Abstract
Phytase can be used in animal’s diets to increase the absorption of several divalent ions, amino acids and proteins and to decrease the excessive phosphorus release in manure to prevent negative effects on the environment. This study aimed to enhance the current submerged fungal phytase productions with a novel fermentation technique by evaluating the effect of the various microparticles on Aspergillus ficuum phytase production. It was observed that microparticles prevented bulk fungal pellet growth, decreased average fungal pellet size and significantly increased phytase activity in the submerged fermentation. Microbial structure imaging results showed that the average fungal pellet radius decreased from 800 to 500 and 200 µm by addition of 15 g/L aluminum oxide and talcum, respectively, in shake-flask fermentation. Also, addition of 15 g/L of talcum and aluminum oxide increased phytase activity to 2.01 and 2.93 U/ml, respectively, compared to control (1.02 U/ml) in shake-flask fermentation. Additionally, phytase activity reached 6.49 U/ml within 96 h of fermentation with the addition of 15 g/L of talcum, whereas the maximum phytase activity was only 3.45 U/ml at 120 h of fermentation for the control in the 1-L working volume bioreactors. In conclusion, microparticles significantly increased fungal phytase activity and production yield compared to control fermentation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytate is the major phosphate source in plants, which is especially abundant in legumes, cereals, pollens and nuts. In plants, phytate is generally used during germination for ATP synthesis [1]. However, phytate is a very strong chelating agent, which has several negative effects on animal and human health. Phytate can bind proteins, amino acids, and divalent ions such as Ca+2, Mg+2, Zn+2, Cu+2, Fe+2, and Mn+2 in vivo and create insoluble salt forms. As a result of this, absorption and utilization of these nutrients decrease [2]. Because of these reasons, consumption of phytate-rich diets may have several health problems such as iron deficiency, bone weakness, tooth decay, and digestion problems [3, 4]. Several environmental issues have also been reported about phytate consumption. Monogastric animals such as chickens and pigs are not able to break phytate, because they do not have the necessary microflora in their digestive systems [5]. Since these monogastric animals are generally fed with phytate-rich compounds such as wheat, rice, and corn, excessive amount of phosphorus accumulates in their manure. This excessive phosphorus secretion causes environmental problems such as water pollution, algal blooms, fish kills, and changing of fauna and flora [5]. However, these problems can be overcome, if the diets are supplemented with phytase to decrease phytate content in the feed. In several studies, a positive effect of phytase application on animal growth and environment has been shown. Aspergillus niger phytase was used to pretreat a corn-soybean diet for broilers and showed that the phosphorus availability increased by 60 % and decreased phosphorus in the manure by 50 % [6]. Phytase also helped to improve body weight of male and female broilers by 13.2 and 5.8 %, respectively, after 21 days on a diet supplemented with phytase. Another positive effect of phytase application was that phytase supplementation increased the relative retention of total P−3, Ca+2, Cu+2, and Zn+2 by 12.5, 12.2, 19.3, and 62.3 %, respectively, in broilers [7].
Phytase can be obtained from plants, but more commonly from microorganisms. Molds were most commonly used for phytase production in several studies. Cowieson et al. [8] used A. niger phytase in animal diets and reduced phytate content by 35–40 %. In another study, Shah et al. [9] reported that optimization of the fermentation medium for phytase production with A. niger NCIM 563 in submerged batch fermentations doubled phytase activity compared to initial values. Similarly, submerged phytase production with A. niger NCIM 56 was performed by Bhavsar et al. [10] and they reported almost sixfold increase in phytase activity after performing mutations on the microorganism and modifications on the fermentation medium. In our previous studies, phytase activity with A. ficuum was increased from 1.02 to 2.27 U/ml and 3.45 U/ml in batch fermentations after optimization of growth parameters and fermentation medium, respectively [11, 12]. However, the cultivation of filamentous microorganisms is commonly accompanied by several problems, such as clumpy growth and insufficient mass transfer, which may result in reduced productivity. To overcome these problems, microparticles can provide a solution. Microparticle addition is a novel approach for cultivation of filamentous microorganisms and product formation to increase the overall yield of the process [13–15]. Additionally, the morphology of filamentous microorganisms during fermentation is an important issue for the desired product formation, supplying substrate, and creating effective agitation in the bioreactors. Therefore, microparticles can provide precise control of filamentous microorganism morphology during fermentation by preventing bulk fungal growth [15]. To date, microparticles such as talcum (magnesium silicate), aluminum oxide, and titanium oxide were used in several studies to increase the yield in fermentations of filamentous microorganisms. Driouch et al. [14] studied A. niger fermentation in the presence of 0–50 g/L TiSiO4. They reported that 25 g/L of TiSiO4 addition increased fructofuranosidase and glucoamylase enzymes productions by 3.7- and 9.5-fold, respectively, compared to control in shake flasks. Additionally, they studied the effect of microparticles on microbial morphology and reported that pellet diameter was reduced from 1.7 to 0.3 mm when 25 g/L concentration of TiSiO4 was added into the fermentation medium [14]. In another study, Kaup et al. [13] studied the effect of aluminum oxide and talcum as microparticles on chloroperoxidase production by Caldariomyces fumago. They observed that particles around 500 µm diameter did not make any difference in growth morphology and production on chloroperoxidase production of Caldariomyces fumago. On the other hand, particles smaller than 42 µm diameter dispersed Caldariomyces fumago to single hyphae, which enhanced enzyme production by fivefold. Driouch et al. [16] also reported that fructofuranosidase production by A. niger was enhanced by 3.5-fold in the presence of microparticles of either 10 g/L of talcum or 20 g/L aluminum oxide in the fermentation medium compared to the control. Currently, there is no study available about the effect of microparticles on fungal phytase production. Therefore, this study is undertaken to enhance A. ficuum phytase activity by evaluating talcum and aluminum oxide as microparticles.
Materials and methods
Microorganism
Aspergillus ficuum (NRRL 3135) was used as the phytase producer [12], which was obtained from USDA Agricultural Research Service Culture Collection (Peoria, IL) and grown on potato dextrose agar (PDA, Difco, Sparks, MD) slants for 6 days at 30 °C and stored at 4 °C as the working culture. To maintain viability, A. ficuum was transferred to sterile fresh agar slant bi-weekly.
Inoculum preparation
A. ficuum spores were prepared by spreading 0.1 ml A. ficuum solution on each 25 PDA plates and grown for 6 days at 30 °C. After incubation, spores were suspended by adding 7 ml of sterile 0.1 % peptone water and the resulting solution (~106 spores/ml) was collected and used as the inoculum for the fermentations.
Shake-flask fermentation
Shake flasks, containing 100 ml of fermentation medium, were supplemented with 0, 5, 10, 15, 20, and 25 g/L of aluminum oxide (Al2O3) or talcum (3MgO·4SiO2·H2O). Base fermentation medium included 126 g of glucose, 0.5 g of KCl, 0.1 g of FeSO4(7H2O), 0.5 g of MgSO4(7H2O), 0.01 g of MnSO4(7H2O), 8.6 g of NaNO3, 3 g (NH4)2SO4, 1.1 g CaSO4, and 14 g of Na-phytate (A&Z Food Additives Co. Ltd., Zhejiang, China) per liter of deionized water, as suggested by our previous study [11]. The pH was adjusted to 6.8 and flasks were autoclaved for 15 min. Thereafter, flasks were inoculated with 3 % prepared spore suspension and incubated at 33 °C and 200 rpm for a total of 144 h. Aliquot samples were collected from each flask every 12 h and analyzed for phytase activity.
Microbial imaging of pellets in shake-flask fermentation
Biomass samples collected from the shake flasks with various microparticle concentrations at 72 h of fermentations were rinsed with 0.9 % NaCl solution to remove excessive microparticles and medium from the pellets. Thereafter, selected average sizes of pellets from each sample were visually analyzed using a light microscope (1242MM, Van Guard, Kirkland, WA). Pellet radius was measured under the microscope.
Batch fermentations in bioreactors
Batch fermentations with talcum as microparticles were performed in Sartorius Biostat B Plus bioreactor (Sartorius, Allentown, PA) equipped with a 2-L vessel with 1-L working volume. The same base fermentation medium with shake-flask fermentations were used, but supplemented with 5, 10, 15, 20, and 25 g/L talcum microparticles. Then, the reactors were autoclaved for 30 min and inoculated with 3 % prepared inoculum after cooling. The fermentations were run at 33 °C, pH 4.5, 0.9 vvm aeration, and 300 rpm agitation as suggested by our previous study [11]. Samples were collected (2 ml) from the reactors every 12 h for 6 days.
Phytase activity analysis
Collected samples from shake flask to bioreactors were centrifuged at 5,200×g for 15 min (Galaxy 5D, VWR, Radnor, PA) to remove the biomass. Then, the supernatant was used for phytase activity analyses. Enzyme assay was performed under the determined optimum temperature and pH as described by Kim et al. [17] with minor modifications. Cell-free broth (0.125 ml) was mixed with 0.125 ml of 1.5 mM Na-phytate in 0.1 M sodium acetate solution, which yielded a final pH of 5.5. Then, the mixture was incubated in the water bath at 55 °C for 30 min. After incubation, the reaction was stopped by adding 0.25 ml of 15 % trichloroacetic acid solution into the tubes. Then, 2 ml of color regent was added, which was prepared freshly with 2:1:1:1 ratio of water:2.5 % ammonium molybdate:6 N H2SO4:10 % ascorbic acid, and tubes were incubated at 55 °C for 30 min. After cooling down to room temperature, absorbances were measured at 700 nm by using a spectrophotometer (Beckman Coulter, Fullerton, CA). Uninoculated fermentation medium was used as the blank for the measurement. The obtained data were used to calculate the activity unit of phytase (U/ml), which was defined as the μmole of phosphorus liberated from 1.5 mM phytate per minute under the set assay conditions.
Statistical analysis
MINITAB Statistical Software package was used for statistical analyses. Two sample t test was used to show if there was a significant (p < 0.05) difference between fermentation results. Additionally, Tukey’s method was used to compare shake-flask and reactor fermentation results. All analyses were replicated with three fermentations.
Results
Effect of aluminum oxide and talcum microparticles on phytase activity in shake-flask fermentation
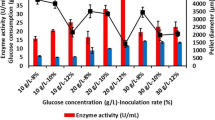
Shake-flask fermentation demonstrated that both aluminum oxide and talcum addition increased phytase activity until 15 g/L of microparticles (Fig. 1). It was calculated that addition of 15 g/L of aluminum oxide and 15 g/L of talcum increased the phytase activity to 2.01 U/ml (97 % increase) and 2.93 U/ml (185 % increase), respectively, compared to control, which yielded only 1.02 U/ml. Therefore, these results suggested that talcum was a better microparticle for phytase production than aluminum oxide. However, a concentration higher than 15 g/L microparticle decreased the maximum phytase activity. Addition of 20 g/L aluminum oxide or talcum in shake-flask fermentation decreased the maximum phytase activity to 1.58 and 1.69 U/ml, respectively. Similarly, 25 g/L talcum or aluminum oxide addition decreased phytase activity to almost control fermentation level (Fig. 1). This can be explained as follows: concentrations higher than 15 g/L microparticle resulted in very small fungal structural growth, which increased the viscosity of the fermentation broth. This high viscosity negatively affected the mass transfer and consequently microbial growth during the fermentation. Similarly, Kaup et al. [13] also reported that the presence of 0.5–10 g/L talcum enhanced chloroperoxidase production in their studies; however, higher concentrations decreased the maximum enzyme activity.
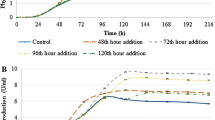
Additionally, it is important to note that maximum phytase activities were obtained at 96 h of fermentation with addition of aluminum oxide and talcum at all levels except for 5 g/L concentration, whereas the control phytase fermentation provided the maximum phytase activity at 120 h of fermentation in shake-flask fermentation (data not shown). Therefore, it is also shown that microparticles do not only increase activity, but also productivity by decreasing the time to reach the maximum phytase activity. Additionally, the highest phytase activities obtained in aluminum oxide- and talcum-added shake-flask fermentation were compared by conducting two-way t test and the result showed that there was a significant difference between phytase activity on addition of 15 g/L of aluminum oxide and talcum in shake-flask fermentations (p value <0.05).
Effect of aluminum oxide and talcum microparticles on fungal morphology in shake-flask fermentations
Fungal pellet size decreased by an increase in microparticle concentration in shake-flask fermentations (Fig. 2). The average fungal pellet radius was measured as 800 ± 65 µm for control fermentations. It was also observed that addition of 5 g of talcum or aluminum oxide did not change the pellet size remarkably. On the other hand, 15 g/L of aluminum oxide and talcum additions decreased the average fungal pellet radius to 500 ± 45 and 200 ± 20 µm, respectively. It was also determined that addition of the same amount of talcum provided smaller fungal pellets compared to aluminum oxide-added shake-flask fermentations (Fig. 3). This can be explained by the differences in mean particle sizes of these two microparticles; since talcum has a smaller diameter compared to aluminum oxide particles, it provides better mass transfer in the fermentation medium (Fig. 4). The average diameter sizes of talcum and aluminum oxide were reported as 10 and 63–200 µm, respectively, by the manufacturers. Driouch et al. [16] also mentioned that the physical properties of the microparticles play an important role in fermentation yield and fungal morphology. They also mentioned the average talcum and alumina they used as ~6 and ~14 µm, respectively.
It was also determined that higher than 15 g/L of microparticle addition negatively affected microbial growth in the fermentation. This was also supported by pH changes based on the different levels of fungal growth in the shake flasks. The initial pH was adjusted to 6.8 in shake flasks and pH decreased to 3.29 in the control flask at 72 h of fermentation. On the other hand, pH values were decreased to 3.49, 3.76, 4.08, 4.39, and 4.60 for 5, 10, 15, 20, and 25 g/L of talcum-added shake-flask fermentations, respectively. Similarly, the pH values were measured as 4.07, 4.64, 4.82, 4.93, and 5.02 for 5, 10, 15, 20, and 25 g/L of aluminum oxide-added shake flask, respectively, at 72 h of fermentation. Therefore, it can be concluded that while microparticles provide smaller pellet size and higher productivity, on the other hand in higher concentrations they can limit microbial growth and decrease the yield of fermentations.
Fermentations in bench-top bioreactor with talcum addition
Since higher phytase productions were obtained with addition of talcum compared to aluminum oxide in shake-flask fermentations, only talcum was studied for batch fermentations in bench-top bioreactor (Fig. 5). The highest phytase activity was measured as 6.49 U/ml in batch fermentations with 15 g/L of talcum addition, whereas phytase activity in the control was only 3.45 U/ml. The maximum phytase activities were obtained as 4.32 and 5.00 U/ml in 5 and 10 g/L talcum additions, respectively. Similar to the shake-flask fermentation, higher than 15 g/L talcum addition decreased phytase production. The highest phytase activities were obtained as 4.00 and 3.56 U/ml in 20 and 25 g/L of talcum additions, respectively, in the bioreactors. Therefore, the maximum phytase activity increased in the bioreactor by 88 % by 15 g/L of talcum addition compared to the control. Additionally, the maximum phytase activity in the bioreactor was 2.2-fold higher than the maximum phytase activity in shake-flask fermentation, which can be explained by the maintenance of pH at 4.5 and more effective agitation and aeration provided in the bioreactors compared to shake-flask fermentations. It was also calculated that the maximum production rate of phytase (steepest slope of the phytase activity curve between 36 and 96 h of fermentation) increased to 0.08 U/ml/h for 15 g/L of talcum addition compared to 0.036 U/ml/h for the control in the bioreactors. Similar to the shake-flask fermentation, the highest phytase activities were obtained at 96 h for all levels except for 5 g/L of talcum addition, while it was observed at 120 h of fermentation for the control (Fig. 5).
Conclusion
In this study, the effects of aluminum oxide and talcum were studied on phytase production with A. ficuum. Phytase activity remarkably increased by the addition of 15 g/L of aluminum oxide and talcum to 2.01 and 2.93 U/ml, respectively, compared to the control (1.02 U/ml) in shake-flask fermentations. Also, microparticle addition prevented bulk fungal growth in the fermentations. Additionally, it was determined that addition of talcum provided smaller fungal pellets compared to aluminum oxide because of smaller particle size. The highest phytase activity was measured as 6.49 U/ml by the addition of 15 g/L of talcum in bioreactors. Additionally, it was found that microparticle addition decreased the time needed to reach the highest phytase activity from 120 to 96 h. In conclusion, this study clearly demonstrated that the application of microparticles in fungal phytase productions was a novel approach, which could significantly enhance fungal phytase production in fermentations.
References
Vohra A, Satyanarayana T (2003) Phytases: microbial sources, production, purification, and potential biotechnological applications. Cr Rev Biotechnol 23:29–60
Haefner SR, Knietsch A, Scholten E, Braun J, Lohscheidt M, Zelde O (2005) Biotechnological production and applications of phytases. Appl Microbiol Biotechnol 68:588–597
Hurrell RF, Reddy MB, Juillerat MA, Cook JD (2003) Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am S Clin Nut 77:1213–1219
Sanson A, Etzion Z, Shanyp S, Berlyne GM, Yawl R (1981) Growth and bone mineralization as affected by dietary calcium, phytic acid and vitamin D. Comp Biochem Physiol 72:43–48
Mullaney EJ, Daly CB, Ulah AHJ (2000) Advances in phytase research. Adv Appl Microbiol 47:157–199
Nelson TS, Shieh TR, Wodzinski RJ, Ware JH (1968) The availability of phytate phosphorus in soybean meal before and after treatment with a mold phytase. Poult Sci 47:1842–1848
Sebastian S, Touchburn SP, Chavez ER, Lague PC (1996) The effects of supplemental microbial phytase on the performance and utilization of dietary calcium, phosphorus, copper, and zinc in broiler chickens fed corn-soybean diets. Poult Sci 75:729–736
Cowieson AJ, O’Neil HM, Bedford MR (2012) Enzymes beyond phytase in poultry nutrition, Poultry Research Foundation. Faculty of Veterinary Science, University of Sydney
Shah P, Bhavsar K, Soni SK, Khire JM (2009) Strain improvement and up scaling of phytase production by Aspergillus niger NCIM 563 under submerged fermentation conditions. J Ind Microbiol Biotechnol 36:373–380
Bhavsar K, Gujar P, Shah P, Kumar VR, Khire JM (2013) Combinatorial approach of statistical optimization and mutagenesis for improved production of acidic phytase by Aspergillus niger NCIM 563 under submerged fermentation condition. Appl Microbiol Biotechnol 97:673–679
Coban HB, Demirci A (2014) Improved submerged Aspergillus ficuum phytase production in bench-top bioreactors by optimization of fermentation medium. Acta Alimentari (In-print)
Coban HB, Demirci A (2014) Screening of phytase producers and optimization of culture conditions for submerged fermentation. Bioprocess Biosyst Eng 37:609–616
Kaup BA, Ehrich K, Pescheck M, Schrader J (2008) Microparticle-enhanced cultivation of filamentous microorganisms: increased chloroperoxidase formation by Caldariomyces fumago as an example. Biotechnol Bioeng 99:491–498
Driouch H, Wittmann C, Hansch R, Wucherpfennig T, Krull R (2012) Improved enzyme production by bio-pellets Aspergillus niger: targeted morphology engineering using titanate microparticles. Biotechnol Bioeng 109:462–471
Walisko R, Wittmann C, Krull R, Schrader J (2012) Microparticle based morphology engineering of filamentous microorganisms for industrial bio-production. Biotechnol Lett 34:1975–1982
Driouch H, Roth A, Dersch P, Wittmann C (2011) Filamentous fungi in good shape microparticles for tailor-made fungal morphology and enhanced enzyme production. Bioeng Bugs 2:100–104
Kim YO, Kim HK, Bae K, Yu JH, Oh TK (1998) Purification and properties of a thermostable phytase from Bacillus sp. DS11. Enz Microb Technol 22:2–7
Acknowledgments
This work was supported in part by the Turkish Ministry of Education which provided scholarship to Hasan Bugra Coban and the Pennsylvania Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coban, H.B., Demirci, A. & Turhan, I. Microparticle-enhanced Aspergillus ficuum phytase production and evaluation of fungal morphology in submerged fermentation. Bioprocess Biosyst Eng 38, 1075–1080 (2015). https://doi.org/10.1007/s00449-014-1349-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1349-4