Abstract
Purpose
Electrical stimulation of the gut has recently been under intensive investigation and various studies have revealed therapeutic potentials of gastrointestinal electrical stimulation for gastrointestinal motility disorders and obesity. While there have been a number of reviews on gastric electrical stimulation, there is a lack of systematic reviews on intestinal electrical stimulation. The aim of this review is to provide an overview on the effects, mechanisms, and applications of intestinal electrical stimulation.
Results
We evaluated published data on intestinal electrophysiology, pathophysiology, and different methodologies on intestinal electrical stimulation and its possible mechanisms in both research and clinical settings using the MEDLINE database for English articles from 1963 to 2008. Based on this systematic review, intestinal electrical stimulation has been reported to alter intestinal slow waves, contractions and transit; the effects were mediated via both vagal and adrenergic pathways. Intestinal electrical stimulation has been reported to have potentials for treating various intestinal motility disorders and obesity.
Conclusions
It is concluded that intestinal electrical stimulation may have promising applications for treating motility disorders associated with altered intestinal contractile activity. The most recent studies have revealed possible applications of intestinal electrical stimulation for the treatment of obesity. Basic research results are promising; however, further clinical studies are needed to bring IES from bench to bedside.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Although the earliest study of electrical stimulation of the gut was reported more than 40 years ago, the development of the field has been slow compared to cardiac pacing or electrical nerve stimulation. In the earliest study on intestinal stimulation, which was published in 1963, Bilgutay et al. [1] reported the use of intraluminal electrical stimulation via the tip of a nasogastric tube to induce peristalsis and shorten the recovery period from ileus after laparotomy; an increase in gastric contractions as well as gastric emptying was reported in both dogs and humans. However, subsequent randomized controlled studies failed to produce consistent or promising results [2–4]. Before the 1960s there was a lack of understanding of gastrointestinal electrophysiology, which only became a topic of interest in the later 1960s and early 1970s [5–9]. From the 1970s to the early 1990s, Kelly and colleagues made significant contributions to the understanding of the electrical stimulation of the gut; a number of studies on intestinal electrical stimulation (IES) and its applications were reported in dogs and humans by the group [10–12]. Research on IES during that period was focused on its potential applications for treating dumping syndrome and short-bowel syndrome by delayed gastric emptying and small intestinal transit and/or enhancing intestinal absorption.
During the past decade, more progress has been made on the methodologies, effects, mechanisms, and clinical applications of gastrointestinal electrical stimulation. Numerous reports are available in the literature on electrical stimulation of various organs of the gastrointestinal tract, such as the stomach, small intestine, colon, and rectum for the treatment or therapeutic potentials of various conditions such as gastroparesis, intestinal pseudo-obstruction and fecal incontinence as well as obesity [13–16]. A number of excellent reviews have been published on gastric electrical stimulation [15–21], whereas few reviews are available on intestinal electrical stimulation in the literature with the most recent one published about 15 years ago [22].
Since electrical nerve stimulation is relatively more matured and clinically applied, gastrointestinal electrical stimulation is often mistakenly compared with nerve stimulation. In reality, however, there are fundamental differences between electrical gut stimulation and electrical nerve stimulation. One of the major differences between electrical gut stimulation and electrical nerve stimulation lies in the affecter and the effecter. With electrical nerve stimulation, nerves or nervous systems are stimulated and organs associated with the nerves or nervous systems are affected. With electrical gut stimulation, an organ of the gut is stimulated and the functions of the organ are altered. These altered organ functions may be attributed to either the local or peripheral effects of the stimulation or the central neural effects of the stimulation or both. Moreover, the stimulation methodologies of the gut or IES are different from those of electrical nerve stimulation, mainly because of the following two reasons: (1) the gastrointestinal organ is composed of smooth muscles. The response of smooth muscles to electrical stimulation is slow and therefore, long pulses are typically required in order to alter the function of the organ being stimulated; Typically, a pulse width of 50 ms or higher is required to activate smooth muscles if single pulses are used; if the stimulus is composed of a train of pulses, the required pulse width is 2 ms or higher for a pulse frequency of 40 Hz. (2) The gastrointestinal organ has intrinsic myoelectrical activity or slow waves and therefore, the electrical stimulation of the gut may be designed to enhance or alter this intrinsic myoelectrical activity.
Electrophysiology of Small Intestine
Small Intestinal Myoelectrical Activity
It is important to understand electrophysiology of the small intestine for IES because (1) intestinal motility (frequency and propagation) is regulated by intestinal myoelectrical activity, and (2) to alter the function of the small intestine, electrical stimuli may have to be designed to enhance or inhibit this intrinsic intestinal myoelectrical activity.
Myoelectrical activity of the small intestine is similar to that of the stomach. It consists of two components: pacesetter potentials or slow waves and spike potentials. A typical recording showing intestinal slow waves and spikes is shown in Fig. 1. Small-intestinal slow waves originate from a region in the proximal 1 cm of the duodenum and propagate as an annular wave front in an aborad direction [7]. It determines the frequency and the direction of propagation of intestinal contractions. Spike potentials are superimposed on the slow waves and are electrical counterparts of contractions [8].
In the dog, the proximal 10–30% of the small intestine [30–115 cm of duodenum and jejunum] maintains the same slow wave frequency: 18–20 cycles/min (cpm), in a region called the “frequency plateau” [6]. Aborad to this point, there is a diminishing slow-wave frequency gradient along the small bowel to a rate of 14 cpm in the distal ileum [5, 23]. In humans, slow waves in the duodenum and proximal jejunum occur at about 12 cpm with an aborad gradient to about 9 cpm in the terminal ileum [24, 25]. Whether a proximal plateau of identical frequencies is present in the human duodenum and proximal jejunum has not been clearly shown [24]. Transection and reanastomosis of the small bowel decrease the slow-wave frequency in the distal segment in both dogs [26] and humans [10]. In addition, at least in dogs, the propagation of slow waves in the distal segment becomes abnormal: with a high percentage of these slow waves propagating in an orad rather than an aborad direction [27].
It has recently been theorized that the intestinal slow waves are generated by interstitial cells of Cajal (ICC) [28]. Like in the stomach, the pacemaker activity to the musculature in the small intestine is a subset of ICC [28] named myenteric ICC (IC-MY), which are arranged in a dense network between the circular and longitudinal muscle layers [29, 30]. Numerous in vitro studies have demonstrated the role of ICC as pacemakers with the evidence that can be summarized as follows: (1) ICC generate slow waves; (2) in the mutant animals where the ICC are absent, there are no slow waves; (3) in a specially prepared gastric tissue where ICC are obliterated by neutralizing antibody to Kit (ACK2), slow waves are absent [31]. A few studies from the groups of Huizinga and Ward using cultured interstitial cells of Cajal provided direct and strong evidence that the generation of pacemaker activity is an intrinsic property of the ICC [32–34]. Recordings from intestinal muscles of the W/Wv mice in which ICC-MY are almost completed absent showed complete loss of slow wave activity [35, 36]. Similar results were obtained from muscles of Sl/Sl mice [37]. However, a recent in vivo study and some in vitro findings have suggested that ICC may not be the sole pacemaker cells that generate slow waves [38–41], which indicated that ICC may not be necessary for the generation of gastrointestinal slow waves, more investigations are required for the later.
Intestinal Slow-Wave Dysrhythmia
Intestinal slow-wave dysrhythmia has been reported in various conditions, including postsurgical conditions, ischemia, and chronic intestinal pseudo-obstruction. During the first few hours after abdominal surgery, intestinal slow waves were found to show a substantial decrease frequency and spike activity [42] but become normal 1 day after abdominal surgery [43]. Intestinal dysrhythmia has been consistently reported in the condition of ischemia. In a rabbit study, intestinal slow-wave frequency fell from 18.2 pm at baseline to 12.2 cpm after 30 min of ischemia and was undetectable by 90 min of ischemia. Tachyarrhythmias were recorded in 55% of the animals as early as 25 min after ischemia was induced and lasted from 1 to 48 min. Frequencies ranged from 25 to 50 cpm. These tachyarrhythmias were seen only during ischemia, suggesting that they are pathognomonic for intestinal ischemia [44]. In patients with intestinal idiopathic pseudo-obstruction, responses of intestinal myoelectrical activity to distention was found to be altered [45].
It should be noted that compared with gastric slow waves, much less is known about the slow wave of the small intestine. The major reason for this is that intestinal slow waves cannot be recorded noninvasively using a similar method like surface electrogastrography [46].
Methodologies of IES
Methodologies of intestinal electrical stimulation are similar to those of gastric electrical stimulation [15], including patterns of stimuli, placement of electrodes, and delivery time of stimuli. Various methods published in the literature are summarized and critically discussed in the following paragraphs.
Long-Pulse Stimulation
This method is most frequently reported in the literature because it is able to “pace” or entrain natural slow waves [11, 47]. It is also called electrical pacing or intestinal pacing. In this method, the electrical stimulus is composed of repetitive single pulses with a pulse width on the order of milliseconds (10–300 ms for IES), and a stimulation frequency in the vicinity of the physiological frequency of the intestinal slow wave (see Fig. 2a). However, it should be noted that currently there are no implantable devices available on the market capable of generating pulses with a width longer than 2 ms.
Short-Pulse Stimulation
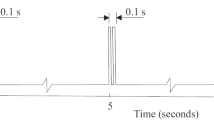
In contrast to long-pulse stimulation, the pulse width in this method is substantially shorter and is on the order of a few 100 μs (see Fig. 2b). Most commercially available cardiac pacemakers or nerve stimulators are capable of generating short pulses.
Pulse Train
In this method, the stimulus is composed of repetitive trains of pulses and is derived from the combination of two signals: (a) continuous short pulses with a high frequency (on the order of 5–100 Hz); (b) a control signal to turn the pulses on and off, such as x seconds “on” and y seconds “off.” The addition of x and y then determines the frequency of the pulse train (Fig. 2c). This kind of stimulation has been frequently used in nerve stimulation. Commercially available stimulators are capable of generating trains of pulses with a pulse width of below 2 ms.
Dual Pulses
A novel method of IES, called dual-pulse IES, has recently been proposed by combining short and long pulses [48]. In this method, the stimulus is composed of one or a few short pulses (on the order of a few 100 μs) followed by a long pulse (on the order of a few 100 ms; see Fig. 3), a long pulse followed by one or a few short pulses. A canine study has shown that dual pulse IES is capable of both normalizing intestinal dsyrhythmia and improving symptoms suggestive of nausea and vomiting induced by infusion of vasopressin [48]. Apparently, the proposed method of dual pulse IES is more attractive than the conventional method of electrical stimulation in which only short pulses or long pulses (but not both) are utilized.
Synchronized Stimulation
Conventionally, electrical stimulation is performed at a fixed frequency delivered at random without consideration of the occurrence of the intrinsic intestinal slow waves. A synchronized method that has been applied in GES and cardiac pacing has recently been adopted: synchronized intestinal electrical stimulation [49]. Synchronized IES requires the implantation of two pairs of electrodes, one for the detection of intestinal slow waves and the other for stimulation. In this proposed method, each electrical stimulus is delivered upon the detection of an intrinsic slow wave peak, that is, IES is performed at the occurrence of cyclic physiological electrical events of the small intestine. By synchronizing each electrical stimulus with the intrinsic physiological electrical activity it is hypothesized that it is capable of enhancing intestinal contractions. A recent canine study showed that synchronized IES in the fed state significantly accelerated intestinal transit [49].
Placement of Stimulation Electrodes
-
(a)
Serosal electrodes. Most commonly, stimulation electrodes are placed on the seromuscularis of the small intestine, approached from the serosal side. The advantage of this method is the guaranteed contact and direct effect on the targeted organ. The disadvantage is its invasiveness. Laparotomy or laparoscopy under general anesthesia is required.
-
(b)
Intraluminal or mucosal electrodes. Alternatively, stimulation electrodes may be placed on the mucosal surface of the small intestine via a nasojejunal tube. Typically, ring electrodes are attached to the tip of a catheter that is placed into the small intestine via the nose [50]. The advantage of this method is that no surgical procedures are required. The intubation of the catheter can be accomplished with or without the aid of endoscopy. The disadvantage is that this method may not be adequate for chronic stimulation.
Effects and Mechanisms of IES on Intestinal Functions
Effects of IES on intestinal motility and possible mechanisms related to IES have been investigated in a number of studies. IES has been shown to be able to pace or entrain intestinal slow waves and normalize intestinal slow wave dysrhythmia. Intestinal contractions can be inhibited with IES of high stimulation energy but may be enhanced with synchronized IES. Similarly, intestinal transit may be inhibited or enhanced with IES of appropriate parameters, resulting in an alteration in absorption. Moreover, the effects of IES on organs along the gut have also been explored (see Table 1).
Effects of IES on Intestinal Slow Waves
The effect of IES or intestinal pacing on intestinal slow waves was investigated systematically in a number of canine models [26, 47, 51, 52]. Long pulses with a pulse width of 50–150 ms are required to entrain or pace the intrinsic intestinal slow waves. It has been reported that intestinal slow waves can only be entrained when IES is delivered at a frequency higher than the frequency of the intrinsic slow waves [47, 51, 52]; a complete entrainment of intestinal slow waves can be achieved only when the stimulation frequency is equal to or below 110% of the intrinsic frequency. When the stimulation frequency is higher than 110% of the intrinsic frequency, only partially entrainment is possible, as shown in Fig. 4. Similarly, entrainment of intestinal slow waves can also be accomplished with IES using intraluminal ring electrodes [53].
Effects of intestinal electrical stimulation on intestinal slow waves. a Entrainment of intestinal slow waves with intestinal pacing. The entrainment is demonstrated by phase-locking between the stimulation artifacts and natural slow waves. b Percentage of slow wave entrainment with intestinal pacing at various pacing frequencies (IF: intrinsic frequency of natural slow waves)
In patients after roux gastretomy or cholecystectomy, however, intestinal pacing at a pulse width of 50 ms and amplitude of 15 mA was not able to entrain intestinal slow waves [10, 24]. It was not reported whether it was attributed to the fact that the stimulation parameters were not optimized or that intestinal pacing was ineffective during the postoperative state in humans.
Effects on Intestinal Slow-Wave Dysrhythmia
Compared to gastric electrophysiology, little is known on intestinal electrophysiology due to lack of non-invasive measurement methods. Unlike gastric slow waves that can be accurately measured using a non-invasive method of electrogastrography [54], intestinal slow waves cannot be accurately measured using abdominal surface electrodes due to the following reasons: (1) the intestinal slow wave signal is much weaker than the gastric slow wave; (2) the frequencies of slow waves in different segments of the small intestine are different and the abdominal surface recording is unable to reflect slow waves at a specific segment of the small intestine [46]. Consequently, little is known about pathophysiology of intestinal slow waves. However, a number of studies did report intestinal slow-wave dysrhythmia in a number of clinical settings such as nausea and vomiting, intestinal pseudo-obstruction and intestinal ischemia [45, 55–59]. Abnormalities in intestinal slow waves include dysrhythmia, reduced frequency, and uncoordinated slow waves along the intestine, and are associated with impaired intestinal contractions [42, 44].
Normalization of intestinal slow-wave dysrhythmia was reported in canine studies [60, 61]. In dogs with Roux gastrojejunostomy, electrical dysrhythmias in the Roux limb occurred and were corrected with electrical pacing [60]. In another study [61], impaired slow waves were induced with intestinal balloon distension. IES with long pulses and parameters similar to those used in pacing intestinal slow waves was able to normalize intestinal dysrhythmia as shown in Fig. 5. In a recent study in dogs, dual-pulse IES was reported to normalize vasopressin-induced intestinal dysrhythmia [48] (Table 1). The improvement or normalization of intestinal dysrhythmia is of clinical significance since intestinal dysrhythmia leads to impaired intestinal motility. Little is known about the effect of IES on intestinal slow-wave dysrhythmia in clinical settings, which is probably attributed to the fact that intestinal slow-wave dysrhythmia is rarely seen in clinical settings where IES is feasible.
Effects and Mechanisms of IES on Intestinal Motility
While intestinal slow-wave dysrhythmia can be reliably and consistently improved with IES or intestinal pacing, conventional IES with long pulses has not been reported to be able to improve intestinal contractions. Only recently was it reported that synchronized IES had an ameliorating effect on intestinal contractions. In this method of synchronized IES, the electrical stimuli were delivered at the occurrence of each slow-wave peak. Each stimulus was composed of a train of pulses lasting 0.5 s, and the pulses in the train had a frequency of 20 Hz, pulse width of 2 ms, and amplitude of 4 mA. It was found that synchronized (but not non-synchronized) IES induced small intestinal contractions in the fasting state in normal dogs. In the fed state, synchronized IES improved glucagon-induced small intestinal postprandial hypomotility. While it was not clear whether the enhancement of intestinal contractions with synchronized IES sustained at length, the small-intestinal transit of length of 1.5 m delayed by glucagon was improved. The excitatory effect of synchronized IES was blocked by atropine [49]. Similarly, gastric contractions were improved with synchronized gastric electrical stimulation [62] and sequential multi-channel gastric electrical stimulation [63, 64].
The excitatory effect of synchronized IES on intestinal contraction is of clinical importance and may be used to treat patients with intestinal hypomotility that is commonly seen in patients with chronic intestinal pseudo-obstruction or postoperative ileus. With the feasibility of intraluminal stimulation, IES may be performed without any surgical procedures by attaching a pair of ring electrodes to an intestinal feeding catheter or a manometric catheter [50].
Most early IES studies were focused on intestinal slow waves, intestinal transit, and absorption, with little attention paid to intestinal contractions. Reiser et al. [65] were probably the first to report that IES might have an inhibitory effect on intestinal contractions. It is clear from a number recent studies that IES with higher stimulation energy inhibits intestinal contractions. In one canine study, IES with a stimulation frequency of 20 cpm, pulse width of 200 ms, and amplitude of 10 mA significantly reduced postprandial intestinal contractions in healthy dogs [66]. Intestinal motility of the entire measured segment (40–220 cm distal to the stimulation electrodes) was inhibited by 60–74% with the single channel IES of long pulses. The percentage of inhibition in intestinal contractions with IES was proportional to the pulse width and amplitude. Hexamethonium, guanethidine, phentolamine, propranolol partially, but not L-NNA, ondansetron and naloxone prevented the inhibitory effect of IES on intestinal motility (Fig. 6). These findings demonstrate that the inhibitory effect induced by IES with long pulses is mediated via sympathetic but not nitrergic, serotoninergic 5-HT3 and opiate pathways. The inhibitory effect of IES on intestinal motility may be applicable to patients with sustained uncoordinated contractions that are seen in a subgroup of patients with CIP patients [67].
Manometric tracings showing the effects of IES with long pulses on small intestinal motility in saline session (a), hexamethonium session (b), and guanethidine (c). The inhibitory effect of IES on intestinal contractions was blocked by guanethidine, suggesting an adrenergic pathway involved with IES
Effects and Mechanisms of IES on Intestinal Transit and Absorption
Regarding intestinal transit and absorption, IES is classified into forward and backward stimulation. If stimulation electrodes are placed in the proximal intestine or the proximal portion of an interested segment, IES is considered as forward IES; if the stimulation electrodes are placed in the distal intestine or the distal portion of an interested segment, IES is regarded as backward stimulation.
Forward IES is able to accelerate intestinal transit [23, 68, 69]. It was reported that forward jejunal electric stimulation accelerated intestinal transit slowed by fat-induced ileal brake in a canine model (See Fig. 7). Backward IES was found to delay intestinal transit [70–73]. IES is applicable to treat dumping syndrome due to its inhibitory effect on intestinal transit [10, 15, 16, 74] and may also be applied to treat obesity due to its excitatory effect on intestinal transit (possibly reducing nutrient absorption) [69].
Intestinal electrical stimulation may also influence small-bowel absorption independent of alterations in the intestinal slow waves. It was reported that backward jejunal electric stimulation slowed or reversed the flow of liquid chyme through the paced segment and led to enhanced absorption of water, nutrients, and electrolytes in canines [47, 75] and in rats [73]. Postprandial backward electric stimulation induced an increase in body weight and a decrease in fecal fat and nitrogen losses during the test period in a canine model of short-bowel syndrome [76]. The enhanced enteric absorption with backward IES was mediated in part by an alpha-adrenergic mechanism [47].
Forward IES slightly decreased the output of water, glucose, and sodium from the jejunal segment [52]. The effects of forward IES on fat absorption and related mechanisms were investigated in a recent rodent study. IES with long pulses or pulse trains accelerated intestinal transit measured by the recovery of phenol red and increased the percentage of triglycerides recovered from the distal segment. IES with trains of short pulses were more effective than IES with long pulses in accelerating jejunal transit and reducing fat absorption. The effects of IES with trains of short pulses on the transit and fat absorption were partially abolished with the treatment of lidocaine, suggesting possible involvement of the enteric nerves [77].
Effects of IES on Other Organs
Reflex among different organs of the gut is well known. For example: ingestion of food into the stomach induces changes not only in the stomach but also in the small intestine and colon. Similarly, distention of the rectum results in alterations in motility functions not only in the rectum but also in the stomach and intestine [78, 79]. The earliest discovery of the inhibitory effect of IES on other organ of the gut was the IES-induced delay in gastric emptying (see Table 1) and this inhibitory effect of proposed for the treatment of dumping syndrome [11, 80, 81]. A number of recent studies have demonstrated similar reflexive effects of electrical stimulation along the gut [50, 82–86]. IES with long pulses inhibits both upper gastrointestinal motility and lower gastrointestinal motility. In dogs, IES with long pulses was found to reduce gastric tone: inhibit antral contractions, and delay gastric emptying [84, 86]. Similarly, rectal tone was also reduced with IES [83]. In humans, long-pulse IES delivered via intraluminal ring electrodes attached to a feeding tube placed in the duodenum reduced gastric accommodation and delayed gastric emptying [50]. The inhibitory effects of IES on upper and lower GI motility were found to be mediated through vagal and sympathetic pathways [66, 84].
It is important to be aware of the cross-organ effects of gastrointestinal electrical stimulation. The cross-organ phenomenon may lead to unwanted side effects. For example, if a particular method of IES, such as synchronized IES, is applied to treat patients with slow intestinal transit, one should make sure that it does not delay gastric emptying. On the other hand, the cross-organ effect may bring convenience in the development of electrical stimulation strategy. For example, if the goal of electrical stimulation is to delay gastric emptying (such as in treating obesity or controlling postprandial glucose level), electrical stimulation may be applied either directly to the stomach or indirectly to the small intestine based on the feasibility of implementation [50, 87].
Potential Applications
Although some promising results have been reported in the literature, demonstrating potential applications of IES for treating gastrointestinal motility disorder and obesity, no FDA-approved device is available at the moment. Almost all of the basic and clinical research studies published in the literature utilized long pulses as stimuli, whereas no commercial implantable devices are available capable of generating long pulses. The lack of an adequate implantable device and the invasive nature of the placement of stimulation electrodes have limited clinical research in exploring applications of IES. On the other hand, the feasibility of utilizing an intraluminal catheter placed naso-intestinally may make it easier for certain clinical research.
IES for the Treatment of Intestinal Motility Disorders
Based on the effects of IES on intestinal motility, forward IES may be used to treat intestinal motility disorders related to slow intestinal transit, such as chronic intestinal pseudo-obstruction. On the other hand, backward IES can be used to delay intestinal transit and therefore may be used to treat patients with dumping syndrome or short-bowel syndrome [10, 15, 16, 74]. A number of early studies also demonstrated the application of IES in enhancing intestinal absorption [47, 52, 71]. In these applications, IES was performed backward and the improvement in short-bowel syndrome or absorption was attributed to a delay in intestinal transit.
With appropriate parameter settings, IES has also been shown to be able to accelerate intestinal transit in animals, especially using the method of synchronized IES. However, little has been studied on the efficacy of appropriate IES on intestinal transit except a preliminary IES study in healthy volunteers in which a significant improvement in intestinal transit was noted [69].
IES for the Treatment of Obesity
Potential applications of IES for the treatment of obesity have been reported in a number of recent studies [76, 84, 85]. Obesity is one of the most prevalent public health problems in the United States, claiming over 400,000 lives and costing over $100 billion in the country every year [88]. It results from an imbalance between energy expenditure and caloric intake. The current therapeutic strategies for the treatment of obesity are not satisfactory. Behavior modification and pharmacotherapy are effective only for a short term [88, 89]. The surgical treatment induces satisfactory long-term weight loss. Its application is, however, very limited because of the substantial risks and complications involved [90]. Recently, there has been a growing interest in electrical stimulation for the treatment of obesity. Gastric electrical stimulation (GES), as a potential therapy for obesity, has been extensively studied in both animals and humans. Previous open-label studies have shown promising results in food intake and weight loss with GES [91–94]. However, a recent multi-center controlled study failed to reach significant weight loss in obese patients treated with GES.
Although there is a lack of clinical studies on the therapeutic potential of IES for obesity, basic and clinical research has yielded interesting results. In rats, IES was reported to reduce food intake and body weight in both lean and diet-induced obese rats (Fig. 8) via the inhibition of gastric emptying, acceleration of intestinal transit, and/or reduction of fat absorption [77, 85]. Mechanisms involving gastrointestinal hormones have also been elucidated. In a recent rodent study, IES was found to decrease a prominent hunger hormone, ghrelin, in gastric tissues and to increase one of the major satiety hormones, cholecystokinin, in the duodenal tissues [95]. Vagal neuronal mechanisms have also been reported with IES [96].
Effect of intestinal electrical stimulation on food intake and weight change in diet-induced obese rats. Top IES significantly reduced food intake (P = 0.03), the reduction was even more with the higher pulse width of 300 ms (P = 0.01). Bottom IES significantly inhibited weight gain (P = 0.049), similar to the food intake, the inhibition was even more with the higher pulse width of 300 ms (P = 0.036)
In dogs, IES was noted to induce gastric distention and the IES-induced gastric distention was correlated with reduced food consumption [86]. Similarly, IES also delayed gastric emptying and the delay in gastric emptying is believed to reduce food intake and increase satiety during inter-meal periods.
A feasibility study of IES was performed in healthy volunteers [50]. IES was performed using long pulses via intraluminal ring electrodes attached to a feeding tube that was placed through the nose into the duodenum. The study was performed in 12 healthy volunteers intubated with a feeding tube in the duodenum under endoscopy. There were three ring electrodes at the end tip of the tube and the two distal electrodes were used for recording and electrical stimulation. On two separate days, each subject underwent a session of IES with various stimulation parameters, a water load test with IES or with sham-IES and a gastric emptying test with IES or with sham-IES. It was found that IES did not induce any noticeable dyspeptic symptoms. The amount of water drunk by the subjects was reduced by 25% with IES. The mean half-time gastric emptying was increased by 56% with IES and the gastric retention at 2 h was increased by 43%. These findings suggested a therapeutic potential of IES for treating obesity.
In another study, IES was shown to accelerate intestinal transit and reduce fat absorption [69]. Twelve healthy volunteers were involved in a four-session (two sessions without IES and two with IES) study. At the beginning of the first session, a nasal-duodenal feeding tube with two ring electrodes (used for IES) was incubated into the duodenum. The duodenum was infused via the feeding tube with intralipid and d-xylose within 30 min. Absorption during the following 24 h was assessed from the stool and urine. The second session was designed to study the intestinal transit and was performed the following day. An isotope labeled non-absorbable solution was infused via the feeding tube within 3 min. These two sessions were repeated 1 week later. Long-pulse IES was performed via the ring electrodes during sessions 1 and 2 or sessions 3 and 4 in a randomized manner. It was found that IES significantly reduced lipid and d-xylose absorption. The fecal lipid was 6.6 ± 4.6 g without IES and almost doubled with IES (11.1 ± 6.5 g, P = 0.047). Similarly, the d-xylose in urine was 3.5 ± 2.2 g with IES, which was significantly lower than that without IES (6.6 ± 5.1 g, P = 0.049). A significant acceleration in intestinal transit was also noted.
Discussion and Conclusions
In typical nerve stimulation, short pulses or trains of short pulses are commonly used. Whereas in IES, repetitive long pulses with a frequency lower than 1 Hz are typically used. This sort of parameter is chosen to alter intestinal muscle functions. Long pulses are needed for IES because the intestinal tissue is composed of smooth muscles that have a large time constant (slow in response to electrical stimulation). Stimulation electrodes can be placed surgically by laparoscopy or laparotomy. However, for temporary IES, intraluminal electrodes may be an excellent alternative, which does not involve any surgical procedure.
Intestinal electrical stimulation with long pulses has been shown to entrain intrinsic intestinal slow waves and improve or normalize intestinal slow-wave dysrhythmia in animals, although little data is available from patients. Intestinal contractions can be consistently inhibited with the conventional method of IES with relatively high energy. However, it seems that only synchronized IES (stimuli are synchronized with intrinsic slow waves) is capable of inducing or enhancing intestinal contractions. Intestinal transit has been reported to be accelerated with forward IES but decreased with backward IES.
Intestinal electrical stimulation may have promising applications for treating motility disorders associated with altered intestinal contractile activity as shown in Table 2. The most recent studies have revealed possible applications of IES for the treatment of obesity. Basic research results are promising, however further clinical studies are needed to bring IES from the bench to the bedside. Human obesity is a complex disorder involving numerous known and unknown pathways, and the evolution of mankind may have resulted in well-developed mechanisms protecting the human from starvation rather than obesity.
The major hindrance in the advancement of IES is similar to that of GES, including the invasive nature of the methodology and the lack of implantable device suitable for IES. Accordingly, a less invasive method of placing stimulation electrodes would be of great significance, such as endoscopical placement of electrodes [97, 98]. The other issue is the lack of suitable implantable stimulator. However, temporary IES can be performed using intraluminal ring electrodes and an external stimulator. Since the intestinal lumen is small and there is no problem with the contact between ring electrodes and the intestinal mucosa, clinical research without any surgical procedures is possible using intraluminal ring electrodes. This may greatly facilitate research and development in the area of intestinal electrical stimulation.
References
Bilgutay AM, Wingrove R, Griffen WO, Bonnabeau RC Jr, Lillehei CW. Gastro-intestinal pacing: a new concept in the treatment of ileus. Ann Surg. 1963;158:338–348.
Quast DC, Beall AC Jr, Debakey ME. Clinical evaluation of the gastrointestinal pacer. Surg Gynecol Obstet. 1965;120:35–37.
Berger T, Kewenter J, Kock NG. Response to gastrointestinal pacing: antral, duodenal and jejunal motility in control and postoperative patients. Ann Surg. 1966;164:139–144.
Moran JM, Nabseth DC. Electrical stimulation of the bowel. A controlled clinical study. Arch Surg. 1965;91:449–451.
Bunker CE, Johnson LP, Nelsen TS. Chronic in situ studies of the electrical activity of the small intestine. Arch Surg. 1967;95:259–268.
Szurszewski JH, Elveback LR, Code CF. Configuration and frequency gradient of electric slow wave over canine small bowel. Am J Physiol. 1970;218:1468–1473.
Hermon-Taylor J, Code CF. Localization of the duodenal pacemaker and its role in the organization of duodenal myoelectric activity. Gut. 1971;12:40–47.
Sarna SK, Bowes KL, Daniel EE. Gastric pacemakers. Gastroenterology. 1976;70:226–231.
Hinder RA, Kelly KA. Human gastric pacesetter potential. Site of origin, spread, and response to gastric transection and proximal gastric vagotomy. Am J Surg. 1977;133:29–33.
Richter HMIII, Kelly KA. Effect of transection and pacing on human jejunal pacesetter potentials. Gastroenterology. 1986;91:1380–1385.
Becker JM, Sava P, Kelly KA, Shturman L. Intestinal pacing for canine postgastrectomy dumping. Gastroenterology. 1983;84:383–387.
Kelly KA, La Force RC. Pacing the canine stomach with electric stimulation. Am J Physiol. 1972;222:588–594.
McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456–461.
Abell T, McCallum R, Hocking M, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–428.
Zhang J, Chen JD. Systematic review: applications and future of gastric electrical stimulation. Aliment Pharmacol Ther. 2006;24:991–1002.
Kelly KA. Pacing the gut. Gastroenterology. 1992;103:1967–1969.
Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of gastroparesis with electrical stimulation. Dig Dis Sci. 2003;48:837–848.
Miedema BW, Sarr MG, Kelly KA. Pacing the human stomach. Surgery. 1992;111:143–150.
Lin Z, Chen JD. Advances in gastrointestinal electrical stimulation. Crit Rev Biomed Eng. 2002;30:419–457.
Eagon JC, Soper NJ. Gastrointestinal pacing. Surg Clin North Am. 1993;73:1161–1172.
Bortolotti M. The “electrical way” to cure gastroparesis. Am J Gastroenterol. 2002;97:1874–1883.
Cullen JJ, Kelly KA. The future of intestinal pacing. Gastroenterol Clin North Am. 1994;23:391–402.
Soper NJ, Geisler KL, Sarr MG, Kelly KA, Zinsmeister AR. Regulation of canine jejunal transit. Am J Physiol. 1990;259:G928–G933.
Soper NJ, Saar MG, Kelly KA. Human duodenal myoelectric activity after operation and with pacing. Surgery. 1990;107:63–68.
Christensen J, Schedl HP, Clifton JA. The small intestinal basic electrical rhythm (slow wave) frequency gradient in normal men and in patients with variety of diseases. Gastroenterology. 1966;50:309–315.
Akwari OE, Kelley KA, Steinbach JH, Code CF. Electric pacing of intact and transected canine small intestine and its computer model. Am J Physiol. 1975;229:1188–1197.
Karlstrom LH, Soper NJ, Kelly KA, Phillips SF. Ectopic jejunal pacemakers and enterogastric reflux after roux gastrectomy: effect of intestinal pacing. Surgery. 1989;106:486–495.
Thuneberg L. Interstitial cells of cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1–130.
Faussone-Pellegrini MS, Pantalone D, Cortesini C. An ultrastructural study of the interstitial cells of cajal of the human stomach. J Submicrosc Cytol Pathol. 1989;21:439–460.
Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S, Sanders KM. C-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97–111.
Ordog T, Ward SM, Sanders KM. Interstitial cells of cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518(Pt 1):257–269.
Lee JC, Thuneberg L, Berezin I, Huizinga JD. Generation of slow waves in membrane potential is an intrinsic property of interstitial cells of cajal. Am J Physiol. 1999;277:G409–G423.
Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of cajal from the murine small intestine. J Physiol. 1998;513(Pt 1):203–213.
Thomsen L, Robinson TL, Lee JC, et al. Interstitial cells of cajal generate a rhythmic pacemaker current. Nat Med. 1998;4:848–851.
Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480(Pt 1):91–97.
Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349.
Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:C1577–C1585.
Hou X, Yin J, Liu J, Pasricha PJ, Chen JD. In vivo gastric and intestinal slow waves in w/wv mice. Dig Dis Sci. 2005;50:1335–1341.
Yin J, Hou X, Chen JD. Roles of interstitial cells of cajal in intestinal transit and exogenous electrical pacing. Dig Dis Sci. 2006;51:1818–1823.
Sarna SK. Are interstitial cells of cajal plurifunction cells in the gut? Am J Physiol Gastrointest Liver Physiol. 2008;294:G372–G390.
Malysz J, Thuneberg L, Mikkelsen HB, Huizinga JD. Action potential generation in the small intestine of W mutant mice that lack interstitial cells of cajal. Am J Physiol. 1996;271:G387–G399.
de Lavernhe-Lemaire MC, Decaud-Laroche J, Boiron M, Thouvenot J. Gastroduodenal electric activity, in situ, during anesthesia and recovery. Study in chronic electrode-carrying rats. Arch Int Physiol Biochim. 1986;94:19–28.
Waldhausen JH, Shaffrey ME, Skenderis BSII, Jones RS, Schirmer BD. Gastrointestinal myoelectric and clinical patterns of recovery after laparotomy. Ann Surg. 1990;211:777–784. discussion 785.
Seidel SA, Hegde SS, Bradshaw LA, Ladipo JK, Richards WO. Intestinal tachyarrhythmias during small bowel ischemia. Am J Physiol. 1999;277:G993–G999.
Sullivan MA, Snape WJ Jr, Matarazzo SA, Petrokubi RJ, Jeffries G, Cohen S. Gastrointestinal myoelectrical activity in idiopathic intestinal pseudo-obstruction. N Engl J Med. 1977;297:233–238.
Chen JD, Schirmer BD, McCallum RW. Measurement of electrical activity of the human small intestine using surface electrodes. IEEE Trans Biomed Eng. 1993;40:598–602.
Bjorck S, Kelly KA, Phillips SF. Mechanisms of enhanced canine enteric absorption with intestinal pacing. Am J Physiol. 1987;252:G548–G553.
Qi H, Liu S, Chen JD. Dual-pulse intestinal electrical stimulation normalizes intestinal dysrhythmia and improves symptoms induced by vasopressin in fed state in dogs. Neurogastroenterol Motil. 2007;19:411–418.
Yin J, Chen J. Excitatory effects of synchronized intestinal electrical stimulation on small intestinal motility in dogs. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1190–G1195.
Liu S, Hou X, Chen JD. Therapeutic potential of duodenal electrical stimulation for obesity: acute effects on gastric emptying and water intake. Am J Gastroenterol. 2005;100:792–796.
Lin X, Peters LJ, Hayes J, Chen JD. Entrainment of segmental small intestinal slow waves with electrical stimulation in dogs. Dig Dis Sci. 2000;45:652–656.
Collin J, Kelly KA, Phillips SF. Absorption from the jejunum is increased by forward and backward pacing. Br J Surg. 1979;66:489–492.
Lin X, Hayes J, Peters LJ, Chen JD. Entrainment of intestinal slow waves with electrical stimulation using intraluminal electrodes. Ann Biomed Eng. 2000;28:582–587.
Chen J, McCallum RW. Electrogastrography: Principles and Applications. New York: Raven; 1995.
You CH, Chey WY, Lee KY, Menguy R, Bortoff A. Gastric and small intestinal myoelectric dysrhythmia associated with chronic intractable nausea and vomiting. Ann Intern Med. 1981;95:449–451.
Abell TL, Kim CH, Malagelada JR. Idiopathic cyclic nausea and vomiting—a disorder of gastrointestinal motility? Mayo Clin Proc. 1988;63:1169–1175.
Schuffler MD. Chronic intestinal pseudo-obstruction syndromes. Med Clin North Am. 1981;65:1331–1358.
Golzarian J, Staton DJ, Wikswo JP Jr, Friedman RN, Richards WO. Diagnosing intestinal ischemia using a noncontact superconducting quantum interference device. Am J Surg. 1994;167:586–592.
Ladipo JK, Bradshaw LA, Halter S, Richards WO. Changes in intestinal electrical activity during ischaemia correlate to pathology. West Afr J Med. 2003;22:1–4.
Morrison P, Miedema BW, Kohler L, Kelly KA. Electrical dysrhythmias in the roux jejunal limb: cause and treatment. Am J Surg. 1990;160:252–256.
Abo M, Kono T, Wang Z, Chen JD. Impairment of gastric and jejunal myoelectrical activity during rectal distension in dogs. Dig Dis Sci. 2000;45:1731–1736.
Zhu H, Sallam H, Chen DD, Chen JD. Therapeutic potential of synchronized gastric electrical stimulation for gastroparesis: enhanced gastric motility in dogs. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1875–R1881.
Mintchev M, Bowes K. Computer model of gastric electrical stimulation. Ann Biomed Eng. 1997;25:726–730.
Mintchev MP, Sanmiguel CP, Amaris M, Bowes KL. Microprocessor-controlled movement of solid gastric content using sequential neural electrical stimulation. Gastroenterology. 2000;118:258–263.
Reiser SB, Schusdziarra V, Bollschweiler E, Holscher AH, Siewert JR. Effect of enteric pacing on intestinal motility and hormone secretion in dogs with short bowel. Gastroenterology. 1991;101:100–106.
Liu S, Liu J, Chen JD. Neural mechanisms involved in the inhibition of intestinal motility induced by intestinal electrical stimulation in conscious dogs. Neurogastroenterol Motil. 2006;18:62–68.
Stanghellini V, Cogliandro RF, de Giorgio R, Barbara G, Salvioli B, Corinaldesi R. Chronic intestinal pseudo-obstruction: manifestations, natural history and management. Neurogastroenterol Motil. 2007;19:440–452.
Chen JD, Lin HC. Electrical pacing accelerates intestinal transit slowed by fat-induced ileal brake. Dig Dis Sci. 2003;48:251–256.
Liu J, Qiao X, Hou X, Chen JD. Effect of intestinal pacing on small bowel transit and nutrient absorption in healthy volunteers. Obes Surg. 2009;19:196–201.
Collin J, Kelly KA, Phillips SF. Increased canine jejunal absorption of water, glucose, and sodium with intestinal pacing. Am J Dig Dis. 1978;23:1121–1124.
O’Connell PR, Kelly KA. Enteric transit and absorption after canine ileostomy. Effect of pacing. Arch Surg. 1987;122:1011–1017.
Hoepfner MT, Kelly KA, Sarr MG. Pacing the canine ileostomy. Surgery. 1988;104:476–481.
Sawchuk A, Nogami W, Goto S, et al. Reverse electrical pacing improves intestinal absorption and transit time. Surgery. 1986;100:454–460.
Karlstrom L, Kelly KA. Ectopic jejunal pacemakers and gastric emptying after roux gastrectomy: effect of intestinal pacing. Surgery. 1989;106:867–871.
Sarr MG, Kelly KA, Gladen HE. Electrical control of canine jejunal propulsion. Am J Physiol. 1981;240:G355–G360.
Layzell T, Collin J. Retrograde electrical pacing of the small intestine—a new treatment for the short bowel syndrome? Br J Surg. 1981;68:711–713.
Sun Y, Chen J. Intestinal electric stimulation decreases fat absorption in rats: therapeutic potential for obesity. Obes Res. 2004;12:1235–1242.
Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G443–G449.
Kerlin P, Zinsmeister A, Phillips S. Motor responses to food of the ileum, proximal colon, and distal colon of healthy humans. Gastroenterology. 1983;84:762–770.
Kelly KA, Code CF. Duodenal-gastric reflux and slowed gastric emptying by electrical pacing of the canine duodenal pacesetter potential. Gastroenterology. 1977;72:429–433.
Cranley B, Kelly KA, Go VL, McNichols LA. Enhancing the anti-dumping effect of roux gastrojejunostomy with intestinal pacing. Ann Surg. 1983;198:516–524.
Liu S, Wang L, Chen JD. Cross-talk along gastrointestinal tract during electrical stimulation: effects and mechanisms of gastric/colonic stimulation on rectal tone in dogs. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1195–G1198.
Xu X, Lei Y, Liu S, Chen JD. Inhibitory effects of gastrointestinal electrical stimulation on rectal tone are both organ-specific and distance-related in dogs. Dis Colon Rectum. 2008;51:467–473.
Yin J, Ouyang H, Chen JD. Potential of intestinal electrical stimulation for obesity: a preliminary canine study. Obesity (Silver Spring). 2007;15:1133–1138.
Yin J, Zhang J, Chen JD. Inhibitory effects of intestinal electrical stimulation on food intake, weight loss and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R78–R82.
Ouyang H, Yin J, Chen JD. Gastric or intestinal electrical stimulation-induced increase in gastric volume is correlated with reduced food intake. Scand J Gastroenterol. 2006;41:1261–1266.
Xu X, Zhu H, Chen JD. Pyloric electrical stimulation reduces food intake by inhibiting gastric motility in dogs. Gastroenterology. 2005;128:43–50.
Bray GA, Greenway FL. Current and potential drugs for treatment of obesity. Endocr Rev. 1999;20:805–875.
AACE/ACE. Position statement on the prevention, diagnosis, and treatment of obesity. Endocr Pract. 1998;4:297–330.
Sagar PM. Surgical treatment of morbid obesity. Br J Surg. 1995;82:732–739.
Cigaina VV, Saggioro A, Rigo VV, Pinato G, Ischai S. Long-term effects of gastric pacing to reduce feed intake in swine. Obes Surg. 1996;6:250–253.
Cigaina V. Gastric pacing as therapy for morbid obesity: preliminary results. Obes Surg. 2002;12(Suppl 1):12S–16S.
De Luca M, Segato G, Busetto L, et al. Progress in implantable gastric stimulation: summary of results of the European multi-center study. Obes Surg. 2004;14(Suppl 1):S33–S39.
Ouyang H, Yin J, Chen JD. Therapeutic potential of gastric electrical stimulation for obesity and its possible mechanisms: a preliminary canine study. Dig Dis Sci. 2003;48:698–705.
Xu J, McNearney TA, Chen JD. Gastric/intestinal electrical stimulation modulates appetite regulatory peptide hormones in the stomach and duodenum in rats. Obes Surg. 2007;17:406–413.
Sun Y, Qin C, Foreman RD, Chen JD. Intestinal electric stimulation modulates neuronal activity in the nucleus of the solitary tract in rats. Neurosci Lett. 2005;385:64–69.
Xu X, Pasricha PJ, Chen JD. Feasibility of gastric electrical stimulation by use of endoscopically placed electrodes. Gastrointest Endosc. 2007;66:981–986.
Elfvin A, Andersson S, Abrahamsson H, Edebo A, Simren M, Lonroth H. Percutaneous implantation of gastric electrodes—a novel technique applied in animals and in patients. Neurogastroenterol Motil. 2007;19:103–109.
Acknowledgments
This work was partially supported by grants from the National Institutes of Health (DK063733, DK055437 and DK075155).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, J., Chen, J.D.Z. Mechanisms and Potential Applications of Intestinal Electrical Stimulation. Dig Dis Sci 55, 1208–1220 (2010). https://doi.org/10.1007/s10620-009-0884-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-009-0884-3