Abstract
Schizophyllum commune is a wood-rotting filamentous fungus that secrets a homopolysaccharide called as schizophyllan. Schizophyllan has several applications such as enhanced oil recovery, pharmaceutical materials and an anti-cancer drug carrier. Biomass growth and schizophyllan production increase the viscosity of the cultivation medium, thus resulting in mass transfer limitation for the substrate. In this study, adding talc and aluminium oxide microparticles into the cultivation medium was studied to improve the fungal growth and morphology. The response surface methodology and one factor at a time were applied to find the effects of microparticles with different sizes and concentrations on the schizophyllan production. The optimum concentration and size of aluminium oxide microparticles were obtained as 20 g L−1 and < 30 µm, respectively. Aluminium oxide microparticles in shake flask culture caused to increase the schizophyllan production from 10 to 15 g L−1 and decrease the cultivation time from 10 to 7 days. The production yield also increased from 0.11 to 0.30 g of schizophyllan/g glucose. Bioreactor cultivation showed a twofold increase in schizophyllan production from 1.5 to 3 g L−1. The results of this study suggested a significant increase in the production of schizophyllan using a low-cost “microparticle-enhanced cultivation” without any further optimization of the culture medium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Filamentous fungi are one of the most important microorganisms in the biotechnological industries because of their biosynthetic capabilities of metabolites such as enzymes, polysaccharides, organic acids, and antibiotics. During their submerged cultivation, filamentous fungi show several morphological forms ranging from dispersed mycelial filaments to dense mycelial masses known as pellets. Due to the complex morphological growth, their industrial application has always been accompanied by extreme operational difficulties such as oxygen transfer limitation and high viscosity of broth. Therefore, the development of cost-effective and feasible cultivation strategies to overcome these operational difficulties is a substantial prerequisite for harnessing the enormous biochemical potential of fungal [1].
One of these filamentous fungi is a wood-rotting fungus named Scizophyllum commune, which can produce schizophyllan (SPG). Schizophyllan is a non-ionic water-soluble extracellular homopolysaccharide with a backbone chain of 1,3-β-d-glucopyranose units and single 1,6-β-d-glucopyranoses at about every third glucose monomer in the main chain. Schizophyllan as a natural polymer has some unique properties such as thermal stability, high viscosity, and the ability of film formation that can prevent oxygen penetration, making it suitable for applying in enhanced oil recovery, cosmetics, food preserver and thickener [2]. Schizophyllan has important therapeutic applications such as immune effect enhancer for anti-cancer drugs and carrier in drug delivery [3, 4].
Several strategies were studied on the improvement of yield and productivity in the cultivation of S. commune. For instance, evaluation of the effects of different impellers and dissolved oxygen on the productivity of fungus was investigated [5]. Controlling the size of forming pellets of fungus was implemented as an effective factor in productivity [6]. A group of researchers screened four different strains of S. commune for higher production yield as well as the best substrates were selected by response surface methodology (RSM) [7]. Effects of adding different components to the culture medium such as sodium carboxymethyl cellulose, l-glutamic acid, VB1, naphthalene acetic acid, oleic acid, and Tween 80 were evaluated and some of them were proved as useful additives for the growth [8]. Utilization of the agricultural biomass as the medium for the production of schizophyllan was used to decrease the final price of biopolymer [9]. Investigation of low-cost agricultural substrates such as date syrup was reported for cost-effective schizophyllan production [10].
A novel morphological engineering technique in the cultivation of filamentous fungi is the microparticle-enhanced cultivation (MPEC) method, which focuses on the morphological development of fungus by the addition of mineral microparticles such as talc or aluminium oxide into the cultivation medium. This method was mostly investigated in Aspergillus genus due to their intriguing and often uncontrollable complex morphology [11, 12]. In the beginning, a group of researchers worked on the effect of talc and aluminium oxide powders on fungi morphology for different species including: Caldariomyces fumago DSM 1256, Penicillium chrysogenum, and Aspergillus niger [13]. Other researchers showed that enzymes secretion of A.niger SKAn 1015 would be increased after the utilization of different types of microparticles, such as titanium silicate, aluminum oxide, and silicate microparticles [14, 15]. Similar phenomena were reported during cultivation of Aspergillus terreus and Aspergillus ficuum while using microparticles; meanwhile, decreasing of the pellets diameters were also resulted [16, 17]. The last study illustrated the effect of aluminium oxide microparticles on four different fungal species (Aspergillus terreus, Penicillium rubens, Chaetomium globosum, and Mucor racemosus) with different mechanisms of agglomerates formation [18]. The aforementioned studies have shown the positive effects of the microparticles on the production of valuable enzymes and organic acids by filamentous fungi. However, there is no study regarding the effect of MPEC method for the production of exopolysaccharide as another valuable fungal metabolite.
Therefore, the microscopic-level comparison between the microparticle-enriched and standard cultures was conducted to improve S. commune productivity. The aim of this study was to provide a quantitative description of the influence of microparticles on schizophyllan production. Accordingly, first, possible factors were screened; response surface methodology (RSM). Then, influential ones were optimized by RSM, a series of experiments were carried out both in shaking flasks and bioreactor in optimum point of factors.
Materials and methods
Strain
Schizophyllum commune ATCC 38548 was employed to produce schizophyllan. The fungus was grown on agar slants supplemented with 39 g L−1 potato dextrose agar and 5 g L−1 yeast extract. The covered slants were stored at 4 °C after 10 days. Subcultures were routinely prepared every two months.
Culture condition
The medium used for cultivation in both shake flasks and bioreactor consisted of (g L−1): 30 glucose, 3 yeast extract, 1 KH2PO4 and 0.5 MgSO4. 7H2O. Cultivations were performed at 27 °C without initial pH adjustment. For the first subculture, a piece of 1 × 1 cm2 agar covered by Schizophyllum commune was used to inoculate 100 mL medium in 250 mL shake flasks. After 4 days on a rotary shaker (180 rpm), the culture suspension was homogenized using a Potter-type homogenizer. Then 3 mL of the culture was transferred to 100 mL medium in shake flask and was cultivated again at 27 °C. Bioreactor cultivations were carried out in a stirred tank fermentor with 1 L working volume (Zist Farayand Sanat Saba Co., Iran), equipped with two baffles mounted inside using two 4-bladed Rushton impellers due to a height to width ratio of 2. The inoculated suspension (10% v/v) was added to the bioreactor after homogenization of subculture. All experiments were carried out in duplicate.
Preparation of microparticles
Microparticles of aluminium oxide (Al2O3, Merck Art.13109) were used with different size and concentrations. Coarse particles of aluminium oxide and talc were grinded by a ball mill and screened into different sizes using the following mesh (in µm): 150, 100, 75, 50, and 32. Microparticle concentrations in the experiments were (g L−1): 0.05, 0.1, 0.5, 2.5, 5, 10, 15, 20, and 25. Microparticles of aluminium oxide and talc with desired size and concentration were added to the culture medium and autoclaved at 15 psi.
Biomass and schizophyllan production
The whole culture suspension was filtered and then centrifuged at 15,000 × g for 20 min at 4 °C to separate mycelium and residual microparticles from culture medium. The mycelium and microparticles residues were dried at 65 °C to a constant weight. This value is reported as the total biomass production after discounting microparticles. To extract the schizophyllan, an equivalent volume of 95% (v/v) ethanol was added to precipitate the schizophyllan from the supernatant. After 1 h, the precipitates were collected by centrifugation at 15,000 × g for 20 min at 4 °C. The polysaccharide precipitates were air-dried overnight to evaporate the ethanol content, and then freeze-dried. The dry weight of schizophyllan was measured and reported.
Glucose measurement
10 µL of supernatant was taken as a sample for calculating glucose consumption. 1000 µL of a solution kit containing glucose oxidase enzyme was added to the sample. Oxygenated water, which was formed by the reaction of glucose and enzyme, reacts with phenol and 4-aminoantipyrine to make kinonimin (red violet). The absorbance of the solution was measured by a spectrophotometer (Cary 100 Varian Co.) at 546 nm that indicating the glucose concentration using a calibration curve.
Production profile of schizophyllan
Ten 250 mL-flasks containing 100 mL medium were inoculated with 5 mL of homogenized S. commune and incubated at 28 °C for 10 days. Each day, one of the flasks was taken out from the shaker and the whole culture suspension from each flask was analysed for biomass, schizophyllan production, and total glucose consumption.
Image processing and analysis
The samples from standard and MPEC runs were subjected to the microscopic observations. The light microscope Euromex “OXION” series was used for this purpose (Euromex microscopen, Netherlands). The microscope was equipped with the high resolution RGB digital camera Euromex VC.3031 and controlled by the computer with the image analysis software ImageFocus 4 English Version (Euromex microscopen, Netherlands). Due to the fact that the size of the observed mycelial objects was changed by several magnitudes, a variety of objectives: 4 × , 10 × , 40 × , and 100 × were used. The slides were prepared by dropping approximately 1 mL of the fungal suspension and observed using phase contrast. At least 10 RGB images of resolution 2592 × 1932 from the different sample were snapped to assure the minimum number of mycelial objects for analysis. In addition, images were analysed by “Imagej” software for measuring pellets core and shells radius.
Optimization of schizophyllan production using response surface methodology (RSM)
A five-level CCD was performed using the statistical software (Design-Expert 7) to determine the optimum levels of microparticle properties. Accordingly, to examine the combined effect of two different factors on the responses, a central composite factorial design of 22 = 4 with 4 centre points and (2 × 2 = 4) star points leading to a total of 12 experiments was conducted. Two factors of microparticles size (A) and microparticles concentration (B) were further investigated at five different levels in the range of 5–150 µm and 0.5–25 g L−1, respectively, to estimate the optimum conditions for product yield, schizophyllan, and biomass production. All experiments were performed in duplicate.
Results and discussion
Microparticles screening
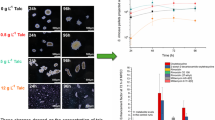
First, the influence of microparticles type and concentration on the schizophyllan biosynthesis in the shaking flask was studied. Figure 1 shows the effect of MPs on the schizophyllan production and S. commune growth at different concentrations of talc and aluminium oxide compared to in the absence of MPs. Schizophyllan production by S. commune was affected by the concentration of microparticles; at 20 g L−1 of talc and aluminium oxide, schizophyllan productions were 1.9 ± 0.06 g L−1 and 1.75 ± 0.05 g L−1, respectively, which were 20% higher than in the absence of MPs (1.45 ± 0.05 g L−1). Consequently, it was determined that microparticles concentration could play a significant role in MPEC method, which was in agreement with other studies in this topic. Almost all of the previous studies mentioned that there was an optimum concentration for the MPs which resulted in the best production [11]. However, due to the fact that talc microparticles are not quite neutral (can leach magnesium to the media), the increased production yield in talc implemented cultivation could be because of biochemical interactions [19].
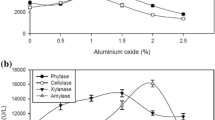
Then, the effect of MPs sizes on schizophyllan production was studied (Fig. 2). Five different sizes of aluminium oxide were added to the culture media at the same MP concentration (20 g L−1). In the run with 30 µm, the best result of schizophyllan production (0.29 g L−1) was achieved, and the whole experiments showed that the smaller particles led to more schizophyllan production, thus being more desirable. Most of the studies showed that adding microparticles to the culture media affected fungal morphology, which basically consisted of a decrease in pellets mean size [20]; but, the mechanism of MPEC method is still unclear. Only one study reported a possible mechanism for microparticles, which illustrated the effect of them on spores agglomeration during the initial phase of fungal growth [15]. Despite the fact that S. commune does not have spore, a morphology change was observed in this study. A probable mechanism for microparticles effect could be the acting as cores, which facilitates fungal grow on them to form pellets (core–shell pellets) instead of mycelial growth. This phenomenon was observed by microscopic analyses from the initial growth phase in Fig. 3 which shows fungal pellets. Based on this probable mechanism, it could be explained that the type of neutral mineral MPs could not make a significant difference in their role in filamentous fungi morphology. Meanwhile, MPs concentration is important since at lower concentration, the availability of them is decreased for each fungus particle which leads to the agglomeration. In addition, according to the mean size of fungal pellets, which is between 300 and 1700 µm [14, 17], microparticles size is important since big particles will participate easily; and the small ones will be out of fungus particles reach. A similar observation was also reported [17].
These two series of experiments, which was conducted for screening the MPs properties, showed the effectiveness of size and concentration of MPs in schizophyllan production, while the type of MPs had not a significant effect.
Optimization of schizophyllan production
According to Table 1, a series of experiments was designed to optimize the effects of MP sizes and concentration on the schizophyllan production.
As it is illustrated in Table 1, two of the best productions have resulted in experiments Std. 3 and 5 (6.49 ± 0.27 g L−1 and 5.26 ± 0.24 g L−1). In both, either the size of microparticles is small or the concentration is relatively high. The reason for this observation could be due to the increase in the chance of availability of MPs to the microorganisms.
According to an ANOVA analysis, Schizophyllan production was fitted to a 2FI equation including microparticles size (A), microparticles concentration (B), and their interaction (AB). The coefficients and P values are listed in Table 2. The obtained model for schizophyllan production based on the two factors is given in Eq. (1):
P values are used for evaluating the significance of each parameter. In this study, the error threshold was considered as 10%, which is good for biological systems. Therefore, P values less than 0.1 indicated that the effect of that factor is significant. The positive sign of B factor shows that increasing the concentration of microparticles will lead to an increase in the schizophyllan production, while the negative sign of AB indicates that production will be decreased if this factor increases. Due to the greatest coefficient for B factor, concentration affects production more than other factors. It was mentioned that the presence of MPs was crucial during the initial phase of growth [15] and could be referred to as the chance of being core for fungal growth. Based on this theory, the availability of microparticles is very important for MPEC method, which is related to their concentration directly. Based on Statistical parameters, ANOVA showed a relatively high coefficient of determination (R2 = 0.82 and Adjusted R2 = 0.7590), which ensures a satisfactory adjustment of the first-order regression model to the experimental data.
Figure 4 shows that increasing the concentration of MPs and decreasing their size will increase production.
The obtained model was used to predict the optimum factor for maximum production. Therefore, the top five optimum amounts for the best production are given in Table 3. According to the Table 3 predictions, microparticles with the sizes less < 30 µm and concentrations around 20 g L−1 have resulted in the best production, which is in agreement with other research.
An abstract of previous studies on MPEC method with their optimum amounts for size and concentration is given in Table 4 in comparison with this work, which shows that other works resulted in the similar amounts for size and concentration of MPs.
Cultivation in optimum point
Shaking flasks cultivation
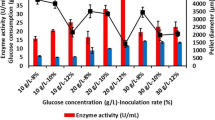
The cultivation of S. commune in the presence of MPs in the optimum amount of size and concentration (30 µm and 20 g L−1) was studied. An experiment for 10 days was conducted in shake flasks with 90 g L−1 glucose as carbon source and 20 g L−1 of aluminium oxide with the size of less than 30 µm. The daily production of schizophyllan and glucose consumption were determined as depicted in Fig. 5.
At the 7th day of cultivation, the results show the maximum production of schizophyllan 15 ± 0.52 g L−1 while 40 g L−1 of glucose was consumed. In the absence of MPs, the maximum production was achieved at 10th day with 10 ± 0.43 g L−1 of schizophyllan production by consuming 85 ± 1.5 g L−1 of glucose. Therefore, the production yield was calculated by Eq. (2), were 0.3 ± 0.01 and 0.11 ± 0.01 g schizophyllan/g glucose in the presence and absence of MPs, respectively. These results show an approximately threefold increase in the production yield in the presence of MPs. A reason for the decrease of schizophyllan after the 7th day may be the severe increase of viscosity and mass transfer limitation which could force microorganism to consume its own production. The effect of microparticles on S. commune morphology was evaluated by the observation of culture samples with a light microscope. Pellets were formed with microparticles in their centre as core (Fig. 3) which resulted in a decrease of fungi radius size up to twofold (Fig. 6). Therefore, in a constant biomass weight, the surface of filamentous fungus increased, leading to an increase in the contact of a fungus with the substrate. Since the cell walls of S. commune were responsible for the production and secretion of schizophyllan [21], the increase of surface/volume ratio could be responsible for higher schizophyllan production in the presence of MPs.
where YP/S, Pt, P0, St, S0 are production yield, schizophyllan at time t, schizophyllan at the start of cultivation, substrate at time t, and substrate at the start of cultivation, respectively.
Bioreactor cultivation
To evaluate the effect of MPEC method in productivity, two experiments were conducted in a bioreactor with 1 L working volume in the presence and absence of MPs (with one replication). The results of daily schizophyllan production and glucose consumption are given in Fig. 7.
In the presence of MPs, a twofold increase in production was obtained from 1.5 to 3 g L−1. To evaluate the effect of these particles on the growth of filamentous fungus, a sample of culture was observed by a light microscope every day during the cultivation (Fig. 8). Using microparticles alters growth type from mycelium to pellet form. In addition, the length of hypha reduced, and pellets became smaller up to threefold in radius size (Fig. 9). Consequently, mass transfer limitation was diminished, and production was increased. Other possible reason for the production increase in the presence of MPs may be related to the role of them as cores of pellets. In the absence of MPs, pellets are totally made of fungi and the inner parts gradually confront to substrate limitation and start to digest themselves, which is mentioned as a common phenomenon in the growth of filamentous fungi [22]. However, in the presence of MPs, there was no fungus in the centre of pellets and there would be less substrate inhibition. Therefore, more of the biomass participated in the metabolite secretion, which resulted in more schizophyllan production. Consequently, the results of this work show high schizophyllan production and yield simultaneously while the usage of microparticles has a low cost, which makes it highly feasible and economical.
Conclusion
MPEC method could be used to increase the production of schizophyllan in S. commune cultivation. In this study, type, size and concentration of aluminium oxide and talc microparticles were screened for schizophyllan production. The effect of microparticles type was not significant; while, size and concentration of aluminium oxide microparticles were significant and were optimized in range of 5–150 µm and 0.5–25 g L−1, respectively, by RSM. The results showed a twofold and threefold increase in the production from 1.5 to 3 g L−1 and yield of schizophyllan from 0.11 to 0.3 g g−1, respectively, using an eco-friendly method without any further optimization of the culture medium under the optimum size and concentration of MPs (< 30 µm and 20 g L−1).
References
Gibbs P, Seviour R, Schmid F (2000) Growth of filamentous fungi in submerged culture: problems and possible solutions. Crit Rev Biotechnol 20:17–48
Mousaviasl S, Saleh T, Shojaosadati SA, Boddohi S (2018) Synthesis and characterization of schizophyllan nanogels via inverse emulsion using biobased materials. Int J Biol Macromol 120:468–474
Jamshidian H, Shojaosadati SA, Mousavi SM, Soudi MR, Vilaplana F (2017) Implications of recovery procedures on structural and rheological properties of schizophyllan produced from date syrup. Int J Biol Macromol 105:36–44
Hamedi S, Shojaosadati SA, Najafi V, Alizadeh V (2020) A novel double-network antibacterial hydrogel based on aminated bacterial cellulose and schizophyllan. Carbohyd Polym 229:115383
Rau U, Gura E, Olszewski E, Wagner F (1992) Enhanced glucan formation of filamentous fungi by effective mixing, oxygen limitation and fed-batch processing. J Ind Microbiol Biotechnol 9:19–25
Shu CH, Chou PF, Hsu I (2005) Effects of morphology and oxygen supply on schizophyllan formation by Schizophyllum commune using a pellet size controlling bioreactor. J Chem Technol Biotechnol 80:1383–1388
Kumari M, Survase SA, Singhal RS (2008) Production of schizophyllan using Schizophyllum commune NRCM. Biores Technol 99:1036–1043
Hao L-m, Xing X-h, Li Z, Zhang J-c, Sun J-x, Jia S-r, Qiao C-s, Wu T (2010) Optimization of effect factors for mycelial growth and exopolysaccharide production by Schizophyllum commune. Appl Biochem Biotechnol 160:621–631
Sutivisedsak N, Leathers TD, Nunnally MS, Price NP, Biresaw G (2013) Utilization of agricultural biomass in the production of the biopolymer schizophyllan. J Ind Microbiol Biotechnol 40:105–112
Jamshidian H, Shojaosadati SA, Vilaplana F, Mousavi SM, Soudi MR (2016) Characterization and optimization of schizophyllan production from date syrup. Int J Biol Macromol 92:484–493
Antecka A, Bizukojc M, Ledakowicz S (2016) Modern morphological engineering techniques for improving productivity of filamentous fungi in submerged cultures. World J Microbiol Biotechnol 32:193
Walisko R, Krull R, Schrader J, Wittmann C (2012) Microparticle based morphology engineering of filamentous microorganisms for industrial bio-production. Biotech Lett 34:1975–1982
Kaup BA, Ehrich K, Pescheck M, Schrader J (2008) Microparticle-enhanced cultivation of filamentous microorganisms: Increased chloroperoxidase formation by Caldariomyces fumago as an example. Biotechnol Bioeng 99:491–498
Driouch H, Hänsch R, Wucherpfennig T, Krull R, Wittmann C (2012) Improved enzyme production by bio-pellets of Aspergillus niger: targeted morphology engineering using titanate microparticles. Biotechnol Bioeng 109:462–471
Driouch H, Sommer B, Wittmann C (2010) Morphology engineering of Aspergillus niger for improved enzyme production. Biotechnol Bioeng 105:1058–1068
Coban HB, Demirci A, Turhan I (2015) Microparticle-enhanced Aspergillus ficuum phytase production and evaluation of fungal morphology in submerged fermentation. Bioprocess Biosyst Eng 38:1075–1080
Gonciarz J, Bizukojc M (2014) Adding talc microparticles to Aspergillus terreus ATCC 20542 preculture decreases fungal pellet size and improves lovastatin production. Eng Life Sci 14:190–200
Kowalska A, Boruta T, Bizukojć M (2018) Morphological evolution of various fungal species in the presence and absence of aluminum oxide microparticles: comparative and quantitative insights into microparticle-enhanced cultivation (MPEC). Microbiologyopen 7:e00603
Etschmann MMW, Huth I, Walisko R, Schuster J, Krull R, Holtmann D, Schrader J et al (2015) Improving 2-phenylethanol and 6-pentyl-α-pyrone production with fungi by microparticle-enhanced cultivation (MPEC). Yeast 32(1):145–157
Yatmaz E, Karahalil E, Germec M, Ilgin M, Turhan İ (2016) Controlling filamentous fungi morphology with microparticles to enhanced β-mannanase production. Bioprocess Biosyst Eng 39:1391–1399
Zhang Y, Kong H, Fang Y, Nishinari K, Phillips GO (2013) Schizophyllan: a review on its structure, properties, bioactivities and recent developments. Bioact Carbohydr Diet Fibre 1:53–71
Papagianni M (2004) Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv 22:189–259
Gonciarz J, Kowalska A, Bizukojc M (2016) Application of microparticle-enhanced cultivation to increase the access of oxygen to Aspergillus terreus ATCC 20542 mycelium and intensify lovastatin biosynthesis in batch and continuous fed-batch stirred tank bioreactors. Biochem Eng J 109:178–188
Coban HB, Demirci A (2016) Enhancement and modeling of micro particle-added Rhizopus oryzae lactic acid production. Bioprocess Biosyst Eng 39(2):323–330
Acknowledgements
The authors are grateful to thank Dr. Alireza Chackoshian Khorasani and Mahdi Soleimani SalehAbadi for their technical supports and assistance during this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alizadeh, V., Shojaosadati, S.A. & Zamir, S.M. Enhancement of schizophyllan production in Schizophyllum commune using microparticles in medium. Bioprocess Biosyst Eng 44, 317–328 (2021). https://doi.org/10.1007/s00449-020-02444-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02444-z