Abstract
Background
Electrical stimulation of the gastrointestinal tract is an attractive concept. In this article we report on a procedure for electrical colonic pacing due to intramuscular electrode placement for slow-transit constipation and some preliminary results.
Methods
From January 2011 to December 2012, all consecutive patients affected by constipation and evaluated in our Pelvic Floor Center were prospectively assessed. Patients who underwent colonic electrical stimulation were evaluated for the present study.
Results
In the study period, 256 patients were evaluated for constipation; 58 % were identified as having obstructed defecation syndrome, 27.3 % with irritable bowel syndrome or mixed forms, 4 % with pelvic floor dyssynergia, and 10.5 % (27 patients) as having slow-transit constipation. After failure of all the maximal conventional therapies, two patients, candidates for colectomy, agreed to undergo colonic electrical stimulation before a resective treatment. Both patients were females, aged 34 and 29 years, and were suffering from severe constipation since childhood. The follow-up was 19 and 6 months. The number of bowel movements per week increased from 0.3 to 3.5 in the first patient and from 0.5 to 2.5 in the second patient. Both patients no longer needed laxatives, enemas, or any other treatment. The hospital stay was 4 days, the mean operative time was 120 min, and no complications were reported.
Conclusions
Colonic pacing seems to be feasible and shows positive results. Further studies are required with a larger number of patients and a longer follow-up period to confirm the role of this promising treatment for slow-transit constipation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Electrical stimulation of the gastrointestinal (GI) tract is an attractive concept. Since these organs have their own natural pacemakers, the electrical signals they generate can be altered by externally delivering electrical currents by intramuscular, serosal, or intraluminal electrodes to specific sites in the GI tract [1].
Over the past decade, some electrical stimulation methods for the treatment of gastric motility disorders have been developed, including implantable electrodes connected to a subcutaneous pacemaker [2]. Although electrical stimulation of the colon has been successful in improving constipation in animal models, there have been few evaluations of its use for constipation in humans. Sacral nerve modulation (SNM) has shown interesting results for the treatment of slow-transit constipation [3, 4], but there are no data about permanent colonic pacing.
Here we report on a procedure for electrical colonic pacing due to intramuscular electrode placement for slow-transit constipation and some preliminary results are given.
Materials and methods
From January 2011 to December 2012, consecutive patients affected by constipation and evaluated in our Pelvic Floor Center/general emergency and minimally invasive surgery unit were prospectively assessed. Patients who underwent colonic electrical stimulation were evaluated for the present study. Patients were considered for colonic electrical stimulation if they met the following criteria:
-
failed all the conventional therapies, including laxatives and/or enemas, dietary and habit modification, biofeedback, prucalopride, or transanal irrigation
-
failed SNM
-
had no evidence of obstructed defecation at defecographic (or magnetic resonance defecography) and clinical evaluation
-
was between 18 and 45 years old
-
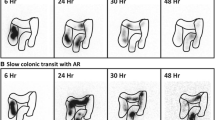
had a slow transit time confirmed radiologically (gut transit time with radiopaque markers with plain abdominal radiography performed at 72 and 120 h after ingestion of the markers) without radiological rectal fecal impaction. The diagnosis of slow-transit constipation was suggested if >20 % of the markers were found in the colon after 120 h
-
had less than one bowel movement per week
-
had absence of bowel movement for more than 1 week without laxatives or enema
-
had absence of fecal residue in ampulla after 1 week without laxatives or enema
-
anorectal manometry had no signs of anorectal dyssynergia
-
had a history of constipation from a young age (or at least for more than 3 years)
-
irritable bowel syndrome (IBS) was excluded
-
psychological disturbance was excluded
Exclusion criteria included congenital anorectal malformations, external rectal prolapse, chronic inflammatory bowel disease, the presence of stoma, pregnancy, neurological disease, and psychiatric or physical inability to comply with study protocol. All patients selected for the study gave written informed consent.
For the slow-transit study, plain abdominal radiographs are taken 3 and 5 days after subjects ingest a capsule containing 20 radioactive markers. The presence in the colon (not accumulated in the rectum) of at least 8 markers on day 3 or at least 5 markers day 5 was considered abnormal [5]. Patients were instructed to abstain from the use of enemas, laxatives, or suppositories of any kind for the 5 days of the study. Radiography on day 5 was considered unnecessary for patients with fewer than 8 markers on day 3.
Before the procedure the patients underwent clinical investigation, including colonoscopy and proctography, to exclude other correctable causes of constipation. A bowel diary was kept from 3 months before to 3 months after the procedure, in which the patient recorded the number of bowel movements per week, the time spent in the bathroom, the presence or absence of a stimulus to assist defecation, and the use of laxatives or enemas. Patients were evaluated after 7 and 15 days, then every 30 days for the first 3 months and subsequently by telephone each month, evaluating the number of bowel movements per week and the maintenance of the effectiveness of the treatment.
Surgical technique
Patients were given a complete bowel preparation (with PEG or saline solution) and preoperative antibiotics (ceftriaxone 2 g+metronidazole 500 mg) were administered. Patients were placed on a beanbag table pad and padded stirrups were utilized. The anus should be lined up with the edge of the bed to facilitate endoscopy. Four trocars were placed: the umbilical port, the upper-right quadrant, the lower-right quadrant, and the lower-left quadrant (optional), as performed for left colonic laparoscopic procedures. The table was then turned on the right side in the Trendelenburg position. After exploration of the abdominal cavity, the sigmoidorectal junction was identified and the optimal area for placement of the electrodes was considered approximately at the level of the confluence of the taeniae anteriorly.
Two parallel 35-cm leads with a 10-mm electrode (Unipolar Intramuscular Lead model 4351, Medtronic, Minneapolis, MN, USA) (Fig. 1) were placed into the muscular layer, 1 cm apart, above the taenia and anchored with a silicone rubber fixation disc. Both the disc and the trumpet anchor on the other side of the electrodes were secured with individual sutures to the bowel wall. Correct electrode position was endoscopically verified before the fixation. A left low inguinal subcutaneous pocket was created (incision about 3 cm) and the leads were placed in an extraperitoneal tunnel, bringing them out at the level of the pocket. The tunneling near the site of insertion was done to avoid possible complications related to the intra-abdominal presence of the leads. The leads were then connected with the neurostimulator (Interstim neurostimulator model 3023, Medtronic) and the neurostimulator was placed in the pocket. The surgical procedure is summarized in Fig. 2. The stimulation parameters were activated by the physician and a remote control was given to the patients to turn stimulation on and off.
Stimulation parameters
After the first activation, for 30 days the stimulation parameters were a pulse width of 150 μs, a rate of 10 Hz, and a voltage of 2 V in continuous mode. After 30 days the mode was cyclically changed, with 2 min ON and 20 min OFF. Voltage could be lowered if required by the patient.
Results
In the study period, 256 patients were evaluated as outpatients for constipation (Fig. 3). A total of 149 patients (58 %) had a main diagnosis of outlet obstruction or obstructed defecation syndrome related to rectal prolapse, rectocele, or intussusception; 70 patients (27.3 %) had IBS or mixed forms; 10 (4 %) patients were identified as having pelvic floor dyssynergia or paradoxical puborectalis muscle contraction; and 27 patients (10.5 %) were diagnosed with slow-transit constipation.
The diet and habits of all 27 patients were reviewed and medical therapy (with fiber or PEG) was proposed. Fifteen patients (55.5 %) failed to benefit from this approach. Six more patients solved their problem with laxatives and enemas (3), transanal retrograde irrigation (2), and biofeedback, pelvic floor rehabilitation, and manual therapies (1). Nine patients (33.3 %) underwent a temporary stimulation period (6–8 weeks) with SNM, and five of those patients (55.5 %) were definitively implanted after the success of the treatment. Of the remaining four patients, one refused any more treatment and one was affected by a neurological syndrome and demolishing surgical treatment was proposed. Two patients, candidates for colectomy, agreed to undergo colonic electrical stimulation before resection. Both patients were females, aged 34 and 29 years, and suffered from severe constipation since childhood.
Follow-up was 19 and 6 months and data of the two patients is given in Table 1. The number of bowel movements per week increased from 0.3 to 3.5 in the first patient and from 0.5 to 2.5 in the second patient. Both patients no longer needed laxatives, enemas, or any other assistance. The hospital stay was 4 days. The mean operative time was 120 min. No perioperative or postoperative complications were reported. The patients did not require changes in the stimulation parameters during the follow-up period.
Discussion
Patients with slow-transit constipation account for 5–15 % of the constipated population [6], and severe constipation (e.g., bowel movements only twice a month) is seen almost exclusively in young women [7], as was seen in our data.
During the past decades, much attention has been paid to abnormalities in autonomic nerves that are associated with colorectal motility disorders. The autonomic nervous system can be divided into parasympathetic and sympathetic components. The parasympathetic, general visceral efferent innervation of the large bowel is derived from the dorsal motor nucleus of vagi and the sacral parasympathetic nucleus. Even though the extent of colonic innervation is still under debate, it is generally believed that vagal innervation to the large bowel terminates at the level of the splenic flexure [8, 9], while the remainder of the colon, including the rectum, receives parasympathetic input from the pelvic nerves [10–12]. A pattern of dual, coordinated, parasympathetic innervation in the left colon may regulate motor activity between the proximal colon and the rectum [13]. The distal colon and rectum also receive sympathetic input from the hypogastric nerves (HGN), derived mainly from the lumbar preganglionic outflow that runs to the inferior mesenteric ganglia (hypogastric ganglion). The innervation and functional roles of the HGN on the internal anal sphincter have been well studied [14]. However, it still remains unclear how the HGN regulates colorectal motility.
Most of the colonic motor activity is represented by single nonpropagated contractions, rarely organized in bursts; this activity is maximal during the day, especially after waking and following meals. In addition, a specialized propagated activity with propulsive features is detectable, represented by high- and low-amplitude propagated contractions. In the severe form of constipation, represented by the slow-transit type, this motor activity is completely deranged. In fact, both basal segmental activity and propagated activity are usually decreased [15].
Based on data generated from colonic manometry studies, there are some primary indicators of abnormal motility that emerge: reduced frequency of high-amplitude propagating sequences, diminished or absent response to eating a high-calorie meal or morning waking, and abnormal colonic response to chemical stimulation or rectal mechanical distension [16]. As the central nervous system is likely to play role in both the increase in propagating pressure waves after a meal and their nocturnal suppression, a diminished or absent response to these stimuli has been proposed as a possible indicator of myopathy or neuropathy [17]. With regard to chemical stimuli, a failed response may indicate an abnormality within the myenteric plexus [18], cholinergic pathways [19], or rectocolonic neural pathways [20].
Considerable progress has been made at the ultrastructural, molecular, and electrophysiological levels in understanding the normal functions of the muscles, nerves, and interstitial cells that generate and control colonic motility. Furthermore, abnormalities in these cell types, and in the interstitial cells of Cajal in particular, have been identified in a number of disease states [21]. In patients with slow-transit constipation, the number of interstitial cells of Cajal was significantly decreased in all layers except the outer longitudinal muscle layer and the myenteric plexus showed a variable grade of hypoganglionosis [21].
Several studies showed the positive effects of direct colonic stimulation in animal models, even if it has never been reported in humans [22–25]. Shafik et al. [26] reported on endoscopic placement of mucosal electrodes connected to a subcutaneous stimulator for direct pacing of the colon. However, direct stimulation in this manner is technically demanding and has never been duplicated by others. Moreover, we preferred the placement of the electrodes in the seromuscularis layer because of the guaranteed contact and direct effect on the targeted organ but the obvious disadvantage is the invasiveness. Laparotomy or laparoscopy under general anesthesia is required. This led us to the proposed procedure just before a colonic resection, considering the medical or minimally invasive treatments (e.g., SNS, colonic irrigation) as preferred treatment of this benign but disabling condition.
Sacral neuromodulation represents a promising alternative via indirect sacral roots stimulation [3, 4], but the results are inconsistent and the mechanism of action is still unclear. Sacral nerve stimulation did not induce major changes in the propulsive capacity of the GI tract in patients successfully treated for fecal incontinence [27], and even though some authors reported that it induces pancolonic propagating pressure waves [28], the clinical implications of this result remain unclear.
According to the colonic innervation, it was demonstrated in a canine model that sacral nerve stimulation elicited contractile movements propagating from the distal colon to the rectum, relaxation response in the rectum, and internal anal sphincter relaxation response, but no proximal colonic effective responses, suggesting its role in modulation of sacral reflexes and confirming its inconsistent outcome in the treatment of constipation [29].
The role of the sigmoid colon in the pathophysiology of slow-transit constipation is well known but not completely clear, considering that megacolon (mainly left) is the main clinical finding in Hirschsprung’s disease, that a dolicho-megacolon (mainly left) is a common feature of patients affected by chronic slow-transit constipation, that diverticular disease manifests in left and sigmoid colon, and an incomplete sigmoid resection for diverticulitis exposes the patient to a higher recurrence rate.
Connell [30] raised the possibility of a sigmoid “brake” by demonstrating that the duration but not the average amplitude of phasic motor activity in the sigmoid colon was greater in constipated subjects but lower in patients with functional diarrhea compared to controls. Similarly, Preston and Lennard-Jones [18] suggested that phasic pressure activity in the sigmoid colon was higher in normal-transit constipation than in controls or in patients with slow-transit constipation. Thus, we believed that the sigmoid colon plays a key role in slow-transit constipation, also taking into account the complex and variable innervation, different from that of the rest of the colon. Moreover, the visionary theories (and experiments) of Shafik et al. [31, 32] suggested the presence of a rectosigmoid junction pacemaker and a colosigmoid functional sphincter, regulated by rectosigmoid and rectocolonic reflexes.
All these observations suggest that slow-transit constipation (or certainly some cases) could be related to conflicting neurogenic input received by the left/sigmoid colon, maybe associated with (or the cause of) hypogangliosis and alteration of interstitial cells. For this reason, we belief that a direct left colonic modulation (maybe at the sigmoidorectal junction) could theoretically resolve the disease and restore a physiological (or regular) electrical transmission.
Regarding the stimulation parameter, considering the lack of consensus about optimal stimulation features and starting from the suggestion of Shafik et al. [33], the studies of Aellen et al. [24], Bertschi et al. [34], and Yin and Chen [35], the gastric Enterra pacing experience [2, 36], and some general principles of electrical stimulation [37], a pulse width of 150 μs, a rate of 10 Hz, and a voltage of 2 V in continuous mode are proposed, with a change in cyclic mode, with 2 min ON and 20 min OFF after 30 days, trying to restore a more physiological stimulation protocol.
Conclusions
Colonic pacing seems to be feasible and has shown positive results. Deeper neurophysiopathological studies need to be performed for a better understanding of colonic motor function, but electrical stimulation seems to be a promising solution for the treatment of slow-transit constipation. Further studies with a larger number of patients and a longer follow-up are required.
References
Lin Z, Sarosiek I, McCallum RW (2007) Gastrointestinal electrical stimulation for treatment of gastrointestinal disorders: gastroparesis, obesity, fecal incontinence, and constipation. Gastroenterol Clin North Am 36(3):713–734 x–xi
Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, Lindberg G, Konturek J, Nowak T, Quigley EM, Tougas G, Starkebaum W (2003) Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology 125:421–428
Naldini G, Martellucci J, Moraldi L, Balestri R, Rossi M (2010) Treatment of slow-transit constipation with sacral nerve modulation. Colorectal Dis 12:1149–1152
Carriero A, Martellucci J, Talento P, Ferrari CA (2010) Sacral nerve stimulation for constipation: do we still miss something? Role of psychological evaluation. Int J Colorectal Dis 25:1005–1010
Bouchoucha M, Devroede G, Arhan P et al (1992) What is the meaning of colorectal transit time measurement? Dis Colon Rectum 35:773–782
Nyam DC, Pemberton JH, Ilstrup DM, Rath DM (1997) Long-term results of surgery for chronic constipation. Dis Colon Rectum 40:273–279; erratum 40:529
Heaton KW, Radvan J, Cripps H, Mountford RA, Braddon FE, Hughes AO (1992) Defecation frequency and timing, and stool form in the general population: a prospective study. Gut 33:818–824
Devroede G, Lamarche J (1974) Functional importance of extrinsic parasympathetic innervation to the distal colon and rectum in man. Gastroenterology 66(2):273–280
Frantzides CT, Condon RE, Schulte WJ, Cowles V (1990) Effects of morphine on colonic myoelectric and motor activity in subhuman primates. Am J Physiol 258(2 Pt 1):G247–G252
Jorge JM, Wexner SD (1997) Anatomy and physiology of the rectum and anus. Eur J Surg 163:723–731
Dorofeeva AA, Panteleev SS, Makarov FN (2009) Involvement of the sacral parasympathetic nucleus in the innervation of the descending colon and rectum in cats. Neurosci Behav Physiol 39:207–210
Gonella J, Bouvier M, Blanquet F (1987) Extrinsic nervous control of motility of small and large intestines and related sphincters. Physiol Rev 67:902–961
Tong WD, Ridolfi TJ, Kosinski L, Ludwig K, Takahashi T (2010) Effects of autonomic nerve stimulation on colorectal motility in rats. Neurogastroenterol Motil 22:688–693
Garrett JR, Howard ER, Jones W (1974) The internal anal sphincter in the cat: a study of nervous mechanisms affecting tone and reflex activity. J Physiol 243:153–166
Bassotti G, de Roberto G, Castellani D, Sediari L, Morelli A (2005) Normal aspects of colorectal motility and abnormalities in slow transit constipation. World J Gastroenterol 11(18):2691–2696
Dinning PG, Di Lorenzo C (2011) Colonic dysmotility in constipation. Best Pract Res Clin Gastroenterol 25:89–101
Rao SS, Sadeghi P, Beaty J, Kavlock R (2004) Ambulatory 24-hour colonic manometry in slow-transit constipation. Am J Gastroenterol 99:2405–2416
Preston DM, Lennard-Jones JE (1985) Pelvic motility and response to intraluminal bisacodyl in slow-transit constipation. Dig Dis Sci 30:289–294
Bassotti G, Chiarioni G, Imbimbo BP, Betti C, Bonfante F, Vantini I et al (1993) Impaired colonic motor response to cholinergic stimulation in patients with severe chronic idiopathic (slow transit type) constipation. Dig Dis Sci 38:1040–1045
Dinning PG, Bampton PA, Kennedy ML, Lubowski DZ, King DW, Cook IJ (2005) Impaired proximal colonic motor response to rectal mechanical and chemical stimulation in obstructed defecation. Dis Colon Rectum 48:1777–1784
Quigley EM (2010) What we have learned about colonic motility: normal and disturbed. Curr Opin Gastroenterol 26:53–60
Sanmiguel CP, Casillas S, Senagore A, Mintchev MP, Soffer EE (2006) Neural gastrointestinal electrical stimulation enhances colonic motility in a chronic canine model of delayed colonic transit. Neurogastroenterol Motil 18:647–653
Sevcencu C, Rijkhoff NJ, Gregersen H, Sinkjaer T (2005) Propulsive activity induced by sequential electrical stimulation in the descending colon of the pig. Neurogastroenterol Motil 17:376–387
Aellen S, Wiesel PH, Gardaz JP et al (2009) Electrical stimulation induces propagated colonic contractions in an experimental model. Br J Surg 96:214–220
Vaucher J, Cerantola Y, Gie O, Letovanec I, Virag N, Demartines N, Gardaz JP, Givel JC (2010) Electrical colonic stimulation reduces mean transit time in a porcine model. Neurogastroenterol Motil 22:88–92
Shafik A, Shafik AA, El-Sibai O, Ahmed I (2004) A therapeutic option for the treatment of constipation due to total colonic inertia. Arch Surg 39:775–779
Damgaard M, Thomsen FG, Sørensen M, Fuglsang S, Madsen JL (2011) The influence of sacral nerve stimulation on gastrointestinal motor function in patients with fecal incontinence. Neurogastroenterol Motil 23:556–e207
Dinning PG, Fuentealba SE, Kennedy ML, Lubowski DZ, Cook IJ (2007) Sacral nerve stimulation induces pan-colonic propagating pressure waves and increases defecation frequency in patients with slow-transit constipation. Colorectal Dis 9:123–132
Hirabayashi T, Matsufuji H, Yokoyama J, Hagane K, Hoshino K, Morikawa Y, Kitajima M (2003) Colorectal motility induction by sacral nerve electrostimulation in a canine model: implications for colonic pacing. Dis Colon Rectum 46:809–817
Connell A (1962) The motility of the pelvic colon. Part II. Paradoxical motility in diarrhoea and constipation. Gut 3:342–348
Shafik A, Shafik AA, El Sibai O, Ahmed I, Mostafa RM (2006) Role of the rectosigmoidal junction in fecal continence: concept of the primary continent mechanism. Arch Surg 141:23–26
Shafik AA, Asaad S, Loka MM, Wahdan M, Shafik A (2009) Colosigmoid junction: morphohistologic, morphometric, and endoscopic study with identification of colosigmoid canal with sphincter. Clin Anat 22:243–249
Shafik A, Shafik AA, Ahmed I, el-Sibai O (2004) Treatment of irritable bowel syndrome with colonic pacing: evaluation of pacing parameters required for correction of the “tachyarrhythmia” of the IBS. Hepatogastroenterology 51(60):1708–1712
Bertschi M, Schlageter V, Vesin JM, Aellen S, Peloponissios N, D’Ambrogio A, Wiesel PH, Givel JC, Kucera P, Virag N (2010) Direct electrical stimulation using a battery-operated device for induction and modulation of colonic contractions in pigs. Ann Biomed Eng 38:2398–2405
Yin J, Chen JD (2010) Mechanisms and potential applications of intestinal electrical stimulation. Dig Dis Sci 55(5):1208–1220
Yin J, Abell TD, McCallum RW, Chen JD (2012) Gastric neuromodulation with Enterra system for nausea and vomiting in patients with gastroparesis. Neuromodulation 15:224–231
Mortimer JT, Kaufman D, Roessman U (1980) Intramuscular electrical stimulation: tissue damage. Ann Biomed Eng 8:235–244
Disclosures
Jacopo Martellucci and Andrea Valeri have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martellucci, J., Valeri, A. Colonic electrical stimulation for the treatment of slow-transit constipation: a preliminary pilot study. Surg Endosc 28, 691–697 (2014). https://doi.org/10.1007/s00464-013-3192-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-3192-0