Abstract
Background
Laparoscopy-assisted distal gastrectomy (LADG) is gaining wider acceptance for treating early gastric cancer (EGC). However, many gastric surgeons are still reluctant to perform LADG, mainly because this procedure entails a considerable learning curve. We aimed to evaluate the technical feasibility and short-term outcomes of performing LADG by a single experienced gastric surgeon who initially had no experience with laparoscopic surgery as compared with open distal gastrectomy (ODG).
Methods
Between January 2006 and December 2007, 177 patients with preoperatively diagnosed EGC located at the middle or lower third of the stomach were enrolled; 102 patients underwent LADG, 4 patients had open conversion, and 71 patients underwent conventional ODG. The operative and early postoperative outcomes from a prospective database were compared between the two groups.
Results
The clinicopathological characteristics were similar between the two groups. No operation-related deaths occurred. Although operation time was significantly longer for LADG than for ODG, time to first flatus was shorter and, consequently, postoperative hospital stay was significantly shorter in the LADG group. There was no significant difference in the overall complication rates between the two groups. On comparing the early (n = 50) and late groups (n = 52) of LADG patients, operation time and postoperative hospital stay were shorter and number of retrieved lymph nodes was greater in the late group (p < 0.05). Major and minor complications were markedly reduced in the late group (p < 0.05).
Conclusions
Although LADG was more time consuming than ODG, it was a feasible, safe procedure that accomplished the oncological requirements. Postoperative morbidity of LADG was similar to that of ODG, and LADG led to faster postoperative recovery. However, LADG should be performed carefully to prevent unexpected complications, especially during the early learning period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Since laparoscopy-assisted distal gastrectomy (LADG) was first reported by Kitano et al. in 1994 [1], this procedure has been sporadically developed by those surgeons who have interest and expertise in laparoscopic surgery [2]. The reduced invasiveness of LADG compared with open distal gastrectomy (ODG) has been demonstrated in several studies that reported less pain, faster recovery, shortened hospital stay, and better cosmesis with LADG [3–5]. Moreover, similar long-term survival rates for treating early gastric cancer (EGC) by ODG and LADG have been reported recently [6–8]. Because of these advantages, the number of laparoscopic-assisted or totally laparoscopic gastrectomies has dramatically increased, especially in Korea and Japan [8, 9].
However, besides major concerns regarding the oncological adequacy of laparoscopic gastrectomy and the high cost of laparoscopic devices, many gastric surgeons are still reluctant to undertake laparoscopic gastrectomy because this procedure is technically sophisticated and entails a learning curve. This is particularly applicable to a senior gastric surgeon who has wide experience with open gastrectomy but limited overall experience with laparoscopic surgery. Except for a few studies in the early era of laparoscopic gastrectomy [10–12], most studies to date have reported beneficial results when LADG is performed by surgeons with modest to substantial laparoscopic experience [13–16].
The purpose of this study was to evaluate the technical feasibility and short-term outcomes of LADG performed by a single experienced gastric surgeon who had no previous experience with laparoscopic surgery, and to compare these results with ODG results during the same period.
Materials and methods
Patients
This study was based on a prospectively designed database of gastric cancer patients at the Department of Surgery, Kangbuk Samsung Hospital. Between January 2006 and December 2007, all patients who had been diagnosed with histologically proven EGC located at the middle or lower third of the stomach were enrolled in this study. The preoperative staging was determined by gastrofiberoscopy, endoscopic ultrasound, abdominopelvic computed tomography (CT) scanning, and assessment of tumor markers [carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9)]. None of the lesions were suitable for endoscopic mucosal resection (EMR), but patients who had been recommended to undergo salvage surgery after incomplete EMR were considered eligible for the study. Patients with cancer in other organs and those with a comorbidity that obviated major surgery, such as severe cardiovascular disease, liver cirrhosis (Child B or C), or renal dysfunction, were not included.
The choice of the surgical procedure (open versus laparoscopic) was based strictly on the patient’s individual decision after he/she was educated as to the methods and risks of each procedure and had provided informed consent. The study’s protocol was approved by the local ethics committee, and contained a critical pathway program to avoid possible bias.
The demographics, surgical risk (ASA status determined according to the standards proposed by the American Society of Anesthesiology), pTNM stage, duration of operation, time to first flatus, time to resumption of liquid diet, length of hospital stay, and postoperative complications were recorded.
Surgical procedure
All operations were performed by one surgeon (C.H.Y.), who had performed more than 1,000 open gastrectomies for treating gastric cancer but had no experience with laparoscopic surgery. Before starting the study, he completed two laparoscopic surgery training steps during a 2-month period: familiarization and skill acquisition. At first, he repeatedly reviewed the available videotapes and digital versatile discs (DVDs) recorded by experienced and novice surgeons. Several laparoscopic devices such as a LigaSureTM (Valleylab Inc., Boulder, CO, USA), a Harmonic ScalpelTM (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA), and an endoscopic stapler were applied to open gastrectomies for familiarization. A video box trainer was used for faster acquisition of the endoscopic technical skills ex vivo before clinical practice. As a last training course, several cases of laparoscopic appendectomy were performed successfully.
LADG and ODG consisted of the following standardized procedures: (1) After gastric resection, Billroth I (B-I) anastomosis using a circular stapler was primarily selected for reconstruction; if B-I was not possible, then extracorporeal handsewn B-II anastomosis was performed. (2) For LADG, D1 + α or D1 + β lymph node dissection was performed according to the rules of the Japanese Research Society for Gastric Cancer [17]. For ODG, D2 lymph node dissection was conducted.
The patient was placed in supine position after induction of general anesthesia. The operator and camera operator stood on the patient’s right side, while the first assistant stood on the patient’s left side. A 12-mm camera port was created above or below the umbilicus by open technique, and pneumoperitoneum was induced by using pressure of up to 15 mmHg carbon dioxide. Four other trocars (one 12-mm and three 5-mm trocars) were inserted under laparoscopic observation. If necessary, gastric air was percutaneously aspirated using a long spinal needle [18], as a nasogastric tube is not routinely inserted at our hospital [19]. The gastrocolic ligament was first divided along the border of the transverse colon using ultrasonic shears (Harmonic ScalpelTM; Ethicon Endo-Surgery Inc., Cincinnati, OH, USA). The left gastroepiploic vessels were divided. Division of the greater omentum was then continued toward the first portion of the duodenum, and the right gastroepiploic vessels were divided at their roots. The soft tissues attached to the duodenum were dissected. The lesser omentum was then opened and the right gastric artery was divided at its origin. The duodenum was transected 1 cm distal to the pylorus using an endoscopic stapling device. The adipose tissue at the anterosuperior border of the pancreas was dissected, and the left gastric vein and artery were exposed and divided using clips and the ultrasonic shears. At this point, the common hepatic artery was exposed and the no. 8a, 9, and 11 lymph nodes were dissected in case of D1 + β dissection. The perigastric lymph nodes were dissected along the lesser curvature up to the esophagogastric junction. A 5-cm incision was made by transverse extension of two epigastric trocar incisions. Through this minilaparotomy, the distal stomach was pulled out of the peritoneal cavity, and the distal two-thirds of the stomach was resected using a linear stapler. For B-I anastomosis, the duodenum was opened and the anvil of a circular stapler was inserted. The body of the circular stapler was introduced into the remnant stomach. After performing a stapling gastroduodenostomy, the opening in the remnant stomach was closed by an additional linear stapler. A closed suction drain was placed through the right upper trocar wound.
For ODG, an upper midline incision was made that measured 10–15 cm, and a standard D2 lymph node dissection was performed. The reconstruction was performed in the same manner as for the LADG.
Statistical analysis
The chi-squared test, Student’s t-test, and linear regression analysis were used to compare the categorical data, parametric data, and the continuous variables, respectively. Values are expressed as mean ± standard deviation. All statistical analyses were performed using SPSS version 11.0 (SPSS Inc., Chicago, IL, USA); p values less than 0.05 were considered to be significant.
Results
During the 2-year period of this study, a total of 177 patients were enrolled; 102 patients underwent LADG, 4 patients (2.3%) had open conversion during their laparoscopic procedure, and 71 patients had conventional ODG. Conversion to open surgery was required due to intraoperative complications of bleeding in three patients and colon ischemia in one patient. Intraoperative bleeding resulted from injury to the right gastroepiploic artery and the right gastric artery in two patients, respectively, and diffuse bleeding from the operative field was seen in one patient with Child A liver cirrhosis. The colon ischemia was caused by accidental ligation of the middle colic artery; therefore, a transverse colectomy was performed after the laparotomy. The conversion cases were excluded from the comparative analysis of completed LADG and ODG.
Details of the clinicopathological characteristics of the patients are shown in Tables 1 and 2. No significant differences were observed between the two groups with respect to demographics, comorbidities, and ASA status. Location and size of tumor, histological differentiation, type of reconstruction, and pTNM stage in the two groups were also similar. Although this study was proposed for pT1 stage cancer, 20 patients had pT2 lesions and one patient had a pT3 lesion. The accuracy rate of the preoperative pT staging was 87%. Lymph node metastasis was found in 9 of the 102 patients who were treated by LADG (8.8%, with 1–10 positive nodes) and in 6 of 71 patients who were treated by ODG (8.4%, with 1–14 positive nodes). The mean number of retrieved lymph nodes in the ODG group was significantly larger than that in the LADG group (37.7 ± 11.9 versus 27.4 ± 11.9, respectively; p < 0.001).
The perioperative outcomes are shown in Table 3. Operation time was significantly longer for the LADG group than for the ODG group (211.4 ± 45.4 min versus 154 ± 25.6 min, respectively; p < 0.001). There was relatively less blood loss in the LADG group than in the ODG group, but the difference was not significant. Mean time to first flatus (3.2 ± 0.8 days versus 3.6 ± 0.7 days, respectively; p = 0.001) and postoperative hospital stay (8.3 ± 2.9 days versus 9.8 ± 2.6 days, respectively; p = 0.001) were significantly shorter in the LADG group.
Seventeen postoperative complications occurred in 16 patients (15.7%) of the LADG group, and 14 complications occurred in 10 patients (14.1%) of the ODG group, not a statistically significant difference in the overall complication rates. In the LADG group, major complications that required either reoperation or more than 30 days of postoperative hospital stay occurred in six patients. Among the two patients with duodenal stump leakage, one patient had massive bleeding from the gastroduodenal artery due to erosion by pancreatic juice. He underwent bleeder ligation and open drainage and was discharged on postoperative day (POD) 54. The other patient underwent primary closure and was discharged on POD 34. Coincidentally, two patients had leakage from the staple line in the remnant stomach that was closed after gastroduodenostomy; the leakage sites were identified and closed through a slight extension of the minilaparotomy wounds. Two patients with leakage from the gastroduodenostomy and the pancreas, respectively, were managed conservatively. Minor complications occurred in 11 patients, including wound infection in 7, delayed gastric emptying in 2, anastomotic bleeding in 1, and anastomotic stenosis in 1. These complications were also treated conservatively.
In the ODG group, major complications occurred in five patients: postoperative bleeding in two, duodenal stump leakage in one, and wound dehiscence in one; they were managed by reoperation without further sequelae. One patient had intractable chylous ascites and the patient was discharged on POD 39 after totally parenteral nutrition. There was no postoperative mortality in either group.
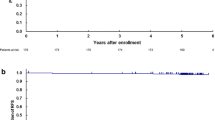
To assess the learning curve of the LADG procedure, changes in operation time and number of retrieved lymph nodes, according to case sequence number, were evaluated by linear regression analysis and construction of a scatter plot. As shown in Figs. 1 and 2, operation time decreased significantly with increasing sequence number (r 2 = 0.15, p < 0.001), whereas the number of retrieved lymph nodes increased significantly with increasing sequence number (r 2 = 0.07, p < 0.001). In both plots, the changes were remarkable after approximately 50 procedures, thus patients were divided into early (n = 50) and late (n = 52) groups to conduct group comparisons. The data are shown in Table 4. Operation time and postoperative hospital stay were shorter, and the number of retrieved lymph nodes was greater, in the late group compared with in the early group (p < 0.05). Major and minor complications were markedly reduced in the late group (p < 0.05).
During the 17-month median follow-up period (range 4–29 months), two patients in the LADG group had recurrences: one patient (pT1N0) had a brain metastasis 15 months after surgery and the other (pT3N1) had a port-site recurrence 10 months after surgery. Since the duration of follow-up was short, all the patients are alive to date.
Discussion
Although laparoscopic gastrectomy is not yet fully accepted worldwide as a standard procedure for gastric cancer, it has already become the general trend for the treatment of EGC in both Korea and Japan [8, 9]. On the contrary, recent issues are moving towards the feasibility and safety of performing laparoscopic gastrectomy for advanced gastric cancer (AGC) [20]. These trends are also reflected in this study. LADG was selected more often by patients as a “good” or a “new operation” despite being informed of the surgeon’s lack of experience with the technique and its cost. Therefore, it has become more difficult for gastric surgeons in our country to insist on performing the “surgeon-friendly” open gastrectomy.
Unlike gastric surgeons in Western countries, where laparoscopic procedures for morbid obesity are popular, most senior gastric surgeons in Asia, including Korea, have had few opportunities for training or clinical experience with laparoscopic surgery because the procedure is relatively new and its main surgical indication is gastric cancer. Laparoscopic surgery requires specialized dexterity that is different from that for open surgery due to the translation of a two-dimensional video image into a three-dimensional working area, decreased tactile feedback, and the need for good eye–hand coordination [21]. These issues make training for laparoscopic skills more difficult, as it has been demonstrated that possessing ample open surgical skills does not translate well to laparoscopic skills. Among several basic training courses, we used a video box trainer for the acquisition of laparoscopic skills, and laparoscopic appendectomies were performed for clinical practice. We believe that the box trainer has the potential advantages of using real laparoscopic instruments, realistic tactile feedback, and time and cost effectiveness. Furthermore, laparoscopic appendectomy, a relatively simple procedure with abundant clinical cases, is suitable for a novice surgeon to gain laparoscopic experience.
The present study showed that LADG is safe and feasible, has a low conversion rate, and provides favorable short-term outcomes compared with ODG. Four patients (2.3%) underwent conversion to open surgery, an acceptable rate compared with previously published data [7, 10, 12, 22]. All conversion cases that occurred in the early period of LADG surgical experience involved males with a relatively large volume of visceral fat. Hyung et al. [23] suggested that surgeons with limited LADG experience should consider the patient and tumor characteristics such as gender and body mass index (BMI) to minimize the learning curve. Therefore, careful patient selection might be one important factor for a successful initial experience with complex LADG procedures.
We found that the LADG group had significantly shorter time to first flatus and shorter postoperative hospital stay, which indicates that LADG was less invasive. Most patients in the LADG group, except patients with complications, were discharged on POD 7 or 8. Although the operation took longer for LADG than for ODG, our mean operation time for LADG is comparable with that reported in other studies [3–8]. While several case–control studies and a few randomized studies have reported no differences between LADG and ODG [3–5, 7, 16, 24], some studies have reported longer operation time for LADG than for ODG [14, 20, 25, 26]. In this study, although the mean operation time was significantly decreased in the late group (189 min) compared with the early group (229 min), LADG still took longer than ODG (154 min), and it seems unlikely that further expertise will result in a significant reduction if we try laparoscopic D2 lymph node dissection in the near future.
The impact of extended lymph node dissection on long-term survival is still under debate because randomized studies have shown no evidence of a statistically significant improvement in survival with D2 lymph node dissection [7]. However, in Korea and Japan, D2 lymph node dissection has long been the standard treatment, even for EGC, with lower morbidity and mortality than in the West [25, 26]. In this study, the mean number of retrieved lymph nodes was significantly greater for the ODG group (37.7 ± 11.9) than for the LADG group (27.4 ± 11.9), which was not surprising considering the difference in the extent of lymph node dissection in each group. Except for a few initial patients with obesity we tried to perform D1 + β dissection for LADG, and more than 15 lymph nodes, the minimum requirement for pN staging, were retrieved in 87 (85.3%) of the 102 LADG patients. Furthermore, there was a definite increase in the number of retrieved lymph nodes in the late group (30.2 ± 12.2) as compared with the early group (24.5 ± 10.8). Kunisaki et al. [27] reported that the incidence of metastasis in the group 2 lymph nodes, such as those along the left gastric artery, the common hepatic artery, the splenic artery, and the celiac trunk, was up to 6%, especially for submucosal cancer. As was shown by our studies and others [6, 11], the accuracy of preoperative pT staging is far from satisfactory. Thus, at least a D1 + β or D2 lymph node dissection is needed for LADG except in the case of a definite small mucosal cancer.
In addition to long-term survival, postoperative complications and mortality are major concerns when performing a LADG procedure because its main indication is EGC rather than AGC. The surgical complications and mortality of LADG have been reported to range from 3.1% to 26.7% and from 0% to 3.3%, respectively [22, 28]. The wide range of complication rates can be attributed to variations in the sample size, the length of the study period, the extent of lymph node dissection, and the surgeons’ experience with laparoscopic surgery. According to the data from the eighth questionnaire survey conducted by the Japanese Society for Endoscopic Surgery in Japan, 4,799 patients underwent laparoscopic gastrectomy in 2004 and 2005 and the complication and mortality rates were 8.7% and 0.083%, respectively. Consistent with other reports, we safely performed LADG with an acceptable complication rate and no postoperative mortality. However, of the six major complications observed in our LADG series, five were related to anastomotic leakage and one was related to pancreatic leakage probably due to pancreatic injury during dissection of the infrapyloric lymph nodes. These results are similar to those of Fujiwara et al. [10], who reported a high (14%) incidence of anastomotic leakage throughout an initial 2-year period. Anastomosis in laparoscopic surgery tends to be more complicated and technically demanding than in open surgery. B-I extracorporeal anastomosis through minilaparotomy incisions may cause forceful tension and injuries to the structures around the anastomosis because of the limited vision of the operative field, especially in an obese patient [29]. Two cases of duodenal stump leakage in our LADG group occurred after substituting B-II anastomosis for B-I anastomosis due to the injuries to the duodenum during purse-string suturing and anvil insertion in obese patients who had thicker abdominal walls. We overcame this problem by primarily performing an extracorporeal B-II anastomosis when expecting difficulty with a B-I anastomosis. Contrary to our expectations, wound infections, which may have been caused by forceful wound retraction and the resultant tissue damage or by contamination by the resident flora in the gastrointestinal tract, occurred in seven patients of the LADG group, but no infections have developed since we started to protect wounds with Alexis Protector (Applied Medical, Rancho Santa Margarita, CA, USA) at the time of minilaparotomy.
Regarding the oncological safety of LADG procedures, this study did not provide enough data to allow a definite conclusion because the follow-up period was too short. Two cases of recurrence after LADG occurred in the follow-up period; one was in the brain of a patient with mucosal cancer and one was a port-site metastasis in a patient with AGC. While brain metastasis is one of the characteristic patterns of failure observed in EGC patients [30] rather than being peculiar to laparoscopic surgery and the creation of a pneumoperitoneum, further long-term study is needed to investigate the association between development of port-site metastasis and peritoneal seeding after laparoscopic manipulation of cancer.
In conclusion, the present study showed that the results obtained by an experienced gastric surgeon with no previous experience in laparoscopic surgery can be at least as good as those obtained in open surgery. Although LADG was more time consuming than ODG, it was a feasible, safe procedure that accomplished the oncological requirements with comparable postoperative morbidity and faster postoperative recovery. However, LADG should be performed carefully to prevent unexpected complications that could detract from the lower degree of invasiveness of the procedure, especially during the early learning period.
References
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4:146–148
Goh PM, Alponat A, Mak K, Kum CK (1997) Early international results of laparoscopic gastrectomies. Surg Endosc 11:650–652
Adachi Y, Shiraishi N, Shiromizu A, Bandoh T, Aramaki M, Kitano S (2000) Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg 135:806–810
Pugliese R, Maggioni D, Sansonna F, Scandroglio I, Ferrari GC, Di Lernia S, Costanzi A, Pauna J, de Martini P (2007) Total and subtotal laparoscopic gastrectomy for adenocarcinoma. Surg Endosc 21:21–27
Varela JE, Hiyashi M, Nguyen T, Sabio A, Wilson SE, Nguyen NT (2006) Comparison of laparoscopic and open gastrectomy for gastric cancer. Am J Surg 192:837–842
Fujiwara M, Kodera Y, Misawa K, Kinoshita M, Kinoshita T, Miura S, Ohashi N, Nakayama G, Koike M, Nakao A (2008) Long-term outcomes of early-stage gastric carcinoma patients treated with laparoscopy-assisted surgery. J Am Coll Surg 206:138–143
Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C (2005) Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 241:232–237
Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N (2007) A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 245:68–72
Song KY, Park CH, Kang HC, Kim JJ, Park SM, Jun KH, Chin HM, Hur H (2008) Is totally laparoscopic gastrectomy less invasive than laparoscopy-assisted gastrectomy? Prospective, multicenter study. J Gastrointest Surg 12:1015–1021
Fujiwara M, Kodera Y, Kasai Y, Kanyama Y, Hibi K, Ito K, Akiyama S, Nakao A (2003) Laparoscopy-assisted distal gastrectomy with systemic lymph node dissection for early gastric carcinoma: a review of 43 cases. J Am Coll Surg 196:75–81
Jin SH, Kim DY, Kim H, Jeong IH, Kim MW, Cho YK, Han SU (2007) Multidimensional learning curve in laparoscopy-assisted gastrectomy for early gastric cancer. Surg Endosc 21:28–33
Shimizu S, Noshiro H, Nagai E, Uchiyama A, Tanaka M (2003) Laparoscopic gastric surgery in a Japanese institution: analysis of the initial 100 procedures. J Am Coll Surg 197:372–378
Fujiwara M, Kodera Y, Miura S, Kanyama Y, Yokoyama H, Ohashi N, Hibi K, Ito K, Akiyama S, Nakao A (2005) Laparoscopy-assisted distal gastrectomy with systemic lymph node dissection: a phase II study following the learning curve. J Surg Oncol 91:26–32
Kim MC, Kim KH, Kim HH, Jung GJ (2005) Comparison of laparoscopy-assisted by conventional open distal gastrectomy and extraperigastric lymph node dissection in early gastric cancer. J Surg Oncol 91:90–94
Lee JH, Kim YW, Ryu KW, Lee JR, Kim CG, Choi IJ, Kook MC, Nam BH, Bae JM (2007) A phase-II clinical trial of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer patients. Ann Surg Oncol 14:3148–3153
Lee SI, Choi YS, Park DJ, Kim HH, Yang HK, Kim MC (2006) Comparative study of laparoscopy-assisted distal gastrectomy and open distal gastrectomy. J Am Coll Surg 202:874–880
Nakajima T (2002) Gastric cancer treatment guidelines in Japan. Gastric Cancer 5:1–5
Hyung WJ, Song C, Cheong JH, Choi SH, Noh SH (2005) Percutaneous needle decompression during laparoscopic gastric surgery: a simple alternative to nasogastric decompression. Yonsei Med J 46:648–651
Yoo CH, Son BH, Han WK, Pae WK (2002) Nasogastric decompression is not necessary in operations for gastric cancer: prospective randomised trial. Eur J Surg 168:379–383
Tanimura S, Higashino M, Fukunaga Y, Kishida S, Nishikawa M, Ogata A, Osugi H (2005) Laparoscopic distal gastrectomy with regional lymph node dissection for gastric cancer. Surg Endosc 19:1177–1181
Clevin L, Grantcharov TP (2008) Does box model training improve surgical dexterity and economy of movement during virtual reality laparoscopy? A randomised trial. Acta Obstet Gynecol Scand 87:99–103
Shiraishi N, Yasuda K, Kitano S (2006) Laparoscopic gastrectomy with lymph node dissection for gastric cancer. Gastric Cancer 9:167–176
Hyung WJ, Song C, Cheong JH, Choi SH, Noh SH (2007) Factors influencing operation time of laparoscopy-assisted distal subtotal gastrectomy: analysis of consecutive 100 initial cases. Eur J Surg Oncol 33:314–319
Kawamura H, Okada K, Isizu H, Masuko H, Yamagami H, Honma S, Ueki S, Noguchi K, Kondo Y (2008) Laparoscopic gastrectomy for early gastric cancer targeting as a less invasive procedure. Surg Endosc 22:81–85
Hayashi H, Ochiai T, Shimada H, Gunji Y (2005) Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc 19:1172–1176
Lee JH, Han HS (2005) A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 19:168–173
Kunisaki C, Shimada H, Nomura M, Akiyama H (2001) Appropriate lymph node dissection for early gastric cancer based on lymph node metastases. Surgery 129:153–157
Ryu KW, Kim YW, Lee JH, Nam BH, Kook MC, Choi IJ, Bae JM (2008) Surgical complications and the risk factors of laparoscopy-assisted distal gastrectomy in early gastric cancer. Ann Surg Oncol 15:1625–1631
Joo YT, Moon HG, Lee SH, Jeong CY, Jung EJ, Hong SC, Choi SK, Ha WS, Park ST, Lee YJ (2007) Laparoscopy-assisted distal gastrectomy with intracorporeal Billroth I stapled anastomosis using a hand access device for patients with gastric cancer. Surg Endosc 21:859–862
Yoo CH, Noh SH, Shin DW, Choi SH, Min JS (2000) Recurrence following curative resection for gastric carcinoma. Br J Surg 87:236–242
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoo, C.H., Kim, H.O., Hwang, S.I. et al. Short-term outcomes of laparoscopic-assisted distal gastrectomy for gastric cancer during a surgeon’s learning curve period. Surg Endosc 23, 2250–2257 (2009). https://doi.org/10.1007/s00464-008-0315-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-008-0315-0