Abstract
Aim
The aim of this study is to evaluate the short- and long-term efficacy of sacral nerve stimulation (SNS) for treating slow-transit constipation (STC).
Method
This is a retrospective cohort analysis of the efficacy of SNS in treating patients affected by STC, who previously failed to respond to conservative therapies. Only patients free of concomitant diseases were enrolled in our study. A temporary stimulation lead was initially implanted; patients with a > 50% symptom reduction were eventually deemed eligible for a permanent implant.
Results
This study enrolled 25 patients who underwent a SNS test stimulation; 21 patients (13 women; median age 32 years) eventually got a permanent implant. The median preoperative Cleveland Clinic Constipation Score (CCCS) was 21 (16–25). Preoperative colorectal transit time recorded a median of 10 markers (7–19) retained in the colorectal tract. At 6-month postoperative follow-up, the total number of markers retained in the colorectal tract decreased to 3 (0–4). The CCCS score improved during the first postoperative year (P < 0.001), but progressively worsened over the longer term. The SF-36 questionnaire showed an improvement in all 8 scales measuring physical and psycho-emotional states; all parameters recorded into the bowel diary also improved. Overall, at 60-month follow up, the overall neuromodulator removal rate was 48%.
Conclusions
The SNS is a minimally invasive surgical procedure that we tested for treating STC. The short-term outcome was promisingly after 6 months; however, there was a declining trend beyond this interval. Thus, the long-term efficacy of SNS needs to be further assessed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic constipation is a common disorder that affects between 2 and 27% of the Western population. It is more frequent in female and elderly patients [1].
The diagnosis of chronic constipation is commonly evaluated according to the Rome III criteria, which require the presence of at least two of the following six symptoms, lasting for six consecutive months: straining to have a bowel movement, passing hard stool, sensation of incomplete emptying, sensation of anorectal obstruction, self-digitation, defecation frequency less than three times per week.
Chronic constipation can be categorized into three main categories: slow-transit constipation, defecatory disorders (i.e., obstructed defecation) or a combination of both [2, 3].
Conservative treatment is first-line to treat chronic constipation. It requires changes in patient’s lifestyle, use of stool softeners or laxatives, and resolution of any psychological disorders that may negatively affect this condition. Surgery is provided as an option only in patients not sufficiently responding to conservative treatment. Surgical options consist either in a subtotal colectomy with ileorectal anastomosis for patients with slow-transit constipation or in an abdominal, perineal, or transanal surgical approach in the occurrence of defecatory disorders [1].
A systematic review of published literature on sacral nerve stimulation (SNS) for chronic constipation found inconsistent reporting and its efficacy remains debated [2, 4,5,6,7].
SNS is a minimally invasive surgical procedure first developed to that has proven to be useful to treat urinary disfunctions. First developed for treating urinary tract, SNS has eventually been applied to evacuation disorders too. In a European consensus conference, SNS was listed among the therapeutic options available for patients suffering from chronic constipation for more than a year, when conservative treatment failed [4]. However, clinical data on the efficacy of SNS for the treatment of slow-transit constipation are based largely on studies with low-level evidence [8].
The aim of this study is to evaluate efficacy of SNS for slow-transit constipation at a 5-year follow-up, in patients with no response to medical and behavioral treatment.
Patients and method
This is a retrospective cohort analysis based on prospectively collected data, which included of 206 consecutive patients, who were referred to the Colorectal and Pelvic Floor Diseases Center in Conegliano (Italy) for chronic constipation between January 2007 and December 2011. Only 25 patients qualified for SNS procedure at the conclusion of a multi-layer selection screening.
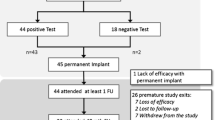
Patient screening flowchart is presented in Fig. 1.
Eligibility
During the time interval above, 206 patients were referred to our Center for chronic constipation, defined according to the Roma III criteria [9].
Our inclusion criteria were as follows: (1) age 18–75 years; (2) a minimum 1-year history of chronic constipation, defined according to Rome III criteria; (3) failure to respond to standard therapies, including laxatives, dietary modification, and physical exercise; (4) normal anorectal physiology, with no evidence of paradoxical sphincter contraction or inability to expel a rectal balloon; (5) normal defecating proctogram; (6) normal colonoscopy.
Our exclusion criteria were as follows: metabolic, neurogenic, or endocrine disorder(s), known to cause constipation (such as hypercalcemia, hypothyroidism, diabetes, multiple sclerosis, Parkinson’s disease, scleroderma); obstructed defecation syndrome; use of drugs that cause constipation as a side effect; history of abdominal radiotherapy; previous abdominal surgery (except cholecystectomy, appendicectomy, or hernia repair); pregnancy; current or previous history of malignancy; congenital anorectal malformations; external rectal prolapse; chronic inflammatory bowel disease; psychiatric or physical inability to comply with a study protocol.
Preoperative evaluation
Patients undergo clinical and proctologic examinations in the preoperative assessment.
The proctologic examination was carried out according to the American Society of Colon and Rectal Surgeons guidelines [10]. All patients with suspected STC were tested for their colorectal transit time and underwent defecography to confirm the diagnosis. Furthermore, all patients underwent colonoscopy to exclude concomitant diseases.
Colorectal transit time was evaluated by performing a plain abdominal X-ray after 5 days from the ingestion of a single gelatin capsule containing 20 radio-opaque markers. Before and during the tests, participants ate normally. From the day before the tests, laxatives, medications (antibiotics, antihistamines, antipyretics), and certain types of food (known to affect gastrointestinal motility, like persimmon and banana) were avoided. Colonic transit delay was defined when > 20% of markers were retained at the time of the abdominal X-ray [11].
Defecography was performed before, during, and after evacuation of a barium paste enema to assess anorectal configuration, pelvic floor position, and structural or functional abnormalities (i.e., occurrence of rectocele, enterocele, rectal intussusception, and/or outlet obstruction).
Patients were asked to fill in a bowel diary on a daily basis, with annotations based on the Cleveland Clinic Constipation Score (CCCS) [12] and the Italian version of the Short Form 36 Health Survey (SF-36) [13]. Test results of the SF-36 survey were subdivided into 8 scales: physical functioning, physical role functioning, bodily pain, general health perceptions, vitality, social role functioning, emotional role functioning, and mental health, where the first four scales are used to determine the physical state and the last four the psycho-emotional state. Patients who refused to complete the pre-operative SF-36 questionnaire were excluded from the questionnaire analysis.
All patients who qualified for SNS did also a psychological consultation to assess potential personality disorders by using the Minnesota Multiphasic Personality Inventory-2 test (MMPI -2) [14]. All tests were recorded, sent, and evaluated by a psychologist who analyzed the patients’ results blind.
The MMPI-2 contains true-or-false 567 items, and takes between 1 and 2 h to complete. Responses are used to assess patient’s personality structure and psychological functioning, describing how effectively the individual is functioning at the interpersonal and intrapersonal level.
Patients who refused to be tested and those affected by psychiatric/psychological disorders did not undergo the neuromodulator implant.
All patients underwent preoperatively anorectal manometry. Anorectal manometry (Polygraf™ ID multi-parametric recorder with POLYGRAM NET® analysis software, Medtronic, USA) recorded the following measures: length of the anal canal, maximum resting pressure (MRP), maximum squeezing pressure (MSP), and presence of the rectoanal inhibitory reflex (RAIR). Rectal sensation to latex balloon distension with air, inflated at a standardized rate of 50 mL/min, was used to measure the rectal sensory threshold, urge threshold, and maximal tolerated rectal volume. Anal and rectal sensitivity to low-amplitude electrical stimulation was measured using a catheter-mounted ring electrode placed within the mid-anal canal and upper rectum, respectively. Sensory threshold to electrical stimulation was defined at first sensation experienced, using stimulation performed at 10 Hz pulse frequency and 500 ms pulse width.
Surgical technique
The SNS implant surgery has been previously described in details [15]. We carried out the SNS procedure under local anesthesia. The percutaneous implant of a permanent quadripolar stimulation electrode (Medtronic InterStim® tined lead model 3889, Minneapolis, MN, USA) was positioned into the third right or left sacral foramina under fluoroscopic guidance. The foramen which elicited the best motor response (bellows contraction of the perineum with plantar flexion of the great toe) at the lowest voltage required was selected for the following test stimulation with the external pulse generator (Medtronic InterStim® model 3625, Minneapolis, MN, USA).
External stimulation (Medtronic InterStim® model 3625, Minneapolis, MN, USA) was begun on the first postoperative day and carried on for 1 month.
Patients were briefed to register all bowel movements, laxative intake, and other symptoms on their bowel diaries during the test stimulation period. Based on their diary registrations, we selected the ones eligible for a permanent implant, provided a 50% symptom reduction was also achieved. A permanent pulse generator (IPG, Medtronic InterStim® model 3023 or model 3058, Minneapolis, MN, USA) was placed subcutaneously in the gluteal region of the eligible patients and then connected to the previously implanted electrode.
Postoperative follow-up
All patients were assessed for postoperative complications at the outpatient clinic 3 and 10 days after the pulse generator implant.
Postoperative follow-up included a proctologic visit every 6 months for the first 2 years, and then once a year. At follow-up visits, pulse generator was re-programmed, if needed.
Patients were asked to register entries into their bowel diaries for the first year after implant and their inputs were analyzed at the 6- and 12-month visits. Moreover, at 6-month follow-up visit, patients took the SF-36 survey again. The CCCS was recorded at 12-month visit and at every next follow-up. The colorectal transit time was reassessed after 6 months, whereas the anorectal manometry was performed at 1-year follow-up.
Therapeutic efficacy of permanent SNM was evaluated by comparing baseline data with post-implant data gathered at follow-up visits.
A prucalopride pharmacological treatment (2 mg daily) was administered to all patients who had a CCCS equal or higher of 13 at follow-up visits [16]. SNS was explanted in patients with a progressive worsening of CCCS (≥ 15) [17], regardless of the prucalopride intake. Once the SNM was removed, patients were considered as dropouts for the purpose of this study.
Statistical analysis
Data were analyzed using SPSS (version 16. for Windows; SPSS Inc., Chicago, USA). Results were reported as mean ± standard deviation and median (range) for continuous variables and number of patients (percentage) for discrete variables. Comparison between preoperative and follow-up data was carried out using nonparametric tests: Wilcoxon test for continuous data for two related samples and Friedman test for more than two related samples. A P value of less than 0.05 was considered statistically significant.
Approval and consent
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration, last amended at the 64th WMA General Assembly, Fortaleza, Brazil, October 2013, or comparable ethical standards. For this type of study, formal consent by the institutional research committee is not required in Italy. Informed consent was obtained from all participants enrolled in this study.
Results
Demographic and preoperative characteristics
Sixty-three patients (of which 42 females, 67%) with STC, complying with the inclusion criteria for SNS treatment, were screened. Their mean age was 38 years (range 21–54 years).
Patients were tested with MMPI-2, except 20 (31.7%), who did not accept the psychological evaluation. As a result, they were excluded from this study.
Out of the 43 patients who completed the MMPI-2 test, 18 patients (44.8%) showed a profile pattern of 1 or 2, leading to their exclusion.
By applying our exclusion criteria, 25 patients (58.1%)—with a MMPI-2 score of 0—underwent a SNS test stimulation. After the screening period (mean 7.80 weeks, range 6–9), 21 patients achieved a > 50% symptom reduction: 8 men (38%) and 13 women (62%) reported an improvement in their bowel movements of more than 50% per week, with a decreased need of laxatives. These 21 patients underwent a permanent implant between 2007 and 2011 and were our sample on which we measured the SNS outcomes.
The median age of the cohort was 32 years (21–50 years). Patients reported symptoms of chronic constipation for a median of 10 years (4–24 years).
The median time spent on the toilet was 15 min (5–20 min). Patients reported 2 episodes per week of abdominal pain (0–5) and 3 episodes per week of abdominal bloating (1–6). Defecation was achieved 2 times per week (0–5) and it required use of laxatives, suppositories, or enemas 2 days per week (1–5/d). Chronic constipation affected patients’ daily activities in a median of 3 days per week (0–5/day). Table 1 shows patients’ bowel diary entries. The median preoperative CCCS was 21 (range 16–25).
Preoperative colorectal transit time recorded a median of 10 markers retained in the colorectal tract (range 7–19).
Six patients out of 21 did not fill in the preoperative SF-36 questionnaire. As a result, they were not included in our questionnaire analysis, but still included in the SNS procedure. Out of the 15 patients who completed it, the preoperative SF-36 reported a median score of 80 for the physical state (30–163) and of 38 for the psycho-emotional state (8–98).
Postoperative data
All patients were discharged during the first postoperative day. One patient (5%) developed a hematoma during the first 3 days postoperatively, which was treated conservatively.
All 5 parameters evaluated in the bowel diary significantly improved after 6 months from the implant. At 12-month follow-up visit, parameters were stable, but no further progress was achieved (Table 1).
After 6 months from SNS procedure, maximum results were achieved: colorectal transit time showed a statistically significant reduction of radio-opaque markers retained in all colorectal segment evaluated (Table 2); the total number of markers retained decreased postoperatively from 10 (7–19) to 3 (0–4); while all patients in the preoperative evaluation had suffered from a colon transit delay, the number dropped to 0 at the postoperative re-evaluation.
Only 15 patients—who had previously completed the preoperative SF-36—filled in the postoperative questionnaire. As shown in Table 3, results improved significantly in all 8 scales, covering both the physical and the psycho-emotional health.
Anorectal manometry performed after 12 months from the implantation did not detect any parameter change, apart from MSP. In fact, the median MSP significantly decreased from 160 (range 120–184) to 154 (range 119–180) (P = 0.012).
Table 4 shows the pre- and postoperative manometric data.
The CCCS improved significantly during the first postoperative year, from 21 (16–25) to 8 (6–11) (P < 0.001). At the following follow-up visits, it progressively worsened (Table 5).
The median follow-up time was 60 months (range, 33–69 months).
Numbers of dropouts and treatment failure at each stage of the follow-up period are highlighted in Fig. 2. The main reason for treatment failure is related to patient dissatisfaction that we could track over time by the gradual worsening of the CCCS score.
One patient had the neuromodulator removed after 33 months because of a traumatic injury requiring neurosurgery (lumbosacral fracture due to a motor vehicle accident). At 36 months, 5 patients were administered prucalopride pharmacological treatment. Three patients refused medication, and at 48 months, 2 out of 3 had their neuromodulator removed, because no more effective. During follow-up visits, 2 more patients refused prucalopride and both had their device removed at 60 months. Five additional patients—under medical treatment from the 36-month follow-up visit—had their neuromodulator removed. At 60 months, 4 patients were administered prucalopride.
Overall, the neuromodulator removal rate was 48% at 60-month follow-up. Table 6 shows the functional results of the subgroup of patients who had the SNM explanted.
Discussion
Treatment for slow-transit constipation is a challenging and controversial matter, clinically debated in literature. Therapeutic options range from conservative management (i.e., change in dietary habits and use of laxative) to major colonic surgery (at risk of potential postoperative complications [18]. Sacral nerve stimulation is a minimally invasive surgical technique consisting in placing electrodes in the S3 foramen. This technique was firstly introduced to treat urinary disorders and fecal incontinence, both pathologies responding well in terms of good clinical outcomes and low complication rates [19].
Results reported in literature are not consistent, mainly because the underlying mechanism of SNS on STC has not been fully understood until now.
Some studies tend to suggest that SNS could not be recommended as standard treatment for chronic constipation [5, 8]. Patton et al. found out that symptom improvement after SNS seems to diminish over time [8] and only 7% of patients (4/53) had still the sacral nerve device implanted and running at 5.7-year follow-up [8]. Similarly, Maeda et al. published a study with a cohort of 62 patients with permanent implantation, where only 22% of patients (14/62) had lasting improvement after 60 months [5].
Our study showed though that 52% of patients (11/21) treated for slow-transit constipation with SNS had a successful outcome at a median follow-up of 60 months.
We are fully aware that it is strenuous to compare results of existing studies available in medical literature since different inclusion criteria were adopted to enroll patients affected by chronic constipation. Besides, follow-up period, patient management during follow-up routine, and criteria adopted to assess outcomes differ significantly across studies.
One concern in literature is patients’ selection. Our team does share the mainstream opinion that inclusion/exclusion criteria could heavily affect the SNS overall effectiveness, so that a systematic screening process has to be in place to come to a homogeneous sample. In our study, we enrolled patients only affected by STC, free of concomitant diseases.
Our outcomes were promising and stable at 6 months and 1 year after implant. However, this short-term improvement did not progress any further. Our results do not differ from findings of other published studies in literature which show that efficacy of SNS for treating slow-transit constipation declines gradually over time [1, 5, 8, 11].
The key advantage of this technique is the relatively low incidence of postoperative complications, compared with more invasive surgical procedures [20, 21]. In our study, we had only 1 patient out of 21 (5%) who developed a hematoma. The overall safety of this technique reduced significantly hospitalization and all associated costs. Similarly, Ratto et al. reported low adverse event rate (16.7%) [3].
Appropriate patient selection for SNS is much debated in literature. Some authors do believe that the mechanism of action of the neuromodulator could help patients with colonic inertia, believing SNS is thus not appropriate in patients with ODS [22]. However, Ratto et al. included in their study also patients affected by both STC and ODS and they reported a greater CCCS improvement in patients with ODS and with mixed constipation rather than patients with STC alone [3]. A comparison between these two studies clearly shows how different inclusion criteria could heavily affect clinical outcomes [3, 22].
Wang et al. reported that slow-transit constipation is mostly caused by disorders of the enteric nervous system [23]. Sacral nerve stimulation is delivered via a percutaneous transforaminal approach, with the electrical current delivered directly to sacral nerve roots known to control the pelvic viscera. Colonic electrical stimulation may lead to positive effects on the electric activity of the interstitial cells of Cajal and/or of the enteric or extrinsic autonomic nerves [24]. It is postulated by some authors that colonic electric stimulation may influence the neuroplasticity of enteric nerves, with induced regeneration of myenteric plexus neurons [23]. It is reasonable enough to assert that a rigorous method of patient selection could increase the success rate of SNS in patients affected by STC.
We deemed useful to assess personality structure and psychological functioning as a further screening criterion. By using MMPI, Wexner and colleagues found a significant increase on the hypochondriasis and depression scales in constipated patients [14]. They came to the conclusion that constipated patients could greatly benefit by adding a psychological component to their treatment regime [14]. However, correlation between psychological conditions and success rate of SNS in patients with constipation has not thoroughly been investigated yet [25]. It is generally known that psychoneurotic disorders, such as stress or anxiety, inhibit the occurrence of intestinal migrating motor complexes [26, 27].
According to Malouf et al., we only enrolled patients without past psychological/psychiatric history [28].
It is very important the identification of this subgroup of patients affected from constipation who may benefit from SNS treatment, even if the working mechanism of SNS has still to be clarified [25].
In our study, patients had a higher preoperative CCCS than those analyzed by Ratto et al. on average: In line with Ratto et al., our CCCS improved significantly after the SNS implant [3]; however, in our cohort, the CCCS progressively worsened after the first year, even if it did not revert back to the preoperative initial score.
In terms of bowel diary parameters, our study recorded a clear improvement in all items at 6-month follow-up, still stable at 1 year; however, no further progress was achieved thereafter, showing that most benefits are achieved in the first 6 months after SNS implant. Our outcome contradicts, at least partially, the results found in Maeda et al., which instead demonstrated that improved spontaneous bowel movements had lasting effects also at 60 months [5].
Colorectal transit time was measured in all patients at 6-month follow-up and showed a statistically significant improvement after implant (P < 0.001), with extension to all large bowel segments (i.e., right colon, left colon, and sigma-rectum), contrary to the study by Maeda and colleagues, where colorectal transit time improved only in 60% of patients with a preoperative slow transit. Besides, they reported a transit time worsening in 3 patients out of 4, with a preoperative normal one [5]. Kamm et al. reported gut transit data of 27 patients, of which 20 (74%) had preoperative delayed transit; at 6-month follow-up, 9 patients out of 27 (33%) still had persistent delayed gut transit time [11].
The Short Form 36 Health Survey measured patients’ health status prior to the modulator implant and used to size changes at 6 months after surgery. We do believe that 6 months is a suitable time interval for recovery after surgery and for consolidating neurostimulation. In our cohort, we recorded a clear improvement in all 8 SF-36 scoring scales. Similarly to our study, Ratto et al. reported a statistically significant improvement in all SF-36 scales. However, they clarified that correlation between clinical improvement and health status was statistically significant for the physical state, but not for the psycho-emotional state [3].
Anorectal manometric evaluation was performed in all patients at baseline and after 1 year from the neuromodulator implant. Comparing the two sets of data, only MSP decreased after the surgical procedure, whereas all other parameters remained unchanged. Ratto et al. showed no change in manometry or rectal sensation postoperatively and Ganio et al. reported an increase of both MRP and MSP [3, 29]. In the abovementioned studies, manometric differences are probably connected to different inclusion criteria adopted. If so, patient selection does possibly explain conflicting data in the literature, as we do believe, in line with other authors’ view.
In our cohort of patients, neuromodulator was removed in 48% of patients at the end of the 5-year follow-up period, because of its declining effectiveness over time. In a review by Sharma et al., they reported that the 89.6% of all permanent neuromodulators implanted were functioning satisfactorily at their last follow-up. This difference in neuromodulator explant rates between their study and ours is possibly deriving by our different follow-up duration (they included in their review studies with shorter median follow-up duration compared with ours [18]).
Our team does believe that one of the key strengths of this study is the screening efforts to come to a homogeneous sample, thanks to a set of strict inclusion/exclusion criteria adopted to enroll patients affected by chronic slow-transit constipation, with no concomitant diseases. Moreover, the 5-year follow-up period, although perhaps not long enough for a functional disease, is one of the longest to be found in literature for this type of study.
The present study has some limitations. First of all, the small number of patients enrolled in our study. On this specific point, we do hope that larger sample sizes could be assessed in the future since large data set on constipation are still lacking, especially ones with a specific focus on mid- and long-term follow-ups. Secondly, the retrospective nature of this study is inherently susceptible to a bias associated with its design. Thirdly, the scoring system used in this study is open to criticism; however, there is not a standard scoring system to refer to yet (and underlying criterion has not been established yet).
To sum up, we are aware that rigorous and large-scale randomized trials are needed to better assess the effects of SNS, based on clear and coherent method of patient selection. For instance, we welcome the efforts of the randomized multicenter controlled trial already running in Denmark, which is expected to end in 2021, whose primary aim is to assess the effectiveness of SNS compared with personalized conservative treatment in patient with idiopathic slow-transit constipation [30].
Conclusions
The SNS is a safe and a well-tolerated surgical procedure, proven to be useful to treat patients affected by slow-transit constipation, where conservative treatment failed to help. The short-term outcome (6 months to 1 year) was promising according to all scoring systems used; however, benefits did progressively worsen over a longer time span.
In wider terms, the underlying mechanism of STC has still to be fully understood. In the future, fine-tuning patient selection—one of the main areas of concern debated in literature—could result in more conclusive and consistent results of sacral neuromodulation used for the treatment of STC.
In light of our results—where positive outcomes were not consolidated over the long term (but this is also the case for other available therapeutic options)—we do think necessary to further investigate the use of sacral neuromodulation and to standardize patient selection as much as possible.
References
Holzer B, Rosen HR, Novi G, Ausch C, Hölbling N, Hofmann M, Schiessel R (2008) Sacral nerve stimulation in patients with severe constipation. Dis Colon Rectum 51:524–530
Thomas GP, Dudding TC, Rahbour G, Nicholls RJ, Vaizey CJ (2013) Sacral nerve stimulation for constipation. Br J Surg 100:174–181
Ratto C, Ganio E, Naldini G, GINS (2015) Long-term results following sacral nerve stimulation for chronic constipation. Color Dis 17:320–328
Maeda Y, O’Connell PR, Lehur PA, Matzel KE, Laurberg S, European SNS Bowel Study Group (2015) Sacral nerve stimulation for faecal incontinence and constipation: a European consensus statement. Color Dis 17:O74–O87
Maeda Y, Kamm MA, Vaizey CJ, Matzel KE, Johansson C, Rosen H, Baeten CG, Laurberg S (2017) Long-term outcome of sacral neuromodulation for chronic refractory constipation. Tech Coloproctol 21:277–286
Pilkington SA, Emmett C, Knowles CH, Mason J, Yiannakou Y, the NIHR capacity working group and Pelvic Floor Society (2017) Surgery for constipation: systematic review and practice recommendations. Color Dis 19:92–100
Dinning PG, Hunt L, Patton V, Zhang T, Szczesniak M, Gebski V, Jones M, Stewart P, Lubowski DZ, Cook IJ (2015) Treatment efficacy of sacral nerve stimulation in slow transit constipation: a two-phase, double-blind randomized controlled crossover study. Am J Gastroenterol 110:733–740
Patton V, Stewart P, Lubowski DZ, Cook IJ, Dinning PG (2016) Sacral nerve stimulation fails to offer long-term benefit in patients with slow-transit constipation. Dis Colon Rectum 59(9):878–885
McCallum IJD, Ong S, Mercer-Jones M (2009) Chronic constipation in adults. BMJ 338:b831
Vogel JD, Johnson EK, Morris AM, Paquette IM, Saclarides TJ, Feingold DL, Steele SR (2016) Clinical practice guideline for the management of anorectal abscess, fistula-in-ano, and rectovaginal fistula. Dis Colon Rectum 59(12):1117–1133
Kamm MA, Dudding TC, Melenhorst J, Jarrett M, Wang Z, Buntzen S, Johansson C, Laurberg S, Rosen H, Vaizey CJ, Matzel K, Baeten C (2010) Sacral nerve stimulation for intractable constipation. Gut 59:333–340
Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD (1996) A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum 39:681–685
Jenkinson C, Coulter A, Wright L (1993) Short Form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ 306:1437–1440
Heymen S, Wexner SD, Gulledge AD (1993) MMPI assessment of patients with functional bowel disorders. Dis Colon Rectum 36:593–596
Schiano di Visconte M, Santoro GA, Cracco N, Sarzo G, Bellio G, Brunner M, Cui Z, Matzel KE (2018) Effectiveness of sacral nerve stimulation in fecal incontinence after multimodal oncologic treatment for pelvic malignancies: a multicenter study with 2-year follow-up. Tech Coloproctol 22(2):97–105
Quigley EMM (2012) Prucalopride: safety, efficacy and potential applications. Ther Adv Gastroenterol 5:23–30
Sharma S, Agarwal BB (2012) Scoring systems in evaluation of constipation and obstructed defecation syndrome (ODS). J Int Med Sci Acad 25:57–59
Sharma A, Bussen D, Herold A, Jayne D (2013) Review of sacral neuromodulation for management of constipation. Surg Innov 20:614–624
Leroi AM, Michot F, Grise P, Denis P (2001) Effect of sacral nerve stimulation in patients with fecal and urinary incontinence. Dis Colon Rectum 44:779–789
Nyam DCNK, Pemberton JH, Ilstrup DM, Rath DM (1997) Long-term results of surgery for chronic constipation. Dis Colon Rectum 40:273–279
Knowles CH, Scott M, Lunniss PJ (1999) Outcome of colectomy for slow transit constipation. Ann Surg 230:627–638
Graf W, Sonesson AC, Lindberg B, Åkerud P, Karlbom U (2015) Results after sacral nerve stimulation for chronic constipation. Neurogastroenterol Motil 27:734–739
Wang Y, Wang Q, Kuerban K, Dong M, Qi F, Li G, Ling J, Qiu W, Zhang W, Ye L (2019) Colonic electrical stimulation promotes colonic motility through regeneration of myenteric plexus neurons in slow transit constipation beagles. Biosci Rep 39(5)
Moore JS, Gibson PR, Burgell RE (2018) Neuromodulation via interferential electrical stimulation as a novel therapy in gastrointestinal motility disorders. J Neurogastroenterol Motil 24(1):19–29
Carriero A, Martellucci J, Talento P, Ferrari CA (2010) Sacral nerve stimulation for constipation: do we still miss something? Role of psychological evaluation. Int J Color Dis 25(8):1005–1010
Valori RM, Kumar D, Wingate DL (1986) Effects of different types of stress and of “prokinetic” drugs on the control of the fasting motor complex in humans. Gastroenterology 90:1890–1900
Pescatori M, Spyrou M, Pulvirenti d’Urso A (2006) A prospective evaluation of occult disorders in obstructed defecation using the ‘iceberg diagram’. Color Dis 8:785–789
Malouf AJ, Wiesel PH, Nicholls T, Nicholls RJ, Kamm MA (2002) Short term effects of sacral nerve stimulation for idiopathic slow transit constipation. World J Surg 26:166–170
Ganio E, Masin A, Ratto C, Altomare DF, Ripetti V, Clerico G, Lise M, Doglietto GB, Memeo V, Landolfi V, del Genio A, Arullani A, Giardiello G, de Seta F (2001) Short-term sacral nerve stimulation for functional anorectal and urinary disturbances: results in 40 patients: evaluation of a new option for anorectal functional disorders. Dis Colon Rectum 44:1261–1267
Heemskerk SCM, Rotteveel AH, Benninga MA et al (2017) Sacral neuromodulation versus personalized conservative treatment in patients with idiopathic slow-transit constipation: study protocol of the No. 2-trial, a multicentre randomized controlled trial. Conference 12th Scientific Annual Meeting European Society of Coloproctology. Color Dis 19:140–143. https://doi.org/10.1111/codi.13799
Author information
Authors and Affiliations
Contributions
MSdV and GB conceived and designed the study; MSdV, GB, AP, and TCM acquired data; MSdV, GB, LB, and LD interpreted data. MSdV, GB, AP, TCM, and LB drafted the manuscript. All authors have read, critically revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration, as last amended at the 64th WMA General Assembly, Fortaleza, Brazil, October 2013, or comparable ethical standards. For this type of study, formal consent by the institutional research committee is not required in Italy.
Informed consent
Informed consent was obtained from all participants enrolled in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schiano di Visconte, M., Pasquali, A., Cipolat Mis, T. et al. Sacral nerve stimulation in slow-transit constipation: effectiveness at 5-year follow-up. Int J Colorectal Dis 34, 1529–1540 (2019). https://doi.org/10.1007/s00384-019-03351-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-019-03351-w