Abstract
Schizophyllan is a homoglucan produced by the fungus Schizophyllum commune, with a β-1,3-linked backbone and β-1,6-linked side chains of single glucose units at every other residue. Schizophyllan is commercially produced for pharmaceutical and cosmetics uses. However, the unique physical properties of schizophyllan suggest that it may have biomaterials applications. Schizophyllan is conventionally produced by submerged culture fermentation using glucose as a carbon source. This study demonstrates for the first time the efficient utilization of agricultural biomass substrates, particularly distiller’s dried grains with solubles, for schizophyllan production. Sugar composition analysis, NMR, and permethylation linkage analysis confirmed that the recovered product was schizophyllan. Schizophyllan produced from agricultural residues was of a high molecular weight and exhibited solution viscosity properties similar to those of commercially produced material. Utilization of biomass substrates could reduce the cost of schizophyllan production and provide a new value-added bioproduct for integrated biorefineries of the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Schizophyllan is a polysaccharide produced by Schizophyllum commune, a white-rot fungus and ubiquitous mushroom. It is a homoglucan with a β-1,3-linked backbone and single β-1,6-linked glucose side chains at every third residue [18, 19]. Schizophyllan acts as a biological response modifier and a non-specific stimulator of the immune system. It is used in vaccines, anticancer therapies, and as a bioactive cosmetics ingredient. However, schizophyllan has many additional potential uses. Its unique physical properties of high viscosity, film formation, and thermal stability suggest bulk biomaterials applications. It has, for example, been tested for use in enhanced petroleum recovery [2, 19]. Schizophyllan can form oxygen-impermeable films for food preservation [21]. Currently, schizophyllan is produced from glucose and available only in small quantities of expensive purified pharmaceutical- and cosmetic-grade materials. Bulk biomaterials applications will call for less expensive production methods, and it may not be necessary to have highly purified products. The utilization of inexpensive agricultural biomass resources could reduce the cost of schizophyllan production. The biorefineries of the future will seek to convert agricultural biomass substrates to fuels and value-added chemicals in a process analogous to today’s petroleum refineries. Bio-based polymers derived from agricultural biomass can enhance the economic viability of biorefineries and reduce the need for imported petroleum by providing substitutes for traditional petroleum-based products.
Although glucose is used in conventional production of schizophyllan, S. commune can utilize a number of sugars and soluble starch for polysaccharide production [10]. Shu and Hsu [22] showed that schizophyllan could be produced from mixed sugars in a detoxified acid hydrolysate of rice hulls. S. commune is well known for production of biomass degrading enzymes, including xylanases and cellulases, and can directly utilize biomass resources [3, 4]. Despite this, little work has been done on schizophyllan production from biomass substrates. Steiner et al. [23] optimized cultures of S. commune for cellulase and xylanase production on purified microcrystalline cellulose, and observed that it also made low amounts of schizophyllan (0.02 g schizophyllan per g cellulose). Leathers et al. [13] reported that S. commune grew well on pretreated corn fiber and produced approximately 0.05 g schizophyllan per g substrate. Gao and Zhou [6] reported that solid-state fermentation of corn bran produced 0.05 g schizophyllan per g substrate.
The current study compares untreated corn fiber, pretreated corn fiber, distiller’s dried grains with solubles (DDGS), and soybean hulls as substrates for schizophyllan production. Conditions are determined for efficient schizophyllan production from these substrates, particularly DDGS, and the recovered product is characterized. Results indicate that agricultural biomass substrates are promising for schizophyllan production.
Materials and methods
Agricultural biomass substrates and culture media
Corn DDGS was obtained from the National Corn-to-Ethanol Research Center (NCERC, Edswardville, IL, USA). Soybean hulls were the kind gift of Archer Daniels Midland (ADM, Galesburg, IL, USA). Corn fiber was the kind gift of Aventine Renewable Energy, Pekin, IL, USA. All biomass substrates were baked to dryness and used directly or, in the case of corn fiber, after pretreatment with alkaline hydrogen peroxide [11].
Biomass substrates were suspended at 1.0 % (w/v) in either yeast extract (YE) or malt extract (ME) basal medium. YE contained 0.3 % (w/v) yeast extract, 0.1 % (w/v) KH2PO4, and 0.05 % (w/v) MgSO4.7H2O. ME contained 2.0 % (w/v) malt extract and 0.1 % (w/v) peptone. ME agar slants contained 2.0 % (w/v) glucose and 2.5 % (w/v) agar.
Strain and culture conditions
Schizophyllum commune ATCC 38548 was grown on ME agar slants (with 2.0 % (w/v) glucose) at 28 °C for 7–10 days. An approximately 7 × 7 mm square of mycelia was used to inoculate 250 ml of ME basal medium with 2.0 % (w/v) glucose in a 500-ml fluted Erlenmeyer flask with three 10-mm glass beads. This preinoculum culture was incubated at 240 rpm for 4–5 days at 30 °C. Experimental cultures containing 1.0 % (w/v) of agricultural biomass substrates in 150 ml of either YE or ME basal medium in 500-ml flasks were inoculated with 1.5 ml of preinoculum (1.0 % v/v) and incubated at 240 rpm for up to 14 days at 30 °C. All experiments were carried out in triplicate and standard deviations are shown.
Isolation of polysaccharide
Whole culture suspensions were transferred into 400-ml centrifuge bottles, homogenized (Power Gen 700, Fisher Scientific) for 20 s, then centrifuged at 8,000 rpm for 1 h at 4 °C. The supernatant was collected and the mycelium and substrate residues were resuspended in an additional 100 ml of deionized water, homogenized, and centrifuged as before. The mycelium and substrate residues were transferred to tared aluminum foil dishes and dried under vacuum for 48 h at 60 °C. The supernatants were combined and one volume of 95 % ethanol was added. After 1 h at 4 °C, precipitates were collected by centrifugation at 8,000 rpm for 1 h at 4 °C. Polysaccharide precipitates were air-dried overnight to reduce the ethanol content and then lyophilized.
Biomass composition analyses
The composition of agricultural biomass substrates was determined as previously described [14]. Approximately 30 mg of agricultural biomass was suspended in 3 ml of 2 N trifluoroacetic acid (TFA) and incubated for 1 h at 120 °C and then air dried to completion. Hydrolysates were dissolved in 2 ml of nanopure water, filtered through 0.2-μm filters, and sugars were resolved by HPLC using an Aminex (Bio-Rad Laboratories, Hercules, CA, USA) fermentation monitoring column (150 × 7.8 mm) eluted with 2.4 mM nitric acid at 0.5 ml/min and 35 °C, with detection by refractive index. Results are presented as means with standard deviations.
Sugar composition of schizophyllan
The sugar composition of isolated schizophyllan was determined as previously described [13]. Lyophilized polysaccharide was hydrolyzed and aldonitrile acetate derivatives were prepared by adapting the method of Price [17]. Approximately 30 mg of schizophyllan was hydrolyzed in 2 N TFA as described above. Hydroxylamine pyridine reagent (0.5 ml) was added and the sample was stirred in a heating block at 60 °C for 1 h prior to the addition of 0.5 ml acetic anhydride. The sample was acetylated for another 30 min. The reaction was quenched with water and extracted with ethyl acetate. The organic layer was analyzed by GC/MS using a Hewlett-Packard 6890 N gas chromatograph equipped with an HP 7683 autoinjector. The GC was interfaced with an HP 5973 Series mass spectrometer configured in electron impact (EI) mode. Chromatography was accomplished with a caterpillar HP-1 column (25 m; 0.2 mm). Helium was used as the carrier. The oven temperature was ramped over a linear gradient from 150 to 250 °C at 4 °C/min. Mass spectra were recorded in positive-ion mode over the range 50–500 mass units. Commercial schizophyllan (cosmetic grade) used as a standard was purchased from European Technologies, Inc. (Denver, CO., USA).
Schizophyllan linkage analysis
Prior to hydrolysis and acetylation, permethylation was performed by adapting the method of Hakamori [7]. Briefly, approximately 10–30 mg lyophilized polysaccharide was dissolved in 1.6 ml dimethyl sulfoxide. The solution was stirred with a magnetic stirrer overnight at ambient temperature. To the solution, 1.5 ml of methyl iodide was added, followed by 190–200 mg of anhydrous sodium hydroxide. This solution was further stirred for 15 min at ambient temperature. The reaction mixture was diluted with water and the methylated product was extracted with chloroform twice. The organic layer was washed with water prior to evaporation under an air stream. The methylated product was hydrolyzed, acetylated, and analyzed by GC/MS as described above.
NMR analysis
Solution NMR spectra were recorded on a Bruker AMX 500 spectrometer at normal probe temperature with standard instrument settings. Deuterated dimethyl sulfoxide (d6-DMSO) was used as the solvent. All chemical shifts were referenced to tetramethylsilane at 0 ppm.
Molecular weight determinations
Polysaccharide molecular weights were determined by size exclusion chromatography as previously described [12]. Briefly, approximately 10 mg of lyophilized polysaccharide were dissolved in 1 ml of nanopure water. The sample solution was filtered through a 0.45-μm filter (Pall, Port Washington, NY, USA), applied to a Shodex SB-806M high-performance size exclusion chromatography (HPSEC) column (Showa Denko, Tokyo, Japan) and eluted with 0.05 M sodium nitrate at a flow rate of 0.5 ml/min. The column was calibrated with a set of eight pullulan molecular weight standards ranging from 5.8 × 103 to 1.66 × 106 Da (Showa Denko, Tokyo, Japan). Separations were monitored using a Shodex OR-1 optical rotation detector (Showa, Denko).
Solution viscosity
Polysaccharide solution viscosity was measured using a TA Instruments (New Castle, DE, USA) ARES LS-1 controlled strain rheometer with a 25-mm titanium parallel plate. All tests were performed at 25 °C using a Peltier plate. Dynamic frequency sweeps (100−0.1 rad/s) at 1 % strain (dynamic strain sweep linear range) were used to determine complex viscosity, G′ (elastic modulus) and G˝ (viscous modulus) of this gel. Steady-rate sweeps were used to determine the viscosity of samples from 0.01 to 100 s−1. The Cross model was used to determine the zero shear viscosity, which was 460 Pa.s.
Surface activity
Surface activity was determined using the pendant drop method [5]. Samples were analyzed using the FTA 4000 surface tension instrument (First Ten Angstroms Inc., Portsmouth, VA, USA). Measurements were made using 22-gauge blunt needles with 7-μl drops. The reported values are the average of triplicate cultures.
Results and discussion
Composition of agricultural biomass substrates
Currently, schizophyllan is commercially produced for cosmetic and pharmaceutical applications. For such high-value uses, it is reasonable to employ purified glucose as a fermentation carbon source. For potential bulk biomaterials applications, it may be desirable to utilize lower-cost agricultural biomass substrates. In the current study, untreated and pretreated corn fiber, DDGS, and soybean hulls are tested as substrates for schizophyllan production. Corn fiber is an operational term used to describe a specific, abundant coproduct of the corn wet milling process, primarily composed of the seed pericarp and adherent starch [8]. Corn fiber may be combined with steep liquor and/or stillage residues to form corn gluten feed for cattle. DDGS is an abundant coproduct of the dry grind process for fuel ethanol production from corn [20] and primarily sold as animal feed. Soybean hulls are an abundant coproduct of soy processing [16]. Hulls may be blended into soybean meal for feed or discarded.
Corn fiber contains about 20 % glucose, 16 % arabinose, and 29 % xylose by dry weight (Table 1). Glucose revealed by this assay would include adherent starch and a portion of cellulose hydrolyzed by TFA. The glucose content of corn fiber will vary somewhat depending on the particular batch, since the efficiency of starch removal varies depending on processing conditions. Pretreatment of corn fiber with alkaline hydrogen peroxide enhances the accessibility of cellulose and hemicellulose to enzymes but is not expected to alter the polysaccharide composition [11], and little or no difference was found between untreated and pretreated corn fiber (Table 1). DDGS contains corn pericarp arabinoxylan and also shows the characteristic 2:1 ratio of xylose to arabinose (Table 1). However, this preparation of DDGS contained much less glucose than corn fiber, reflecting a lower starch content. Soybean hulls also included glucose, xylose, and arabinose (Table 1). S. commune can utilize these sugars as well as soluble starch for polysaccharide production [10].
Production of schizophyllan from agricultural biomass substrates
Schizophyllan for pharmaceutical and cosmetic applications is produced from glucose [18]. Yields are about 0.33 g schizophyllan per g glucose in a basal medium containing yeast extract. A previous study showed coproduction of schizophyllan and arabinoxylan from 1.0 % w/v alkaline hydrogen peroxide-pretreated corn fiber in a defined basal medium, with yields of 0.05 g schizophyllan and 0.2 g arabinoxylan per g corn fiber [13]. Similar low yields of schizophyllan were previously reported from other biomass substrates [6, 23]. In the current work, untreated corn fiber, pretreated corn fiber, DDGS, and soybean hulls are compared as substrates for schizophyllan production. Two different basal media were compared for this study. Yeast extract medium (YE) is conventionally used for production of schizophyllan on glucose [18]. ME basal medium is used (with 2.0 % (w/v) glucose) for preparation of the S. commune preinoculum.
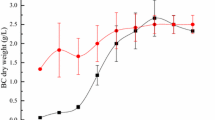
Cultures were grown in either YE or ME basal medium containing 1.0 % (w/v) agricultural biomass substrates, and total biomass and extracellular polysaccharide yields (dry weight) were measured over a 14-day time course (Fig. 1). Total biomass includes the weight of both insoluble residual agricultural substrates and fungal biomass. In YE, total biomass yields showed gradual losses over time, suggesting that all four substrates were utilized to some degree (Fig. 1a). Fungal growth was apparent in YE cultures with agricultural substrates. However, no growth or biomass accumulation took place in cultures containing YE with no added carbon source (Fig. 1a). ME basal medium cultures showed sequential decreases and increases in total biomass, reflecting net differences between the solubilization of agricultural biomass substrates and fungal growth (Fig. 1b). Increases in total biomass in late cultures may also reflect reutilization of schizophyllan produced up to that point. Fungal growth was obviously heavier in these ME basal medium cultures than in the corresponding YE cultures. Furthermore, S. commune showed growth in medium containing ME plus glucose, and even in ME with no added carbon source (Fig. 1b). ME basal medium contains 2.0 % malt extract, which includes about 60 % reducing sugars, particularly maltose [1].
S. commune cultures were grown in either YE (a, c) or ME (b, d) basal medium containing 1.0 % (w/v) agricultural biomass substrates, and total biomass (a, b) and extracellular polysaccharide (c, d) yields (dry weights) were measured over 14 days (YE yeast extract basal medium, ME malt extract basal medium, AHPCF alkaline hydrogen peroxide pretreated-corn fiber, UCF untreated corn fiber, DDGS distiller’s dried grains with solubles, SBH soybean hulls)
In YE medium, S. commune produced maximal yields of approximately 2.2 g extracellular polysaccharide/liter at day 6 from pretreated corn fiber, and approximately 1.1 g polysaccharide/liter at day 4 from DDGS (Fig. 1c). Since cultures contained 1.0 % substrates, these yields are equivalent to 0.22 and 0.11 g polysaccharide/gram substrate, respectively, somewhat greater than previously reported schizophyllan yields from agricultural biomass [6, 13, 23]. Maximal extracellular polysaccharide yields from untreated corn fiber, soybean hulls, and no carbon controls in YE were approximately 0.5 g/l, at days 8, 2, and 12, respectively (Fig. 1c).
S. commune cultures in ME basal medium produced much higher maximal yields of extracellular polysaccharide than did cultures in YE (Fig. 1d). Cultures containing untreated corn fiber or glucose produced similar yields of 10.8 g polysaccharide/liter at days 8 and 10, respectively. Pretreated corn fiber produced approximately 18.5 g/l at day 14, while cultures with DDGS produced 15.4 g/l at day 8. Cultures with no added carbon source produced 7.4 g/l at day 14, presumably reflecting the contribution of maltose in malt extract. Interestingly, cultures grown on soybean hulls in ME produced very little polysaccharide (maximal yield of about 0.9 g/l at day 4), much less than ME basal medium with no added substrate. This suggests that soybean hulls are actually inhibitory to schizophyllan production. Although pretreated corn fiber produced the highest yields of polysaccharide, DDGS is the most promising substrate for schizophyllan production, because pretreatment of corn fiber adds considerable cost to the process. Maximal yields also occurred earlier (day 8) on DDGS than on pretreated corn fiber (day 14), saving production time.
Since cultures produced approximately 5.8 g exopolysaccharide/liter in ME basal medium with no added carbon source at day 8, this would imply that cultures produced an additional 9.6 g/l polysaccharide from DDGS, or 0.96 g polysaccharide/g DDGS. This 96 % conversion rate seems unlikely, suggesting that there may be synergistic co-utilization of DDGS and malt extract in ME basal medium. Besides hemicelluloses and cellulose, DDGS contains protein, amino acids, and other nutrients derived from corn and yeast [15]. These nutrients could facilitate more complete utilization of the malt extract in ME basal medium.
Effect of medium components on schizophyllan production from DDGS in ME
The effect of medium components was further investigated in cultures grown for 8 days on modified ME basal media. As reported above, cultures grown on 1.0 % DDGS in standard ME basal medium produced more than 15 g extracellular polysaccharide/liter at day 8, while those grown on ME with no added carbon source produced nearly 6 g/l polysaccharide (Table 2). Cultures grown on modified medium containing DDGS but no malt extract produced only about 0.6 g polysaccharide/liter, proving that malt extract is essential for the utilization of DDGS. Cultures grown on DDGS with half-strength malt extract produced about 3.0 g polysaccharide/liter, still much less than that produced from DDGS with standard ME (Table 2). Cultures grown on medium with no peptone produced less than a third of the polysaccharide produced from DDGS with standard ME, showing that this component is also important, probably as a nitrogen source. Thus, it appears that standard ME basal medium is a particularly well suited for production of schizophyllan from DDGS.
Chemical characterization of schizophyllan produced from DDGS
A time course of S. commune growth on DDGS in ME basal medium showed the gradual appearance of extracellular polysaccharide over time, suggesting that schizophyllan was synthesized de novo (Fig. 1). However, it is also possible that degradative enzymes from S. commune gradually liberated polysaccharides, particularly starch and arabinoxylan, from DDGS. Indeed, culture supernatants of S. commune grown under other conditions on pretreated corn fiber were found to contain four times as much arabinoxylan as schizophyllan [13]. Thus, it was important to perform chemical characterizations of the extracellular polysaccharides produced in the current study.
The monosaccharide components of extracellular polysaccharides produced by S. commune grown on DDGS in ME were analyzed by GC/MS after hydrolysis (Table 3). Results were compared with those from a sample of commercial schizophyllan. Both samples were primarily composed of glucose, characteristic of schizophyllan. Importantly, polysaccharides appeared to contain a low percentage of xylose and arabinose, suggesting little contamination by arabinoxylan.
Extracellular polysaccharides produced on DDGS in ME were also analyzed by Heteronuclear Single Quantum Coherence-NMR (HSQC-NMR, Fig. 2). As shown, the spectra were essentially identical to a commercial standard. The HSQC 1H-13C correlation spectrum of this polysaccharide precipitated from 50 % (v/v) ethanol showed two anomeric sugar signals at 4.55 ppm and 4.20 ppm, due to the β-1,3-linked glucose and β-1,6-linked glucose, respectively [9]. These signals were clear in the HSQC spectrum, and are correlated to overlapping 13C signals at 103.4 ppm, consistent with β-linked glucosyl residues. Other carbohydrate signals are apparent in the 2.7–4.0 ppm region for 1H, and 55–85 ppm for 13C nuclei. Characteristic methylene –CH2 signals are apparent at 3.40 and 3.60 ppm, coupled to a single 13C signal at 61 ppm. These are assigned to the C-6 position of the backbone glucose residues carrying a 1,6-Glc branch. These data are consistent with the isolated polysaccharide being schizophyllan.
The NMR data were confirmed by carbohydrate permethylation linkage analysis (Fig. 3). As analyzed by GC/MS, two peaks were detected at 7.10 and 9.08 min that integrated to 56.4 and 43.6 %, respectively. Analysis of the mass fragmentations showed that these peaks are due to 2,4,6-methylglucose and 2,4-methylglucose, arising from the 3-linked and 3,6-linked sugars of the schizophyllan backbone. A larger peak was also observed at 5.91 min due to 2,3,4,6-tetramethylglucose, arising from the terminal branched glucose residues. Taken together with the NMR analysis, these data indicated that the precipitated polysaccharide is schizophyllan.
Physical characterization of schizophyllan produced from DDGS
HPSEC was used to characterize the molecular weight of schizophyllan produced from DDGS in ME. Schizophyllan was predominantly represented by a high molecular weight peak of 1.3 × 107. Commercial schizophyllan similarly showed peaks consistent with literature values of 6–12 × 106 [18].
The solution viscosity properties of 0.6 % (w/v) aqueous solutions of DDGS-derived and commercial schizophyllan also were found to be identical or nearly identical (Fig. 4). Aqueous solutions of schizophyllan exhibit pseudoplastic flow behavior, characterized by decreasing viscosity with increasing shear rates [18].
The interfacial tension of 0.6 % (w/v) aqueous solutions of DDGS-derived and commercial schizophyllan were 69.7 ± 2.1 and 61.9 ± 4.4 dy/cm, respectively (standard deviations shown). Values represent an equilibrium surface tension determined 60 s after drop formation and are not significantly different. By comparison, pure water provides an interfacial tension of 72 dy/cm [5]. This means that, as expected, schizophyllan solutions have little or no surface activity. More importantly, DDGS-derived schizophyllan appears to be equivalent to commercial schizophyllan with regard to surface activity, and it does not contain contaminants that modify surface activity.
In conclusion, agricultural biomass substrates, particularly distiller’s dried grains with solubles (DDGS), can be used efficiently for the production of the polysaccharide schizophyllan. The recovered DDGS-derived polysaccharide was confirmed to be schizophyllan by chemical and structural analyses, and solutions of this material showed high molecular weight and solution viscosity properties similar to those of commercial schizophyllan. The use of low-cost biomass substrates could reduce the cost of schizophyllan production for bulk biomaterials applications such as in biodegradable films and in enhanced oil recovery.
References
Anonymous (1998) Difco Manual, 11th edn. Difco Laboratories, Sparks
Anonymous (2011) Fungus could boost crude output by 45 %. Petrol. Econ. June 2011, General OneFile, Web, 20 June 2012
Biely P, MacKenzie CR, Schneider H (1988) Production of acetyl xylan esterase by Trichoderma reesei and Schizophyllum commune. Can J Microbiol 34:767–772
Desrochers M, Jurasek L, Paice MG (1981) Production of cellulase, β-glucosidase, and xylanase by Schizophyllum commune grown on a cellulose-peptone medium. In: Underkofler LA, Wulf ML (eds) Developments in industrial microbiology, vol 22. Society for Industrial Microbiology, Arlington, pp 675–684
Dunlap CA, Schisler DA, Price NP, Vaughn SF (2011) Cyclic lipopeptide profile of three Bacillus subtilis strains; antagonists of Fusarium head blight. J Microbiol 49:603–609
Gao L, Zhou S (2008) Optimization of extraction technology of schizophyllan produced by solid fermentation. Shipin Gongye Keji 29:214–216
Hakamori S (1964) A rapid permethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J Biochem Tokyo 55:205–208
Johnson LA, May JB (2003) Wet milling: the basis for corn biorefineries 2nd Edition. In: White PJ, Johnson LA (eds) Corn: chemistry and technology. American Association of Cereal Chemists, St. Paul, pp 449–494
Kim Y-T, Kim E-H, Cheong C, Williams DL, Kim C-W, Lim S-T (2000) Structural characterization of β-D-(1→3, 1→6)-linked glucans using NMR spectroscopy. Carbohydr Res 328:331–341
Kumari M, Survase SA, Singhal RS (2008) Production of schizophyllan using Schizophyllum commune NRCM. Biores Technol 99:1036–1043
Leathers TD, Gupta SC (1996) Saccharification of corn fiber using enzymes from Aureobasidium sp. strain NRRL Y-2311-1. Appl Biochem Biotechnol 59:337–347
Leathers TD, Nunnally MS, Cote GL (2010) Optimization of process conditions for enzymatic modification of alternan using dextranase from Chaetomium erraticum. Carbohydr Pol 81:732–736
Leathers TD, Nunnally MS, Price NPJ (2006) Co-production of schizophyllan and arabinoxylan from corn fiber. Biotechnol Lett 28:623–626
Leathers TD, Price NPJ (2007) Effect of oil extraction method on enzymatic digestibility of corn germ arabinoxylan. Cereal Chem 84:243–245
Loy DD, Wright KN (2003) Nutritional properties and feeding value of corn and its by-products. In: White PJ, Johnson LA (eds) Corn: chemistry and technology, 2nd edn. American Association of Cereal Chemists, St. Paul, pp 571–603
Lusas EW (2004) Soybean processing and utilization. In: Boerma HR, Specht E (eds) Soybeans: improvement, production, and uses, 3rd edn. American Society of Agronomy, Madison, pp 949–1046
Price NPJ (2004) Acylic sugar derivatives for GC/MS analysis of 13C-enrichment during carbohydrate metabolism. Anal Chem 76:6566–6574
Rau U (1999) Production of schizophyllan. In: Bucke C (ed) Methods in biotechnology, carbohydrate biotechnology protocols, vol 10. Humana Press, Totowa, pp 43–55
Rau U (2002) Schizophyllan. In: Vandamme EJ, De Baets S, Steinbuchel A (eds) Biopolymers polysaccharides II: polysaccharides from eukaryotes, vol 6. Wiley-VCH, Weinheim, pp 61–91
Rosentrater KA, Ileleji K, Johnston DB (2012) Manufacturing of fuel ethanol and distillers grains: current and evolving processes. In: Liu K, Rosentrater KA (eds) Distillers grains: production, properties, and utilization. CRC Press, Boca Raton, pp 73–102
Schulz D, Rau U, Wagner F (1992) Characteristics of films prepared from native and modified branched β-1,3-D-glucans. Carbohydr Pol 18:295–299
Shu C, Hsu H (2011) Production of schizophyllan glucan by Schizophyllum commune ATCC 38548 from detoxificated hydrolysate of rice hull. J Taiwan Inst Chem Eng 42:387–393
Steiner W, Lafferty RM, Gomes I, Esterbauer H (1987) Studies on a wild strain of Schizophyllum commune: cellulase and xylanase production and formation of the extracellular polysaccharide schizophyllan. Biotechnol Bioeng 30:169–178
Acknowledgments
Expert technical assistance was provided by Erika Hertenstein, Suzanne Unser, Trina Hartman, Karl E. Vermillion, and Andrew J. Thomas. The authors sincerely thank Christopher Dunlap for surface tension measurements. This project was supported by Agriculture and Food Research Initiative Competitive Grant no. 2010-65504-20377 from the USDA National Institute of Food and Agriculture.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of any trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Rights and permissions
About this article
Cite this article
Sutivisedsak, N., Leathers, T.D., Nunnally, M.S. et al. Utilization of agricultural biomass in the production of the biopolymer schizophyllan. J Ind Microbiol Biotechnol 40, 105–112 (2013). https://doi.org/10.1007/s10295-012-1208-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1208-8