Abstract
Background
The aim of this study was to explore the feasibility and early outcomes of laparoscopy-assisted total gastrectomy with a modified splenic hilar lymphadenectomy for upper- and middle-third stage cT1-2 gastric cancer.
Methods
A total of 97 patients diagnosed with upper- and middle-third stage cT1-T2 gastric cancer were enrolled. Patients were assigned to the laparoscopy-assisted total gastrectomy group (LATG, n = 41) or the open total gastrectomy group (OTG, n = 56). All patients underwent total gastrectomy with modified splenic hilar lymphadenectomy. The operative and postoperative measures, number of retrieved lymph nodes (LNs), and complications were compared between the two groups.
Results
The mean number of dissected LNs was not significantly different between the two groups: 23.1 ± 8.0 in the LATG group versus 24.2 ± 7.5 in the OTG group. Compared with the OTG group, the LATG group had less operative blood loss [104.2 ± 42.9 vs. 355.6 ± 51.3 ml (p < 0.0001)], shorter time to out-of-bed activities [14.4 ± 3.2 vs. 16.5 ± 1.2 h (p < 0.0001)], shorter time to first flatus [72.2 ± 16.2 vs. 78.4 ± 8.6 h (p = 0.017)], earlier resumption of soft diet [52.8 ± 21.6 vs. 74.2 ± 12.2 h (p < 0.0001)], and shorter postoperative hospital stay [9.7 ± 2.2 vs. 13.6 ± 3.6 days (p < 0.0001)]. However, LATG had a slightly longer operating time than OTG [235.7 ± 38.5 vs. 211.5 ± 33.2 min (p = 0.001)]. The operative complications rates for the LATG and OTG groups were not significantly different: 4.9 versus 5.4 %.
Conclusion
For upper- and middle-third stage cT1-2 gastric cancer, a limited splenic hilar lymphadenectomy strategy seems to be safe and feasible, particularly for the number of retrieved LNs. However, this technique is not suitable for cT3 disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopy-assisted distal gastrectomy (LADG) has become widely used for lower-third stage cT1 gastric cancer because of its minimally invasive approach compared with open distal gastrectomy (ODG). LADG potentially results in a reduction in perioperative morbidity and mortality, has a shorter recovery time, and similar long-term results compared to ODG [1–5]. Moreover, numerous investigators had reported similar long-term survival when comparing LADG with ODG combined with D2 lymphadenectomy treatment for advanced lower-third gastric cancer [6–9]. Several previous studies have addressed the use of laparoscopy-assisted total gastrectomy (LATG) for the treatment of upper- or middle-third gastric cancer, including partial advanced stage [10, 11]. However, to our knowledge, there are limited data on dissection of lymph nodes (LNs) from the splenic hilum in combination with LATG for upper- or middle-third gastric cancer while preserving the spleen and pancreas [11–14].
Currently, an extended D2 lymphadenectomy is the standard approach for advanced gastric cancer in Eastern countries. For gastric cancer located in the upper or middle third of the stomach, according to the guidelines of the Japanese Research Society of Gastric Cancer (JRSGC), LNs of the splenic hilum (No. 10) are grouped into the second level (N2) [15]. Barriers to widespread acceptance of LATG for advanced gastric cancer have been its technically challenging nature and concerns about the ability to achieve a clear resection of the splenic hilum No. 10 LNs when it is performed for advanced gastric cancer located in the upper or middle third.
Recent Guidelines from the National Comprehensive Cancer Network (NCCN) suggest that a minimum of 15 nodes should be examined to ensure accurate staging. In the prelaparoscopy era, to achieve a complete tumor resection (R0), splenectomy with or without distal pancreatectomy was performed in 70 % of patients to dissect the No. 10 LNs [16]. However, due to the complicated anatomic relationship between the LNs and vessels of the splenic hilum, it seems very difficult for the surgeon to perform a splenic hilar lymphadenectomy without a splenectomy using a laparoscopic approach. Moreover, multiple previous investigators have documented the superiority of preserving the spleen and pancreas during extended lymphadenectomy for gastric cancer, as it decreases complications and there is no clear evidence that it is detrimental to overall survival.
Results from retrospective studies have demonstrated that no LN metastasis in the No. 10 nodal station occurs in stage pT1 cancer and there is a reported 0 and 8.2 % incidence of metastasis with stage pT2 upper or middle gastric cancer [17, 18]. Lymphatic drainage of upper and middle gastric cancers by sentinel LNs has been determined. In pT1-2 gastric cancers, most metastatic LNs are restricted to the N1 level, while No. 1 and No. 3 are the common metastatic sites. The frequency of N2 level involvement was much higher in stage T2b rather than in T2a and T1 tumors; in addition, skip metastasis often occurred to stations Nos. 7, 8a, 9, 10, and 11p in T2b tumors, but it seldom occurred with T2a and T1 tumors [19]. Therefore, it seems rational to perform a limited number of station No. 10 LN dissections for patients with upper- or middle-third stage cT1-2 gastric cancer.
Based on these findings, we conducted a case–control study of LATG with limited splenic hilar lymphadenectomy, with dissection of the LNs alongside the plane of the vessels of the splenic hilum. We compared LATG to the open total gastrectomy (OTG) technique for patients with stage cT1 and stage T2 gastric cancer located in the upper and middle third of the stomach. The aim was to explore the feasibility and early outcomes of LATG with modified dissection of the LNs of station No. 10, being especially concerned about the number of dissected LNs. Preliminary results are presented here.
Patients and methods
Patients
From March 2007 to August 2010, a total of 97 patients with upper- or middle-third gastric cancer in stage cT1-2, proven by pathology, were enrolled in this study. According to patients’ wishes, 41 patients were treated with laparoscopy-assisted total gastrectomy (LATG group) and 56 were treated with open total gastrectomy (OTG group). The pretreatment invasive depth and the extension of LN metastasis were evaluated by endoscopy, ultrasound endoscopy, abdominal CT scan, and ultrasound examination. The patients with a tumor size ≥5 cm and with severe cardiopulmonary comorbidities were excluded. The LNs were grouped according to the guidelines of Japanese Classification of Gastric Carcinoma [15]. Clinical and pathological staging was in accordance with the American Joint Committee on Cancer (AJCC) seventh edition of Gastric Cancer TNM Staging [20]. This study was approved by the institutional review board of the Union Hospital of Fujian Medical University, and written informed consent was obtained from each patient enrolled in our study.
Surgical technique
LATG procedure
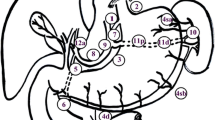
The patients who underwent LATG were given general anesthesia and placed in the supine position with the legs abducted on the boards. A 10-mm trocar port for the camera was made via the umbilicus using an open method. On the left anterior axillary line, a 10-mm trocar port for the main operating device was placed 1 cm below the costal margin. On the left midclavicular line, a 5-mm trocar port 1 cm above the umbilicus was opened for assisting in the operation. At the opposite side, two 5-mm trocar ports were placed for the assistant (as shown in Fig. 1). The surgeon stood on the patient’s left or between the patient’s legs, depending on the surgical procedure.
It was routine to first determine if there was metastasis to the liver, abdominal cavity, or pelvic cavity. All 97 patients underwent a modified D2 lymphadenectomy in accordance with the Japanese Gastric Cancer Treatment Guidelines (13th ed.). The greater omentum was turned over cephalad to the surface of stomach, and its dissection began at the left side of the middle of the transverse colon using an Ultracision Harmonic Scalpel. After division of the greater omentum, the lesser sac was seen; division was then continued in order to dissect the anterior layer of the transverse mesocolon between the flexura lienalis coli and flexura hepatica coli. The right gastroepiploic vein was cut at its root and the No. 6 LNs were dissected. The dissection was continued to the duodenum under the anterior pancreaticoduodenal fascia. The anterior pancreaticoduodenal fascia was then dissected from the inferior margin of the pancreas to the superior margin of the pancreas. The right gastroepiploic artery was skeletonized and cut at its root. The paries posterior of the duodenal bulb was mobilized to expose the gastroduodenal artery. The right gastric artery and the proper hepatic artery were exposed by dissecting along the gastroduodenal artery and the common hepatic artery. The right gastric artery was ligated with a ligating clip and cut at its roots, and the No. 5 LNs were dissected.
The greater omentum was removed under the liver, the stomach was turned cephalad, and the gastropancreatic fold was maintained vertically by the assistant. After dissection of the anterior pancreaticoduodenal fascia, the common hepatic artery, the proximal splenic artery, and the celiac trunk artery were all exposed and it was easy to dissect the No. 8a LNs from the anterior and superior margins of the common hepatic artery. Dissection was continued along the celiac artery trunk, and the left gastric artery and vein were skeletonized and cut at the root where the No. 9 and No. 7 LNs were dissected. The right diaphragmatic crura were exposed to the cardiac orifice with the use of the Ultracision Harmonic Scalpel. Then the sheath of the splenic artery was opened and the No. 11p and No. 11d LNs were dissected. After that, the posterior gastric artery and vein were identified and cut. To better expose the pancreatic tail, we elevated the patient we elevated and cut the left gastroepiploic vessels. Dissection was continued cephalad to cut the short gastric vessels and remove the No. 4sb and No. 4sa LNs. Alongside the surface of the splenic vessels near the hilus lienis, parts of the No. 10 LNs were dissected (as shown in Fig. 2). To dissect the No. 2 LNs, the left diaphragmatic feet were mobilized to the left side of the cardia orifice. We moved the greater omentum back to its normal position, and the liver was elevated by the assistant using a bowel grasper to expose the hepatogastric ligament. The LNs of the hepatoduodenal ligament (No. 12a LNs) were removed after the hepatogastric ligament was dissected with the Ultracision Harmonic Scalpel. The lower segment of the esophagus was mobilized to dissect the No. 1 LNs.
A 6–8-cm incision was made and protected by using an incision protector. The mobilized stomach was pulled out of the incision, and the duodenum was closed with a stapler. The lower segment of the esophagus was clamped by a purse string instrument and cut about 2–3 cm from the verge of the tumor. The proximal cut end of the esophagus was routinely verified by intraoperative frozen-section examination. The Roux-en-Y procedure was performed to reconstruct the digestive tract. Peritoneal lavage was routinely performed by using 3,000 ml distilled water at 43 °C to eliminate cancer cells that became exfoliated during the operation.
OTG procedure
Briefly, in the OTG group operation, after dissection of the anterior pancreaticoduodenal fascia, node dissection was performed in the supraduodenal areas and along the hepatoduodenal ligament (No. 12a LNs) and the common hepatic (No. 8a LNs) and celiac (No. 9 LNs) arteries. The left gastric artery (No. 7 LNs) was ligated at its origin and node dissection (No. 11 LNs) was extended along the splenic artery to the splenic hilum, while fatty connective tissue and nodes (No. 10 LNs) alongside the surface of the splenic vessels were removed en bloc with the stomach, gastric omentum, and perigastric nodes (Nos. 1–6 LNs).
Evaluation parameters
The operative parameters assessed included operating time, operative blood loss, and risk of a blood transfusion. Tumor clearance was evaluated by the length of the esophageal resection, the number of LNs in each group, and the number of LNs dissected from each patient. Postoperative parameters evaluated were the time of gastrointestinal recovery (time to first passage of gas by the anus), the time to first soft diet intake, and the length of hospital stay. Morbidity was assessed by the overall number of complications and was stratified into stomach resection–related (e.g., anastomotic and intraperitoneal hemorrhage, anastomotic leakage, postoperative celiac infection, and pancreatic leakage) and general complications (e.g., pneumonia, urinary tract infection, incisional and peritoneal infection, and bowel obstruction). All of the clinical data from the LATG group were compared to the data from the OTG group.
Statistical analyses
All statistical analyses were carried out using Statistical Package for the Social Sciences (SPSS) ver. 12.0 for Windows (SPSS, Inc., Chicago, IL). The χ2 or Fisher’s exact test was used for comparing incidence rates and category variables where appropriate. Student’s t-test was used for comparing the mean data of continuous variables. A p value <0.05 was considered statistically significant.
Results
Characteristics of the patients
The patients’ characteristics and tumor stage distribution are given in Table 1. The mean age was 60.7 ± 9.1 years (range = 25–80 years), and the study group included 54 males (60.0 %) and 36 females (40.0 %). We identified stage cT1 cancer in 25 patients and stage cT2 in 72. Of the 97 patients, 41 underwent LATG and 56 underwent OTG.
Oncologic outcomes
All patients had a histologically complete resection (R0 resection). There was no significant difference in the length of esophageal resection between the two groups, 3.4 ± 1.0 cm in the LATG group vs. 3.3 ± 0.4 cm in the OTG group. The mean number of LNs recovered from all patients was 23.7 ± 7.7 (median = 20, range = 12–48). The average number of LNs retrieved was 23.1 ± 8.0 for the LATG group and 24.2 ± 7.5 for the OTG group, with no significant difference between the two groups. Subset analysis based on the depth of tumor invasion showed that the mean numbers of LNs dissected in the LATG group and in the OTG group were similar for both stage pT1 and pT2 patients. However, with stage pT3 disease, the OTG group had slightly more LNs dissected than the LATG group (29.4 ± 5.3 vs. 22.0 ± 8.5), but the difference was not significant (Table 2).
No significant difference was noted between the two study groups with respect to the numbers of each LN group removed (Table 3). No LN metastasis was found in No. 10 LNs in either stage pT1 or pT2 patients. However, in stage pT3 patients, we found that each group had one case of metastasis to the No. 10 LN group for a metastatic rate of 25 % (2/8) in stage pT3 patients (Table 4).
Operative parameters
Among the 46 patients treated with LATG, one patient was converted to open surgery because of extensive abdominal adhesions, for a conversion rate of 2.4 %. The operating time was significantly longer for the LATG group than for the OTG group (235.7 ± 38.5 vs. 211.5 ± 33.2 min, p = 0.001). However, the LATG group had less operative blood loss (104.2 ± 42.9 vs. 355.6 ± 51.3 ml, p < 0.0001) (Table 5).
Postoperative parameters
The overall postoperative parameters and complications are given in Table 5. Compared with the OTG group, the LATG group showed superior postoperative recovery, with a shorter time to out-of-bed activities (14.4 ± 3.2 vs. 16.5 ± 1.2 h, p < 0.0001), shorter time to first flatus (72.2 ± 16.2 vs. 78.4 ± 8.6 h, p = 0.017), shorter time to first soft food intake (52.8 ± 21.6 vs. 74.2 ± 12.2 h, p < 0.0001), and shorter postoperative hospital stay (9.7 ± 2.2 vs. 13.6 ± 3.6 days, p < 0.0001).
In all, no serious postoperative complications or deaths occurred in either of the two groups. In the LATG group, there were two surgical complications: pancreatic leakage and peritoneal infection. There were three patients, 5.4 % of the OTG group, who suffered complications: pancreatic leakage, postoperative wound infection, and pneumonia. There was no significant difference in complications or morbidity between the two groups.
Discussion
Lymph node metastasis (LNM) is one of the most important poor prognostic factors and causes of death in patients with gastric cancer. The incidence ranges from 1.9 to 19.4 % in early disease [21, 22], but increases to 70 % in advanced disease [23]. Aggressive resection is usually performed by surgeons in an effort to remove the metastatic LNs. For advanced tumors in the upper or middle third of the stomach, splenectomy with or without distal pancreatectomy is usually performed to dissect the LNs around the splenic artery (LN group No. 11) and the splenic hilum (LN group No. 10) to achieve complete tumor resection. However, several random trials and meta-analyses have documented that prophylactic splenectomy to remove negative LNs near the spleen did not increase the long-term survival rate when compared with spleen-preserving surgery; in contrast, it resulted in higher postoperative morbidity and mortality [24–30]. Therefore, a strategy of pancreas- and spleen-preserving lymphadenectomy has been widely used in OTG for advanced disease by moving the spleen and body/tail of the pancreas out of the abdominal cavity [16]. Is this feasible and safe in LATG? In recent years, some investigators have performed a D2 extended lymphadenectomy with pancreas and spleen preservation during LATG. However, their studies had small sample sizes: only 18 in Hur et al. [31], 15 in Hyung et al. [32], 30 in Sakuramoto et al. [33], and 53 in Okabe et al. [34]. Some of these studies did not assess metastasis to the splenic hilar nodes [31, 33]. In this study we developed a modified splenic hilar lymphadenectomy for LATG for stage cT1-2 gastric cancer to determine whether a limited lymphadenectomy is really safe and feasible and whether LATG improves the quality of life when compared with OTG.
Our results have shown that a similar average number of LNs can be dissected from the LATG group (23.1 ± 8.0) and the OTG group (24.2 ± 7.5); the total number of dissected LNs in both groups was ≥ 15, a number enough to perform an accurate staging. A retrospective study conducted by Sasada et al. [35] examined records from 201 patients who had undergone OTG with splenectomy and assessed LN metastasis to the splenic hilus. They reported the incidence of LN metastasis to the splenic hilus in 31 cases (15.4 %), and no LN metastasis to the splenic hilus was detected in any stage T1 or T2 tumors at the lesser curvature or the anterior wall. Shin et al. [17] reported on 319 patients with proximal gastric cancer who had undergone curative total gastrectomy with simultaneous splenectomy and D2 LN dissection. Splenic hilar node metastasis was noted in 41 patients (12.9 %), with no splenic hilar node metastasis in those with stage T1a, T1b, or T2a tumors and in only 9.7 % of patients with T2b tumors. Recently, Li et al. [36] reported results from a retrospective study of 131 patients with advanced middle-third gastric carcinoma who had undergone OTG with D2 lymphadenectomy and LN dissection, and 62 had undergone simultaneous splenectomy. Splenic hilar node metastasis was found in 3 (1.57 %) T2 patients and in 33 (25.2 %) T3 patients. In the present study, no group No. 10 LN metastasis occurred with stage pT1 and pT2 tumors, consistent with previous reports. Therefore, for the upper- or middle-third gastric cancers of stage cT1 or cT2, the limited No. 10 station LN dissection developed by us seems to remove enough LNs and does not leave residual metastatic LNs behind.
In our series, eight patients were staged with pT3 tumors, and two of these patients had metastasis to the splenic hilus, for an incidence of 25 %. The rate of metastasis to the splenic hilus in pT3 cancer is reported to range from 5.8 to 21.6 % [17, 18, 37]. Several retrospective studies have demonstrated that LNM to the splenic hilus presents a poor prognosis, with a cumulative 5-year survival rate of only 11.0–16.9 %, whereas for patients without metastasis, the 5-year survival is 38.7–51.1% [16, 17, 35]. Therefore, for the patients with stage cT3 gastric cancer located in the upper or middle third of the stomach, a lymphadenectomy with OTG instead of LATG is recommended to completely dissect group No. 10 LNs, for the purpose of achieving an R0 resection.
Another concern of laparoscopic resection for gastric cancer is obtaining clear margins at the proximal esophageal resection. In this study, there was no significant difference in the length of the esophageal resection between the two groups: 3.4 ± 1.0 versus 3.3 ± 0.4 cm. All patients had a complete resection without microscopic residue based on histopathological analysis. Compared with OTG, the LATG procedure can also dissect an adequate proximal esophagus.
Improvement of early surgical outcomes with LATG compared with the standard open gastric resection for upper- and middle-third gastric cancer patients has been documented in several studies [11, 38]. In our study, the mean operating time for the LATG group was slightly but significantly longer compared to that for OTG (235 vs. 211 min). This result is similar to the average operating time of 211–370 min for LATG reported by others [31–34]. However, the LATG group had significantly less blood loss, shorter time to out-of-bed activities, shorter time to first flatus, earlier time to resumption of soft diet, and a shorter postoperative hospital stay. In the LATG group, there were only two patients with complications (one pancreatic fistula and one postoperative celiac infection), and there was no mortality. Compared with the OTG group, the total morbidity and mortality rates in the LATG group were 4.9 and 0 % vsersus 5.4 and 0 %, respectively. These results indicate that LATG is a safe procedure with a high success rate.
Our findings were obtained from a nonrandomized study in a single center, and there are several limitations that must be considered when interpreting these results. First, there may be selection bias. In a group of patients with more comorbidities, more patients would likely have been referred for conventional OTG rather than the novel and more difficult LATG. Second, the follow-up time of the present study was short so we were unable to obtain long-term survival data. Thus, longer follow-up and prospective randomized controlled studies are needed in the future to confirm these results.
Conclusions
Our present study demonstrated that a limited lymphadenectomy with partial dissection of the splenic hilar LN is rational and possible with LATG for stage cT1-2 gastric cancer located in the upper or middle third of the stomach. A D2 LN dissection can be achieved in all cases. However, it is not suitable to use this technique in patients with stage cT3 as it would miss postoperative tumor residue to the spleen hilum LNs which would result in recurrence and a poor prognosis compared with patients without this metastasis.
References
Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N (2007) A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 245:68–72
Memon MA, Khan S, Yunus RM, Barr R, Memon B (2008) Meta-analysis of laparoscopic and open distal gastrectomy for gastric carcinoma. Surg Endosc 22:1781–1789
Fujiwara M, Kodera Y, Misawa K, Kinoshita M, Kinoshita T, Miura S, Ohashi N, Nakayama G, Koike M, Nakao A (2008) Long-term outcomes of early-stage gastric carcinoma patients treated with laparoscopy-assisted surgery. J Am Coll Surg 206:138–143
Song J, Lee HJ, Cho GS, Han SU, Kim MC, Ryu SW, Kim W, Song KY, Kim HH, Hyung WJ (2010) Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group Recurrence following laparoscopy-assisted gastrectomy for gastric cancer: a multicenter retrospective analysis of 1,417 patients. Ann Surg Oncol 17:1777–1786
Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C (2005) Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 241:232–237
Hwang SI, Kim HO, Yoo CH, Shin JH, Son BH (2009) Laparoscopic-assisted distal gastrectomy versus open distal gastrectomy for advanced gastric cancer. Surg Endosc 23:1252–1258
Lee J, Kim W (2009) Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: analysis of consecutive 106 experiences. J Surg Oncol 100:693–698
Huang JL, Wei HB, Zheng ZH, Wei B, Chen TF, Huang Y, Guo WP, Hu B (2010) Laparoscopy-assisted D2 radical distal gastrectomy for advanced gastric cancer. Dig Surg 27:291–296
Lee SW, Nomura E, Bouras G, Tokuhara T, Tsunemi S, Tanigawa N (2010) Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg 211:33–40
Tanimura S, Higashino M, Fukunaga Y, Kishida S, Ogata A, Fujiwara Y, Osugi H (2007) Laparoscopic gastrectomy with regional lymph node dissection for upper gastric cancer. Br J Surg 94:204–207
Mochiki E, Toyomasu Y, Ogata K, Andoh H, Ohno T, Aihara R, Asao T, Kuwano H (2008) Laparoscopically assisted total gastrectomy with lymph node dissection for upper and middle gastric cancer. Surg Endosc 22:1997–2002
Lee SE, Ryu KW, Nam BH, Lee JH, Kim YW, Yu JS, Cho SJ, Lee JY, Kim CG, Choi IJ, Kook MC, Park SR, Kim MJ, Lee JS (2009) Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. J Surg Oncol 100:392–395
Kawamura H, Yokota R, Homma S, Kondo Y (2009) Comparison of invasiveness between laparoscopy-assisted total gastrectomy and open total gastrectomy. World J Surg 33:2389–2395
Jeong GA, Cho GS, Kim HH, Lee HJ, Ryu SW, Song KY (2009) Laparoscopy-assisted total gastrectomy for gastric cancer: a multicenter retrospective analysis. Surgery 146:469–474
Japanese Gastric Cancer Association (1998) Japanese classification of gastric carcinoma - 2nd english edition. Gastric Cancer 1:10–24
Zhang CH, Zhan WH, He YL, Chen CQ, Huang MJ, Cai SR (2007) Spleen preservation in radical surgery for gastric cardia cancer. Ann Surg Oncol 14:1312–1319
Shin SH, Jung H, Choi SH, An JY, Choi MG, Noh JH, Sohn TS, Bae JM, Kim S (2009) Clinical significance of splenic hilar lymph node metastasis in proximal gastric cancer. Ann Surg Oncol 16:1304–1309
Kosuga T, Ichikawa D, Okamoto K, Komatsu S, Shiozaki A, Fujiwara H, Otsuji E (2011) Survival benefits from splenic hilar lymph node dissection by splenectomy in gastric cancer patients: relative comparison of the benefits in subgroups of patients. Gastric Cancer 14:172–177
Huang B, Wang Z, Sun Z, Zhao B, Xu H (2011) A novel insight of sentinel lymph node concept based on 1–3 positive nodes in patients with pT1-2 gastric cancer. BMC Cancer 11:18
Washington K (2010) 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 17:3077–3079
Haruta H, Hosoya Y, Sakuma K, Shibusawa H, Satoh K, Yamamoto H, Tanaka A, Niki T, Sugano K, Yasuda Y (2008) Clinicopathological study of lymph-node metastasis in 1,389 patients with early gastric cancer: assessment of indications for endoscopic resection. J Dig Dis 9:213–218
An JY, Baik YH, Choi MG, Noh JH, Sohn TS, Kim S (2007) Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg 246:749–753
Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, Okajima K, Japan Clinical Oncology Group (2008) D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 359:453–462
Csendes A, Burdiles P, Rojas J, Braghetto I, Diaz JC, Maluenda F (2002) A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery 131:401–407
Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I (2004) Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 22:2069–2077
McCulloch P, Nita ME, Kazi H, Gama-Rodrigues J (2004) Extended versus limited lymph nodes dissection technique for adenocarcinoma of the stomach. Cochrane Database Syst Rev (4):CD001964
Yu W, Choi GS, Chung HY (2006) Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg 93:559–563
Yang K, Chen XZ, Hu JK, Zhang B, Chen ZX, Chen JP (2009) Effectiveness and safety of splenectomy for gastric carcinoma: a meta-analysis. World J Gastroenterol 15:5352–5359
Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ (2010) Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 11:439–449
Ding J, Liao GQ, Zhang ZM, Pan Y, Ni Q, Hao LS (2011) Necessity of splenectomy in radical resection of gastric cancer: a meta-analysis. Zhonghua Wei Chang Wai Ke Za Zhi 14:120–124
Hur H, Jeon HM, Kim W (2008) Laparoscopic pancreas- and spleen-preserving D2 lymph node dissection in advanced (cT2) upper-third gastric cancer. J Surg Oncol 97:169–172
Hyung WJ, Lim JS, Song J, Choi SH, Noh SH (2008) Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg 207:e6–e11
Sakuramoto S, Kikuchi S, Futawatari N, Katada N, Moriya H, Hirai K, Yamashita K, Watanabe M (2009) Laparoscopy-assisted pancreas- and spleen-preserving total gastrectomy for gastric cancer as compared with open total gastrectomy. Surg Endosc 23:2416–2423
Okabe H, Obama K, Kan T, Tanaka E, Itami A, Sakai Y (2010) Medial approach for laparoscopic total gastrectomy with splenic lymph node dissection. J Am Coll Surg 211:e1–e6
Sasada S, Ninomiya M, Nishizaki M, Harano M, Ojima Y, Matsukawa H, Aoki H, Shiozaki S, Ohno S, Takakura N (2009) Frequency of lymph node metastasis to the splenic hilus and effect of splenectomy in proximal gastric cancer. Anticancer Res 29:3347–3351
Li C, Kim S, Lai JF, Oh SJ, Hyung WJ, Choi WH, Choi SH, Zhu ZG, Noh SH (2009) Lymph node dissection around the splenic artery and hilum in advanced middle third gastric carcinoma. Eur J Surg Oncol 35:709–714
Aoyagi K, Kouhuji K, Miyagi M, Imaizumi T, Kizaki J, Shirouzu K (2010) Prognosis of metastatic splenic hilum lymph node in patients with gastric cancer after total gastrectomy and splenectomy. World J Hepatol 2:81–86
Shinohara T, Kanaya S, Taniguchi K, Fujita T, Yanaga K, Uyama I (2009) Laparoscopic total gastrectomy with D2 lymph node dissection for gastric cancer. Arch Surg 144:1138–1142
Acknowledgments
We are grateful to our patients and staff who were involved in the patient care to make this study possible.
Disclosures
G. Guan, W. Jiang, Z. Chen, X. Liu, H. Lu, and X. Zhang have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guan, G., Jiang, W., Chen, Z. et al. Early results of a modified splenic hilar lymphadenectomy in laparoscopy-assisted total gastrectomy for gastric cancer with stage cT1-2: a case–control study. Surg Endosc 27, 1923–1931 (2013). https://doi.org/10.1007/s00464-012-2688-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-012-2688-3