophageal reflux disease [2.1(1.4–3.0)], DJD [3.4(2.0–5.6)], diabetes [3.4(2.2–5.4)], apnea [3.1(1.9–5.3)], HTN [5.5(3.4–8.8)], and dyslipidemia [6.3(3.5–11)] compared to AGB.
Conclusion
Our results support previous studies that have observed a greater weight loss associated with RYGB as compared to AGB and provide further evidence toward the long-term sustainability of this weight loss. Additionally, RYGB appears to result in a greater reduction of medical comorbidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Approximately, 80 million Americans are obese [i.e., body mass index (BMI) ≥ 30] [1]. Obesity is associated with heart disease, cancer, and stroke and costs the United States nearly $150 billion annually [2]. Weight loss surgery may be an option for patients with BMI ≥ 40 or ≥ 35 with comorbid conditions, when less invasive weight loss alternatives have failed [3].
Roux-en-Y gastric bypass (RYBG) and adjustable gastric banding (AGB) are the two most commonly performed weight loss surgeries worldwide [4, 5]. Studies comparing outcomes between the two methods are conflicting [6–25]. Furthermore, long-term studies (≥ 5 years) are few [7, 8, 15, 17–21]. The purpose of this study was to compare long-term postoperative outcomes between RYGB and AGB at our institution.
Methods
Patients and follow-up
Details of the study database and methodology have been previously described and are summarized below [26]. A retrospective cohort analysis of a prospectively maintained database of all patients undergoing bariatric surgery at our institution from 1985 to 2013 was performed. Institutional review board approval was obtained before review of data was initiated. For the purposes of this study, patients from 1998 to 2012 were chosen based upon Current Procedural Terminology code, electronic medical record, and follow-up availability (i.e., 1-, 3-, and 5-year follow-up). Patients were subsequently stratified and compared by procedure type (i.e., RYGB vs. AGB).
Preoperative patient demographics and comorbidities evaluated included: age, gender, weight, BMI, gastroesophageal reflux disease (GERD), degenerative joint disease (DJD), diabetes (DM), obstructive sleep apnea (OSA), hypertension (HTN), dyslipidemia, asthma, heart disease, and hemoglobin A1c (HgbA1c). Perioperative data evaluated included: operative (OR) time, intensive care unit (ICU) admission, hospital length of stay (LOS), 30-day bleeding, 30-day anastomotic leak, small bowel obstruction (SBO), pulmonary embolism, 30-day mortality, and anytime mortality. 1-, 3-, and 5-year follow-up data evaluated included: percent total body weight loss (PTBWL), GERD, DJD, DM, OSA, HTN, dyslipidemia, and heart disease.
Definitions
RYGB was defined as laparoscopic, open, or laparoscopic converted to open Roux-en-Y. AGB was defined as laparoscopic or laparoscopic converted to open gastric banding. OSA was defined by sleep study center evaluation. GERD, DJD, DM, HTN, dyslipidemia, asthma, and heart disease were defined by medical history documentation and/or associative medication within the electronic medical record. PTBWL was defined as total weight loss (weight at follow-up subtracted from preoperative weight) divided by preoperative weight. Mortality was defined as all-cause for both 30-day and anytime.
Setting
The University of Virginia Health System is a tertiary care center located in Charlottesville, VA and has been recognized by the American College of Surgeons as a Level 1a Accredited Bariatric Center of Excellence. Between 1985 and 2013, approximately, 3,000 bariatric surgeries were performed at our institution.
RYGB operative description and postoperative management
Open and laparoscopic RYGB are performed in a similar fashion. After obtaining intraperitoneal access, the omentum is retracted cephalad, and the ligament of Treitz is identified. A 100-cm (BMI <50) or 150-cm (BMI ≥50) Roux limb is constructed with the distance from the ligament of Treitz to the jejunojejunostomy measuring approximately 50 cm. The common enterotomy is hand sewn closed. The Roux limb is anastomosed to a 15–30 cc pouch created using a linear cutting stapler in a retrocolic and retrogastric fashion. The anastomosis is tested for air leak using an endoscope and water. A cholecystectomy is performed if preoperative imaging identifies gallstone presence. On postoperative day 1, patients receive a gastrografin swallow study. If no leak or obstruction is demonstrated, a gastric bypass phase I diet (liquid) is initiated. If this is tolerated, a gastric bypass phase II diet (pureed foods) is initiated. The patient is discharged home usually by postoperative day 2 or 3. Patients are seen back in clinic for their first postoperative visit at 3 weeks and are advanced to a gastric bypass phase III diet (soft, solid foods). Following this, patients are scheduled to be seen back at 3 months from surgery, 6 months from surgery, 1 year from surgery, and then every year thereafter unless a complication arises.
AGB operative description and postoperative management
Open and laparoscopic AGB are performed in a similar fashion. Intraperitoneal access is obtained. Using a pars flaccida technique, the diaphragmatic crus are identified, and a retroperitoneal space is created at the base of the crus. The band device (Original, VG, AP Standard, AP Large, or Realize) is then brought through the newly created retrogastric tunnel, passed around the esophagus and upper stomach, buckled, and secured with three plication sutures. Band tubing is subsequently brought out of the epigastric port site and secured within a subcutaneously created pocket. Patients are started on a gastric bypass phase I diet following surgery and are usually discharged home on the same day of the procedure. Patients may advance to a gastric bypass phase II diet if phase I is tolerated. Patients are seen back in clinic for their first postoperative visit at 3 weeks and are advanced to a gastric bypass phase III diet. Following this, patients are seen back every 6–8 weeks for band adjustments to optimize weight loss until a plateau is reached. At this point, patients may be seen every 6 months to a year unless a complication arises.
Statistical analysis
Categorical data were analyzed using either χ 2 or Fisher's exact test depending upon the size of data for each respective category. Continuous data were analyzed using either Student’s t test or Wilcoxon rank sum depending upon the normalcy of distribution. Continuous variables not previously categorized were divided into quartiles prior to statistical analysis. Quartile categorization is beneficial because it limits the influence of outliers and allows for the assessment of trend across categories. For completeness, we also provided mean and median estimates. Unadjusted and adjusted Cox proportional hazard regression modeling was performed to compute cure ratios (CR) and 95 % confidence intervals (CI) for preoperative comorbidities (i.e., GERD, DJD, DM, OSA, HTN, dyslipidemia, and heart disease) following RYGB relative to AGB (referent). Additionally, unadjusted and adjusted mixed effects repeated measures modeling was performed to evaluate the PTBWL experienced following RYGB relative to AGB (referent). Variables deemed clinically relevant and statistically significant were included in the multivariable analysis models (excluding stratifying variables of interest for Cox regression models). Analysis was performed using SAS Version 9.3© (Cary, NC, USA) programming software. Statistical significance was defined as a p-value of less than 0.05.
Results
Two thousand four hundred twenty patients (AGB = 380 vs. RYGB = 2,040) were identified by CPT code. Mean follow-up for patients was 12 ± 16 months (AGB = 20 ± 19 vs. RYGB = 10 ± 15 months).
Preoperative patient demographics and comorbidities are presented in Table 1 and stratified by procedure type. The two groups were similar; however, patients undergoing RYGB were younger, weighed more, and had a lower prevalence of HTN, dyslipidemia, and asthma compared with AGB. Although a difference in diabetic prevalence was not observed between groups, HgbA1c levels were lower between AGB patients compared with RYGB.
Perioperative data stratified by procedure type are presented in Table 2. The two groups were similar; however, patients undergoing RYGB experienced longer OR times, greater incidence of ICU admission, longer hospital LOS, and greater incidence of SBO compared with AGB. Notably, incidence of mortality was similar between groups.
Postoperative outcomes recorded at 1-, 3-, and 5-year follow-up and stratified by procedure type are presented in Table 3. One thousand sixty five (AGB = 296/349 vs. RYGB = 769/1,912) out of 2261, 497 (AGB = 135/267 vs. RYGB = 362/1697) out of 1964, and 288 (AGB = 56/121 vs. RYGB = 232/1457) out of 1,578 patients were present for their 1-, 3-, and 5-year follow-up, respectively. Multivariable, mixed effects repeated measures modeling revealed that RYGB was independently associated with greater PTBWL compared with AGB. Additionally, multivariable, Cox proportional hazard modeling revealed that RYGB was independently associated with GERD, DJD, DM, OSA, HTN, and dyslipidemia cure compared with AGB.
Discussion
We present one of the largest cohorts evaluating RYGB and AGB over a long-term period of time (i.e., ≥5 years) [7, 8, 15, 17–21]. Our study observed an independent and significant advantage of RYGB over AGB regarding PTBWL as well as resolution of GERD, DJD, DM, OSA, HTN, and dyslipidemia over time. Previous studies have observed conflicting results [6–25].
RYGB patients in our study experienced a 34–35 % total body weight loss compared with AGB patients who experienced a 16–19 % total body weight loss. This difference was maintained throughout each follow-up period. Previous studies have reported similar findings at 2-year [9–12], 3-year [13, 16], 5-year [6, 8, 17, 18, 21], and 10-year follow-up [7, 19] and have observed greater frequency of comorbid resolution (i.e., DM, HTN, hyperlipidemia, OSA) with RYGB compared to AGB [11, 13, 16–18]. Our study supports these findings and additionally reports a greater GERD and DJD cure rate among RYGB patients.
One possible explanation for the observed benefits may involve changes in gastrointestinal hormones [i.e., glucagon-like peptide-1 (GLP-1), peptide YY (PYY), leptin, and ghrelin] [27, 28]. Up-regulation of GLP-1, PYY, and leptin (via ileum, colon, and adipocyte stimulation) is thought to reduce hunger, impart satiety, and increase energy expenditure by decreasing gastric emptying, inhibiting gastric acid secretion, promoting insulin secretion and sensitivity, and acting upon the hypothalamus. Down-regulation of ghrelin (via pituitary and gastric stimulation) is thought to suppress appetite by increasing insulin secretion and decreasing gastrointestinal motility. Previous studies have shown a greater increase in GLP-1 and PYY among patients following RYGB compared with AGB [27, 28]. This increase is thought to be attributable to the additional intestinal bypass component of RYGB as opposed to the purely gastric-restrictive banding procedure. Additionally, the greater degree of gastric restriction seen with RYGB may result in a greater decrease in ghrelin levels. A recent randomized clinical trial by Chronaiou et al. [29] evaluated weight loss and hormonal secretion in 24 patients (12 laparoscopic Roux-en Y gastric bypass vs. 12 laparoscopic Roux-en Y gastric bypass with additional fundus resection). The authors observed that fundal resection (i.e., increased gastric restriction and manipulation) was associated with persistently lower fasting ghrelin levels and higher GLP-1, PYY, and insulin responses. The applicability of this physiological alteration has been described by multiple studies surveying patients and their postoperative hedonic response to food [30–32]. The authors observed a dramatic difference in taste changes experienced by RYGB compared with AGB resulting in a greater repulsion to high caloric food and a resultant adoption of healthy eating behavior.
Conversely, other studies have not observed a difference in weight loss between groups [14, 15, 20]. Jan et al. [15] reported on 898 patients (492 laparoscopic RYGB vs. 406 laparoscopic AGB) and observed a greater weight loss among RYGB within the first 5 years following surgery at which point weight loss became equivalent between groups. It is important to note, however, that over 50 % of their available patient population was lost to follow-up (LTFU) at that time point, and thus selection bias may be a factor.
Previous studies have largely observed longer perioperative OR times and HLOS and greater incidence of early postoperative complications (i.e., ≤30 days) among RYGB recipients compared with AGB [6–8, 10, 15, 17, 18, 23–25]. Common, early postoperative complications include: anastomotic stricture, SBO, laparoscopic port site wound, gastrointestinal bleeding, marginal ulcer, perforation, abscess, pneumonia, deep vein thrombosis, pulmonary embolism, sepsis, and urinary tract infection [6–8, 10, 15, 17, 18, 23, 24]. This may result in readmission (median time 8 days following surgery) [24] and/or need for reparative operation. Similarly, our study observed longer perioperative OR times and HLOS and greater incidence of ICU admission and SBO but not 30-day leak, gastrointestinal bleed, or pulmonary embolism. Interestingly, other studies have not observed a difference between groups regarding early postoperative complications [11, 16, 21]. It has been speculated that this discrepancy in outcome may in part be due to surgical technique rather than some inherent flaw with the operation itself and suggests a learning curve to RYGB [11, 33].
Long-term postoperative complications appear to be more frequent among AGB and include: band erosion, slippage, leak, migration, infection, and stenosis, and port discomfort, dislocation, and infection [7, 11, 16, 18–21]. Treatments for AGB-associated complications range from band adjustment (5–20 times per patient on average) to revision (1.7–18 % incidence) and/or removal (0–10 % incidence) [34]. Long-term complications reported for RYGB include: marginal ulceration, anastomotic strictures, internal hernia, and gallstones [7, 11, 16, 18–21]. Treatments range from upper gastrointestinal endoscopy with balloon dilation to laparotomy with revision (1.4–16.2 % incidence) and/or cholecystectomy (5–20 % incidence) [34].
Given the apparent differences in weight loss, comorbid resolution, and frequency of postoperative complication between groups, why is AGB still being performed? Ternovits et al. [35] surveyed 120 consecutive patients who had undergone AGB or RYGB approximately 3–24 months prior to ascertain why they chose either procedure, and how they rated their postoperative outcome. The top two reasons for choosing RYGB were greater and quicker weight loss, while the top two reasons for choosing AGB were low surgical risk and quicker recovery. Interestingly, AGB patients felt that they would have experienced similar weight loss regardless of the procedure, while RYGB patients felt that they would have experienced an inferior outcome. Furthermore, RYGB patients showed a significant trend toward overall greater satisfaction with their operation compared with AGB patients. Finally, cost-efficacy models reported within the literature appear to be equivalent [34, 36]. Both analyses determined that RYGB and AGB were cost effective to below $25,000 per quality-adjusted life year gained.
Strengths and weaknesses
Our study is strengthened by its large sample size and long-term follow-up. However, its retrospective design may be considered a limitation, as these studies are known to be susceptible to recall and selection bias. For example, our study had a high attrition rate, and thus may not be reflective of the complete bariatric surgery population. Previous retrospective studies have observed follow-up ranging from 22 to 94 % at 1 and 2 years [9–12, 15], 37–45 % at 3 years [13, 15, 16], and 44–92 % at 5 years [15, 17, 18] compared with our 47 % at 1 year, 25 % at 3 years, and 18 % at 5 years. Our slightly lower follow-up rate may be explained by the use of in-person, bariatric clinic reminders rather than a combination of in-person, phone, primary care provider, and/or EMR reminders. Consequently, this may have introduced selection bias, as patients may be more or less inclined to follow-up based upon their surgical outcome. However, while a substantial number of patients were LTFU, the distribution between the two study groups at each follow-up period was similar, indicating that the data were missing at random between both groups. Furthermore, we feel that our data characterize the population of patients that return to our clinic and provide useful information for postoperative management of this group. Although our analyses adjusted for relevant variables, additional unmeasured factors could have influenced our results due to the non-randomized nature of the study. Finally, external validity may be limited in generalizing our results to other centers as the demographics and comorbidities of our patient population may differ.
Conclusion
Our results support previous studies that have observed a greater weight loss associated with RYGB compared with AGB and provide further evidence toward the long-term sustainability of this weight loss. Additionally, RYGB appears to result in a greater reduction of medical comorbidity. Our data combined with the current literature have resulted in a substantial decrease in the frequency of AGB performed at our institution. Presently, we only offer AGB to patients if they directly request it and are adamantly opposed to RYGB.
References
Ogden CL, Carroll MD, Kit BK, Flegal KM (2012) Prevalence of obesity in the United States, 2009–2010. United States Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. http://www.cdc.gov/nchs/data/databriefs/db82.pdf. Accessed 10/12/2013
Centers for Disease Control and Prevention (8/3/2010) Cdc vital signs [updated 8/3/2010]. Available www.cdc.gov/vitalsigns/adultobesity/index.html. Accessed 03/10/2014
National Heart Lung and Blood Institute (1998) Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Available www.nhlbi.nih.gov/guidelines/obesity/ob-gdlns.pdf. Accessed 10/13/2013
Tice JA, Karliner L, Walsh J, Petersen AJ, Feldman MD (2008) Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med 121(10):885–893
Nguyen NT, Sloan J, Nguyen XM (2010) Laparoscopic gastric bypass or gastric banding: which operation is best? Adv Surg 44:49–57
Nguyen NT, Slone JA, Nguyen XM, Hartman JS, Hoyt DB (2009) A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity: outcomes, quality of life, and costs. Ann Surg 250(4):631–641
Angrisani L, Cutolo PP, Formisano G, Nosso G, Vitolo G (2013) Laparoscopic adjustable gastric banding versus Roux-En-Y gastric bypass: 10-year results of a prospective, randomized trial. Surg Obes Relat Dis 9(3):405–413
Angrisani L, Lorenzo M, Borrelli V (2007) Laparoscopic adjustable gastric banding versus Roux-En-Y gastric bypass: 5-year results of a prospective randomized trial. Surg Obes Relat Dis 3(2):127–132 (discussion 32–33)
Puzziferri N, Nakonezny PA, Livingston EH, Carmody TJ, Provost DA, Rush AJ (2008) Variations of weight loss following gastric bypass and gastric band. Ann Surg 248(2):233–242
Biertho L, Steffen R, Ricklin T, Horber FF, Pomp A, Inabnet WB, Herron D, Gagner M (2003) Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding: a comparative study of 1,200 cases. J Am Coll Surg 197(4):536–544 (discussion 544–545)
Weber M, Muller MK, Bucher T, Wildi S, Dindo D, Horber F, Hauser R, Clavien PA (2004) Laparoscopic gastric bypass is superior to laparoscopic gastric banding for treatment of morbid obesity. Ann Surg 240(6):975–982 (discussion 982–983)
Lee DY, Guend H, Park K, Levine J, Ross RE, McGinty JJ, Teixeira JA (2012) Outcomes of laparoscopic Roux-En-Y gastric bypass versus laparoscopic adjustable gastric banding in adolescents. Obes Surg 22(12):1859–1864
Cottam DR, Atkinson J, Anderson A, Grace B, Fisher B (2006) A case-controlled matched-pair cohort study of laparoscopic Roux-En-Y gastric bypass and lap-band patients in a single us center with 3-year follow-up. Obes Surg 16(5):534–540
Jan JC, Hong D, Pereira N, Patterson EJ (2005) Laparoscopic adjustable gastric banding versus laparoscopic gastric bypass for morbid obesity: a single-institution comparison study of early results. J Gastrointest Surg 9(1):30–39 (discussion 40–41)
Jan JC, Hong D, Bardaro SJ, July LV, Patterson EJ (2007) Comparative study between laparoscopic adjustable gastric banding and laparoscopic gastric bypass: single-institution, 5-year experience in bariatric surgery. Surg Obes Relat Dis 3(1):42–50 (discussion 51)
Bowne WB, Julliard K, Castro AE, Shah P, Morgenthal CB, Ferzli GS (2006) Laparoscopic gastric bypass is superior to adjustable gastric band in super morbidly obese patients: a prospective comparative analysis. Arch Surg 141(7):683–689
Boza C, Gamboa C, Awruch D, Perez G, Escalona A, Ibanez L (2010) Laparoscopic Roux-En-Y gastric bypass versus laparoscopic adjustable gastric banding: 5 years of follow-up. Surg Obes Relat Dis 6(5):470–475
Romy S, Donadini A, Giusti V, Suter M (2012) Roux-En-Y gastric bypass vs gastric banding for morbid obesity: a case-matched study of 442 patients. Arch Surg 147(5):460–466
Spivak H, Abdelmelek MF, Beltran OR, Ng AW, Kitahama S (2012) Long-term outcomes of laparoscopic adjustable gastric banding and laparoscopic Roux-En-Y gastric bypass in the United States. Surg Endosc 26(7):1909–1919
O’Brien PE, MacDonald L, Anderson M, Brennan L, Brown WA (2013) Long-term outcomes after bariatric surgery: 15-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg 257(1):87–94
Zuegel NP, Lang RA, Huttl TP, Gleis M, Ketfi-Jungen M, Rasquin I, Kox M (2012) Complications and outcome after laparoscopic bariatric surgery: Lagb versus Lrygb. Langenbeck’s Arch Surgy/Deutsche Gesellschaft fur Chirurgie 397(8):1235–1241
Saunders J, Ballantyne GH, Belsley S, Stephens DJ, Trivedi A, Ewing DR, Iannace VA, Capella RF, Wasileweski A, Moran S, Schmidt HJ (2008) One-year readmission rates at a high volume bariatric surgery center: laparoscopic adjustable gastric banding, laparoscopic gastric bypass, and vertical banded gastroplasty-Roux-En-Y gastric bypass. Obes Surg 18(10):1233–1240
Lancaster RT, Hutter MM (2008) Bands and bypasses: 30-day morbidity and mortality of bariatric surgical procedures as assessed by prospective, multi-center risk-adjusted Acs-Nsqip data. Surg Endosc 22(12):2554–2563
Saunders JK, Ballantyne GH, Belsley S, Stephens D, Trivedi A, Ewing DR, Iannace V, Capella RF, Wasielewski A, Moran S, Schmidt HJ (2007) 30-Day readmission rates at a high volume bariatric surgery center: laparoscopic adjustable gastric banding, laparoscopic gastric bypass, and vertical banded gastroplasty-Roux-En-Y gastric bypass. Obes Surg 17(9):1171–1177
Stephens DJ, Saunders JK, Belsley S, Trivedi A, Ewing DR, Iannace V, Capella RF, Wasielewski A, Moran S, Schmidt HJ, Ballantyne GH (2008) Short-term outcomes for super–super obese (Bmi ≥ 60 Kg/m2) patients undergoing weight loss surgery at a high-volume bariatric surgery center: laparoscopic adjustable gastric banding, laparoscopic gastric bypass, and open tubular gastric bypass. Surg Obes Relat Dis 4(3):408–415
Davies SW, Efird JT, Guidry CA, Penn RI, Sawyer RG, Schirmer BD, Hallowell PT (2014) Long-term diabetic response to gastric bypass. J Surg Res 190(2):498–503
Cummings DE (2009) Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes 33(Suppl 1):S33–S40
Beckman LM, Beckman TR, Earthman CP (2010) Changes in gastrointestinal hormones and leptin after Roux-En-Y gastric bypass procedure: a review. J Am Diet Assoc 110(4):571–584
Chronaiou A, Tsoli M, Kehagias I, Leotsinidis M, Kalfarentzos F, Alexandrides TK (2012) Lower ghrelin levels and exaggerated postprandial peptide-Yy, glucagon-like peptide-1, and insulin responses, after gastric fundus resection, in patients undergoing Roux-en-Y gastric bypass: a randomized clinical trial. Obes Surg 22(11):1761–1770
Ernst B, Thurnheer M, Wilms B, Schultes B (2009) Differential changes in dietary habits after gastric bypass versus gastric banding operations. Obes Surg 19(3):274–280
Tichansky DS, Boughter JD Jr, Madan AK (2006) Taste change after laparoscopic Roux-En-Y gastric bypass and laparoscopic adjustable gastric banding. Surg Obes Relat Dis 2(4):440–444
Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, Ismail NA, Durighel G, Ahmed AR, Olbers T, Vincent RP, Alaghband-Zadeh J, Ghatei MA, Waldman AD, Frost GS, Bell JD, le Roux CW, Goldstone AP (2013) Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 63:891–902
Oliak D, Ballantyne GH, Weber P, Wasielewski A, Davies RJ, Schmidt HJ (2003) Laparoscopic Roux-En-Y gastric bypass: defining the learning curve. Surg Endosc 17(3):405–408
Salem L, Devlin A, Sullivan SD, Flum DR (2008) Cost-effectiveness analysis of laparoscopic gastric bypass, adjustable gastric banding, and nonoperative weight loss interventions. Surg Obes Relat Dis 4(1):26–32
Ternovits CA, Tichansky DS, Madan AK (2006) Band versus bypass: randomization and patients’ choices and perceptions. Surg Obes Relat Dis 2(1):6–10
Campbell J, McGarry LA, Shikora SA, Hale BC, Lee JT, Weinstein MC (2010) Cost-effectiveness of laparoscopic gastric banding and bypass for morbid obesity. Am J Manag Care 16(7):174–187
Disclosures
Stephen W. Davies, Christopher A. Guidry, and Robert G. Sawyer were supported by NIH Grant 5T32AI078875-05. Jimmy T. Efird, Rachel I. Penn, Bruce D. Schirmer, and Peter T. Hallowell have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davies, S.W., Efird, J.T., Guidry, C.A. et al. Twenty-first century weight loss: banding versus bypass. Surg Endosc 29, 947–954 (2015). https://doi.org/10.1007/s00464-014-3758-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3758-5