Abstract
To improve the efficiency of ε-poly-l-lysine (ε-PL) production by Streptomyces sp. M-Z18, batch and fed-batch fermentations with glucose and glycerol (co-fermentations) were performed. The batch fermentations showed that the initial ratio of glucose to glycerol plays an important role in glucose/glycerol co-fermentation. The optimal glucose/glycerol weight ratio was 30/30; this resulted in a maximum ε-PL productivity of 5.26 g/L/d. Glucose and glycerol were consumed synergistically during the co-fermentation process, and the length of time during which the substrate was exhausted was significantly shortened compared with the single carbon source fermentation. Under optimized conditions, fed-batch fermentations with glucose and glycerol as a mixed carbon source achieved maximum ε-PL concentration and productivity values of 35.14 g/L and 4.85 g/L/d, respectively. These values were respectively 1.43- and 1.39-, and 1.17- and 1.16-folds higher than those obtained from fermentations with glucose and glycerol as single carbon sources. The present study is the first to suggest that glucose/glycerol co-fermentation may be an efficient strategy for ε-PL production by Streptomyces sp. M-Z18.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
ε-Poly-l-lysine (ε-PL), a naturally occurring homopolymer of Streptomyces or Kitasatosporia, consists of several residues of l-lysine with an isopeptide linkage between the α-carboxyl and ε-amino groups. ε-PL has recently attracted significant attention due to its wide antimicrobial spectrum and high safety. ε-PL shows a strong antimicrobial activity against Gram-positive and Gram-negative bacteria, fungi, and some viruses [1, 2]. Moreover, the safety of ε-PL has been demonstrated in experiments using rats; thus, the use of ε-PL in cooked or sushi rice was approved in 2004 by the US Food and Drug Administration (FDA) [3, 4]. ε-PL is widely used as a natural food preservative in a number of countries, including Japan, the United States, and Korea [5, 6]. There has been a great interest in the medical applications of ε-PL and its derivatives, among others. Furthermore, ε-PL has been applied as an emulsifying agent, disinfectant, drug delivery carrier, endotoxin remover, and biosensor [7].
Fermentation is almost the sole known method for ε-PL production. Many studies on ε-PL biosynthesis have focused on the use of glucose as a carbon source. M3G, the common medium for ε-PL production in the flask- or fermenter-scale, consists of 5% glucose as a sole carbon source [8–12]. In a combined two-stage pH control strategy for feeding 1% glucose fermentation technology, Streptomyces albulus S410 achieved the highest ε-PL concentration at 48.3 g/L; this record remains up to the present [10]. The same strategy was successfully applied in S. albulus IFO 14147 and S. albulus TUST2 [11, 13]. Zhang et al. [12] recently performed repeated fed-batch experiments with immobilized cells of Kitasatospora sp. MY 5-36 in loofah sponge using glucose as a carbon source. Glycerol and glucose were proven to be the best carbon sources by Shima and Sakai [14] through medium composition optimization experiments in 1981. The positive effect of glycerol as a carbon source in ε-PL production was also observed by Bankar and Singhal [15]. However, glycerol as a carbon source for ε-PL production has only been used in flask experiments, probably because glycerol as a raw material is more expensive than glucose. Today, glycerol is co-produced at a weight ratio of about 10% of the biodiesel [16]. Therefore, ε-PL has the economic feasibility of fermentation from glycerol, and the production of ε-PL from glycerol is beneficial to the production of low molecular weight ε-PL [17, 18]. ε-PL with fewer lysine residues (larger than ten) was found to exhibit a more pleasant taste when used as food preservative [19]. Recently, we determined industrial glycerol to be a carbon source for ε-PL production by medium optimization technology using Streptomyces sp. M-Z18 [20]. We also developed a novel two-stage pH control strategy, and improved ε-PL production from glycerol based on the analysis of kinetic parameters [21].
Based on the above-mentioned discussion, ε-PL production has been performed using glucose and glycerol as individual carbon sources. However, only a few reports regarding the influence of glucose and glycerol as a mixed carbon source on ε-PL production have been published. In the current study, we attempt to develop a glucose/glycerol co-fermentation strategy for enhancing ε-PL concentration and productivity by Streptomyces sp. M-Z18.
Materials and methods
Microorganism
Streptomyces sp. M-Z18 was isolated from the soil as described by Nishikawa and Ogawa [22], preserved in our lab, and subjected to ultraviolet and nitrosoguanidine mutagenesis as described by Hiraki et al. [8].
Media and culture conditions
The agar slant medium contained the following (g/L): glucose, 10; yeast extract, 5; beef extract, 5; MgSO4·7H2O, 0.5; K2HPO4, 1; agar, 20. The pH was adjusted to 7.0. The pre-culture medium, M3G, consisted of the following (g/L): glucose, 50; yeast extract, 5; (NH4)2SO4, 10; KH2PO4, 1.36; K2HPO4, 0.8; MgSO4·7H2O, 0.5; ZnSO4·7H2O, 0.04; FeSO4·7H2O, 0.03. The initial pH of the medium was adjusted to 6.8 with 2 M NaOH and/or 1 M H2SO4. The fermentation medium consisted of the following (g/L): industrial glycerol (Kerry Oleochemical Industrial (Shanghai) Co., Ltd.), 60; (NH4)2SO4, 5; beef extract, 10; KH2PO4, 4; MgSO4·7H2O, 0.8; FeSO4·7H2O, 0.05. The media were sterilized in an autoclave for 20 min at 121 °C. Mixed carbon source fermentations were performed with the fermentation medium described above, except that glycerol was replaced with 60 g/L of the mixed carbon source (glucose + glycerol). To prevent Maillard reactions from occurring, the glucose in the agar slant and the M3G medium were separately autoclaved.
The strain maintained on the agar slants was incubated at 30 °C for 5–7 days. After growth and sporulation, two loopfuls of Streptomyces sp. M-Z18 spores were transferred to 80 mL of the M3G medium in a 500-mL flask and incubated at 30 °C and 200 rpm for 24 h on a rotary shaker. This culture was used as the seed in subsequent experiments.
Batch and fed-batch fermentations were performed in a 5-L jar-fermenter (Baoxing Corp., Shanghai, China) with a working volume of 3.5 L. About 300 mL of the pre-cultured seed was inoculated into 3.2 L of the sterilized fermentation medium at an initial pH of 6.8. Two Rushton turbines provided agitation, which varied from 200 to 900 rpm, to control dissolved oxygen (DO) to 10–30%, as monitored by a DO electrode (Mettler Toledo). Aeration was provided by a ring sparger with a range of 0.5 − 3.0 vvm. The pH change was detected by a pH electrode (Mettler Toledo) during cultivation. The fermentation temperature was maintained using a re-circulating water bath at 30 °C.
Glucose and glycerol co-fermentation of ε-PL in batch cultures
To investigate the effect of glucose and glycerol as a mixed carbon source on ε-PL production, three weight ratios of glucose to glycerol were chosen (i.e., 40/20, 30/30, and 20/40). In these three combinations, the total volume of carbon sources was 60 g/L. To prevent Maillard reactions from occurring, glucose was added separately after sterilization. During the cultivation, when the pH naturally dropped from 6.8 to 3.8, 12.5% (v/v) NH4OH solution was added to the culture broth to maintain the pH at 3.8 until cultivation was completed.
Glucose and glycerol co-fermentation of ε-PL in fed-batch cultures
To enhance the production of ε-PL, fed-batch fermentation was carried out via glucose/glycerol co-fermentation at a weight ratio of 30/30. When the concentrations of both glucose and glycerol were below 5 g/L, sterilized pure glycerol and 800 g/L of glucose were added to the culture broth using a peristaltic pump at set rates, to maintain glucose or glycerol levels at about 5 g/L, and maintain the amount of the total carbon source at about 10 g/L. Residual ammonia nitrogen (NH3-N) was also maintained at about 1 g/L by feeding (NH4)2SO4 solution during the fed-batch fermentation. The pH control strategy was similar to that used during batch fermentation.
Analyses of cell mass, ε-PL, glucose, glycerol, and NH3-N
The samples were withdrawn from the fermenter for analysis at regular intervals. The broth was centrifuged (4,500×g, 10 min), and the precipitate was collected, washed twice with distilled water, and then dried at 105 °C to a constant weight to determine the biomass of the culture. The supernatant was used to determine the ε-PL concentration according to the procedure described by Kahar et al. [10]. The concentrations of glucose and glycerol were determined using an HPLC system (DIONEX, U-3000, US) with a refractive index detector (Shodex RI-101, Japan) and an ion exchange column (Aminex HPX-87H, 300 × 7.8 mm, Hercules, CA). The column was eluted with 5 mM H2SO4 at a temperature of 60 °C and a flow rate of 0.6 mL/min. NH3-N was analyzed by a calorimetric method using Nessler reagent [23].
Results and discussion
Effect of single carbon sources (glucose or glycerol) on ε-poly-l-lysine fermentation by Streptomyces sp. M-Z18
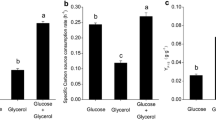
To evaluate the effect of glucose and glycerol on ε-PL production, batch cultures performed with Streptomyces sp. M-Z18 were carried out on 60 g/L of glucose or glycerol. The maximum concentrations of ε-PL in the fermentation broth using glycerol and glucose as single carbon sources were 7.22 and 5.0 g/L, respectively (Fig. 1). ε-PL productivity and yield were 2.86 g/L/d and 0.083 g/g, respectively, when glucose was used as a carbon source. With glycerol, both values were higher (3.89 g/L/d and 0.117 g/g, respectively) (Table 1). Glycerol appears to be superior to glucose for ε-PL fermentation. However, when the substrate exhaustion time and the time at which the pH dropped from 6.8 to 3.8 were considered, glucose was the preferred carbon source. When glucose was used, the substrate exhaustion time and the time at which the pH dropped were 42 and 11.28 h, respectively, shorter by 1.50 and 3.27 h, respectively, than when glycerol was used (Table 1). ε-PL production was promoted when the pH naturally dropped to the optimal value at the soonest possible time, which mainly resulted from the degradation of NH4 + [20]. Furthermore, Streptomyces sp. M-Z18 cells grew slightly better on glucose than on glycerol during fermentation (Fig. 1). Thus, investigating the effects of glycerol and glucose mixtures on ε-PL production by Streptomyces sp. M-Z18 is logical.
Comparison between glucose and glycerol as carbon sources for cell growth and ε-PL production in a 5-L fermenter by Streptomyces sp. M-Z18. Filled symbols and solid lines are used for the glucose medium: glucose (filled squares), cell growth (filled triangles), ε-PL production (filled circles), and pH (solid lines). Empty symbols and dashed lines are used for the glycerol medium: glycerol (empty squares), cell growth (empty triangles), ε-PL production (empty circles), and pH (dotted lines)
Considering ε-PL concentration, productivity, and yield, glycerol appeared to be a better carbon source than glucose. Several investigations indicate that glycerol is a more efficient carbon source than glucose for the production of some microbial products, such as propionic acid and lycopene [24, 25]. However, the specific reasons behind why glycerol is better than glucose as a carbon source for ε-PL production have yet to be completely elucidated. Based on the above-mentioned results, as well as summarized literature data, glycerol appears to be superior to glucose for ε-PL production because of the following possible effects: glycerol and l-lysine essentially have the same redox state (reductance degree, 4.67), and can thus maintain a redox balance without the production of another compensating metabolite [26]. In contrast, l-lysine production from glucose (reductance degree, 4) necessarily leads to the formation of a more oxidized co-metabolite to achieve the redox balance. Thus, a portion of the carbon flow is directed towards co-metabolite formation from glucose, resulting in lower ε-PL yields. This hypothesis is consistent with our experimental results, which show that the ε-PL yield from glycerol was enhanced by 41% compared with that from glucose (0.117 vs. 0.083 g/g) (Table 1).
Effect of the ratio of glucose to glycerol in mixed carbon source fermentation on ε-PL production during batch fermentation
To evaluate the effect of the ratio of glucose to glycerol on ε-PL production, batch cultivations with initial weight ratios of glucose to glycerol of 40/20, 30/30, and 20/40 (w/w) were conducted. The results of the experiment indicated that the initial glucose/glycerol weight ratio plays an important role in glucose/glycerol co-fermentation (Fig. 2). As shown in Fig. 2a, the times at which the pH dropped from 6.8 to 3.8 in the mixed carbon source fermentations were all shortened to about 12 h. In particular, at 30/30 glucose/glycerol, the pH dropped fastest. To monitor the consumption of carbon sources by Streptomyces sp. M-Z18, the total amount of carbon sources was used as an indicator. The results showed that the time of carbon source depletion was 33.5 h when the initial glucose/glycerol was set to 30/30, much shorter than the depletion times at ratios of 40/20 (39 h) and 20/40 (37.5 h) (Fig. 2b). Although the highest ε-PL concentration of 7.34 g/L was attained when the glucose/glycerol was 30/30 (Fig. 2c), statistical analysis showed that cell growth did not exhibit significant differences when the ratio of glucose to glycerol was varied (Fig. 2d). We thus conclude that the optimal weight ratio of glucose/glycerol is 30/30 for ε-PL production using glucose/glycerol co-fermentation by Streptomyces sp. M-Z18.
Effects of the ratio of glucose to glycerol on a pH, b total carbon source, c cell growth, and d ε-PL production by Streptomyces sp. M-Z18 in batch cultures using glucose and glycerol as mixed carbon sources. Glucose/glycerol = 40/20 (dotted lines and filled triangles), glucose/glycerol = 30/30 (solid lines and filled squares), and glucose/glycerol = 20/40 (dashed lines and filled circles)
To analyze the consumption of carbon sources during co-fermentation, a comparison of the glucose and/or glycerol depletion between single carbon source fermentation and co-fermentation is shown in Fig. 3. Glucose and glycerol were consumed almost simultaneously during mixed carbon source fermentation, similar to the reported glucose and glycerol co-fermentation of propionic acid by Propionibacterium acidipropionici [24]. When the weight ratio of glucose to glycerol was either 40/20 or 20/40, the carbon source of low proportion was depleted first. However, at 30/30 glucose/glycerol, both glucose and glycerol were consumed completely within about 33.5 h (Fig. 3a, b). The carbon sources in the co-fermentation were easier to consume than single carbon sources when the total weights of the individual carbon sources of co-fermentation were equal (Fig. 3c). This finding proves that the utilization of glucose and glycerol produced a synergistic effect during the glucose/glycerol co-fermentation of ε-PL by Streptomyces sp. M-Z18. This effect was also found during lycopene production using glycerol–glucose/l-arabinose as mixed carbon sources [27].
Comparison of glucose or/and glycerol depletion between single and mixed carbon source fermentation by Streptomyces sp. M-Z18 in batch cultures. a Glucose; b glycerol; c total carbon source. Single carbon source: glucose (filled squares), glycerol (empty squares); mixed carbon source: glucose/glycerol = 40/20 [glucose (filled diamond), glycerol (empty diamond), glucose + glycerol (half-filled diamond)], glucose/glycerol = 30/30 [glucose (filled circles), glycerol (empty circles), glucose + glycerol (half-filled circles)], glucose/glycerol = 20/40 [glucose (filled triangles), glycerol (empty triangles), glucose + glycerol (half-filled triangles)]
As shown in Table 1, compared with single carbon source fermentation, the synergistic effect of glucose and glycerol during co-fermentation resulted in a significant decrease in the substrate exhaustion time and the time at which the pH dropped from 6.8 to 3.8. The substrate exhaustion time was shortened from 42 and 44.5 h under single carbon source fermentations with glucose and glycerol, respectively, to 33.5 h in 30/30 glucose/glycerol. Enhancing ε-PL productivity by shortening the substrate exhaustion time appears to be beneficial to the glucose/glycerol co-fermentation process. Furthermore, the time at which the pH dropped from 6.8 to 3.8 was also reduced from 14.5 h in glycerol to 11.28 h in 30/30 glucose/glycerol, similar to that found when glucose was used as a carbon source (11.28 h). Thus, starting ε-PL synthesis in advance also appears to be helpful to the fermentation. Meanwhile, compared with glucose and glycerol single carbon source fermentations, the productivity of ε-PL increased by 83.9 and 35.2%, respectively, when co-fermentation was performed with 30/30 glucose/glycerol. Therefore, the increase in ε-PL concentration and the decrease in substrate exhaustion time during 30/30 glucose/glycerol co-fermentation led to the enhancement of ε-PL productivity.
Effect of 30/30 glucose/glycerol co-fermentation on ε-PL production by Streptomyces sp. M-Z18 during fed-batch fermentation
To further improve the ε-PL production via 30/30 glucose/glycerol co-fermentation by Streptomyces sp. M-Z18, a fed-batch fermentation strategy was investigated. The time course is shown in Fig. 4. When both glucose and glycerol concentrations were below 5 g/L, a feeding technology was applied to ensure that they were maintained at a level of about 5 g/L. After 174 h of fermentation, a ε-PL concentration and productivity of 35.14 g/L and 4.85 g/L/d, respectively, were attained (Fig. 4). Compared with glucose and glycerol as sole carbon sources for ε-PL production by Streptomyces sp. M-Z18 during fed-batch fermentation, the concentration of ε-PL during co-fermentation increased by 43.2 and 16.7%, respectively (Table 2), and the productivity of ε-PL increased by 38.6 and 16.0%, respectively. The significant improvement in these parameters proved the effectiveness of the glucose/glycerol co-fermentation strategy on ε-PL fermentation by Streptomyces sp. M-Z18. Although the concentration and productivity of ε-PL obtained from glucose/glycerol co-fermentation were less than those of S. albulus S410 and Kitasatospora sp. MY 5-36 from glucose, in comparison, the biomass of Streptomyces sp. M-Z18 was 1.54- and 1.81-fold higher, respectively (Table 2). Obviously, ε-PL productivity could be further improved through enhancement of the capacity of the biomass for ε-PL synthesis.
A fed-batch culture of glucose/glycerol = 30/30 for ε-PL production by Streptomyces sp. M-Z18 keeping both glucose and glycerol at about 5 g/L; NH3-N at about 1 g/L. Glucose (filled squares), glycerol (empty squares), NH3-N (filled diamond), cell growth (filled circles), ε-PL production (filled triangles)
Mixed carbon source fermentation is an effective strategy for enhancing subject metabolic products, and it has been widely applied in the fermentation industry. For example, glycerol/glucose co-fermentation has been employed to improve propionic acid [24] and 1,3-propanediol production [28], glucose/glycerol/xylose co-fermentation has been carried out to enhance p-hydroxybenzoate production [29], and a glucose–corn oil mixture has been used as a mixed carbon source to increase the gibberellic acid production [30]. Several explanations have been made with respect to the mechanism of mixed carbon sources for enhancing the microbial products: (1) They supply different carbon sources for biomass formation and product synthesis. Glucose provides reducing equivalents and ATP for biomass formation, and glycerol is mainly used for propionic acid and 1,3-propanediol production [24, 28]. (2) They provide a specific carbon source for the synthesis of metabolic precursors. Xylose is easily metabolized through the pentose phosphate pathway and produces erythrose-4-phosphate, the key precursors of p-hydroxybenzoate. Therefore, p-hydroxybenzoate production from mixed carbon sources containing glucose and xylose is efficient [29]. Specific to the ε-PL production from glucose/glycerol co-fermentation, we speculate that glucose/glycerol mixed carbon sources reduce the carbon catabolite repression derived from glucose metabolism and regulate the requirement of reductance degree for cell growth and ε-PL synthesis. Nevertheless, further research is necessary to determine the true mechanism of glucose/glycerol co-fermentation in enhancing ε-PL production.
Conclusions
The current study showed that glucose/glycerol co-fermentation can serve as an efficient strategy for ε-PL production by Streptomyces sp. M-Z18. Co-fermentation with glucose and glycerol stimulated the consumption of the total carbon source compared with the single carbon source during batch fermentations, resulting in the improvement of ε-PL productivity due to the substantial reduction of the substrate exhaustion time. Fed-batch fermentation with 30/30 glucose/glycerol as a mixed carbon source achieved a maximum ε-PL concentration and productivity of 35.14 g/L and 4.85 g/L/d, respectively. Therefore, glucose/glycerol co-fermentation could be an alternative to conventional ε-PL production.
References
Hiraki J (2000) ε-Polylysine, its development and utilization. Fine Chem 29:18–25
Shima S, Matsuoka H, Iwamoto T, Sakai H (1984) Antimicrobial action of ε-poly-l-lysine. J Antibiot 37:1449–1455
Hiraki J, Ichikawa T, Ninomiya S, Seki H, Uohama K, Seki H, Kimura S, Yanagimoto Y, Barnett JW Jr (2003) Use of ADME studies to confirm the safety of ε-polylysine as a preservative in food. Regul Toxicol Pharmacol 37:328–340
US Food and Drug Administration (USFDA) (2004) GRAS Notice 000135: ε-Poly-lysine. Office of Food Additive Safety. http://www.fda.gov. Cited 1 Mar 2011
Oppermann-Sanio FB, Steinbuchel A (2002) Occurrence, functions and biosynthesis of polyamides in microorganisms and biotechnological production. Naturwissenschaften 89:11–22
Yoshida T, Nagasawa T (2003) ε-Poly-l-lysine: microbial production, biodegradation and application potential. Appl Microbiol Biotechnol 62:21–26
Shih IL, Shen MH, Van YT (2006) Microbial synthesis of poly(l-lysine) and its various applications. Bioresour Technol 97:1148–1159
Hiraki J, Hatakeyama M, Morita H, Izumi Y (1998) Improved ε-poly-l-lysine production of an S-(2-aminoethyl)-l-cysteine resistant mutant of Streptomyces albulus. Seibutsu-Kogaku 76:487–493
Ouyang J, Xu H, Li S, Zhu HY, Chen WW, Zhou J, Wu Q, Xu L, Ouyang PK (2006) Production of ε-poly-l-lysine by newly isolated Kitasatospora sp. PL6–3. Biotechnol J 1:1459–1463
Kahar P, Iwata T, Hiraki J, Park YE, Okabe M (2001) Enhancement of ε-polylysine production by Streptomyces albulus strain 410 using pH control. J Biosci Bioeng 91:190–194
Jia SR, Wang GL, Sun YF, Tan ZL (2009) Improvement of ε-poly-l-lysine production by Streptomyces albulus TUST2 employing a feeding strategy. In: The 3rd International Conference on Bioinformatics and Biomedical Engineering (iCBBE 2009). Beijing, China. 1–4. doi:10.1109/ICBBE.2009.5162940
Zhang Y, Feng XH, Xu H, Yao Z, Ouyang PK (2010) ε-Poly-l-Lysine production by immobilized cells of Kitasatospora sp. MY 5–36 in repeated fed-batch cultures. Bioresour Technol 101:5523–5527
Shih IL, Shen MH (2006) Optimization of cell growth and poly(ε-lysine) production in batch and fed-batch cultures by Streptomyces albulus IFO 14147. Process Biochem 41:1644–1649
Shima S, Sakai H (1981) Poly-l-Lysine produced by Streptomyces. Part II. Taxonomy and fermentation studies. Agric Biol Chem 45:2497–2502
Bankar SB, Singhal RS (2010) Optimization of poly-ε-lysine production by Streptomyces noursei NRRL 5126. Bioresour Technol 101:8370–8375
da Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27:30–39
Nishikawa M (2009) Molecular mass control using polyanionic cyclodextrin derivatives for the epsilon-poly-l-lysine biosynthesis by Streptomyces. Enzyme Microb Technol 45:295–298
Hirohara H, Saimura M, Takehara M, Miyamoto M, Ikezaki A (2007) Substantially monodispersed poly(ε-l-lysine)s frequently occurred in newly isolated strains of Streptomyces sp. Appl Microbiol Biotechnol 76:1009–1016
Takehara M, Aihara Y, Kawai S, YoshikabuH, Inoue Y, Hirohara H (2001) Strain producing low molecular weight ε-poly-l-lysine and production of low molecular weight ε-poly-l-lysine using the strain. Japan patent 2001-017159
Chen XS, Tang L, Li S, Liao LJ, Zhang JH, Mao ZG (2011) Optimization of medium for enhancement of ε-poly-l-lysine production by Streptomyces sp. M-Z18 with glycerol as carbon source. Bioresour Technol 102:1727–1732
Chen XS, Li S, Liao LJ, Ren XD, Li F, Tang L, Zhang JH, Mao ZG (2011) Production of ε-poly-l-lysine using a novel two-stage pH control strategy by Streptomyces sp. M-Z18 from glycerol. Bioprocess Biosyst Eng 34:561–567
Nishikawa M, Ogawa K (2002) Distribution of microbes producing antimicrobial ε-poly-l-lysine polymers in soil microflora determined by a novel method. Appl Environ Microbiol 68:3575–3581
AOAC International (formerly the Association of Official Analytical Chemists) (1995) Official Methods of Analysis Arlington. AOAC International, VA
Liu Y, Zhang YG, Zhang RB, Zhang F, Zhu JH (2011) Glycerol/Glucose co-fermentation: one more proficient process to produce propionic acid by Propionibacterium acidipropionic. Curr Microbiol 62:152–158
Kim YS, Lee JH, Kim NH, Yeom SJ, Oh DK (2011) Increase of lycopene production by supplementing auxiliary carbon sources in metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 90:489–497
Erickson LE, Minkevich IG, Eroshin VK (1979) Utilization of mass-energy balance regularities in the analysis of continuous-culture data. Biotechnol Bioeng 21:575–591
Kim YS, Lee JH, Kim NH, Yeon SJ, Kim SW, Oh DK (2011) Increase of lycopene production by supplementing auxiliary carbon sources in metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 90:489–497
Saint-amans S, Girbal L, Andrade J, Ahrens K, Soucaille P (2001) Regulation of carbon and electron flow in Clostridium Butyricum VPI 3266 grown on glucose-glycerol mixtures. J Bacteriol 183:1748–1754
Meijnen JP, Verhoef S, Briedjlal AA, de Winde JH, Ruijssenaars HJ (2011) Improved p-hydroxybenzoate production by engineered Pseudomonas putida S12 by using a mixed-substrate feeding strategy. Appl Microbiol Biotechnol 90:885–893
Rios-Iribe EY, Flores-Cotera LB, González Chávira MM, González-Alatorre G, Escamilla-Silva EM (2011) Inductive effect produced by a mixture of carbon source in the production of gibberellic acid by Gibberella fujikuroi. World J Microbiol Biotechnol 27:1499–1505
Acknowledgments
This work was supported by a grant from Wuxi Science & Technology Support Program (CYE21N1107), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and 111 Project (111-2-06).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, XS., Ren, XD., Dong, N. et al. Culture medium containing glucose and glycerol as a mixed carbon source improves ε-poly-l-lysine production by Streptomyces sp. M-Z18. Bioprocess Biosyst Eng 35, 469–475 (2012). https://doi.org/10.1007/s00449-011-0586-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0586-z