Abstract
Gibberellic acid has been known since 1954 but its effect on rice still remains very important in the agricultural world. Gibberellic acid (GA3) is the main secondary metabolite produced by the Gibberella fujikuroi fungus. This hormone is of great importance in agriculture and the brewing industry, due to its fast and strong effects at low concentrations (μg) on the processes of growth stimulation, flowering, stem elongation, and germination of seeds, among others. Plant promoters of growth production such as the gibberellins, especially the GA3 are a priority in obtaining better harvests in the agricultural area and by extension, improving the food industry. Three routes to obtaining GA3 have been reported: extraction from plants, chemical synthesis and microbial fermentation. The latter being the most common method used to produce GA3. In this investigation, glucose-corn oil mixture was used as a carbon source on the basis of 40 g of carbon in a 7 L stirred tank bioreactor. A pH of 3.5, 29°C, 600 min−1 agitation and 1 vvm aeration were maintained and controlled with a biocontroller connected to the bioreactor, throughout the entire culture time. The carbon source mixture affected the fermentation time as well as the production of the GAs. The production of 380 mg GA3L−1 after 288 h of fermentation was obtained when the glucose-corn oil mixture was employed contrasting the 136 mg GA3L−1 at 264 h of culture when only glucose was used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
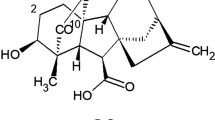
Since the discovery of gibberellins in 1935 by Yabuta (1935), there has been continued interest in the study of their biosynthesis and how they act. The enzymes involved in each stage of the metabolism are not known systematically nor how the genes that initiate their production shoot off. With regards to the production process of gibberellins there are many points to study. To date, more than 126 GAs have been identified from these natural sources (Tudzynski 1999; Shukla et al. 2003). Some of these GAs act as natural plant hormones, controlling developmental processes such as the induction of hydrolytic enzyme activity during seed germination, stem elongation, flower induction and pericarp growth (Rojas et al. 2001). As a result of these properties and their commercial applications, there has been considerable interest in the GAs over the last half-century, leading to many reviews of their biosynthesis (MacMillan 1997). The commercial source of the GAs, especially the bioactive compound gibberellic acid (GA3; Fig. 1), is obtained by fermentation of the rice pathogen fungus, Gibberella fujikuroi, from which the GAs were originally discovered (Phinney 1983) and capable of producing large amounts of GA3 during the secondary metabolism (Linnemannstönns et al. 1999; Escamilla-Silva et al. 1999). Various investigations have shown that the choice of carbon source during the processes of microbial fermentation for the production of metabolites directly affects their performance. The majority of experimental developments on fermentative processes have chosen glucose as the carbon source. This is based on the fact that glycolysis is present in a large variety of living organisms and is the fastest pathway to meeting the energy needs of cell functions. The production of GAs through G. fujikuroi has been no exception, since their main lines of investigation are based on the use of glucose; even the maximum reported production of GA3 used this source of carbon (Escamilla et al. 2000). However, the need to improve the production of GAs through G. fujikuroi, has led to the search for new sources of carbon, focusing primarily on the use of natural oils. Some reports suggested that flour and oil plant extracts might contain precursors or inductors of the GA pathway (Rademacher 1994; Vass and Jefferys 1979). In fact, Muromtsev and Agnistova (1984) and Gancheva et al. (1984) have successfully used plant oils, e.g. sunflower oil, for GA productions. In our laboratory we have already conducted research using sesame, safflower, corn, and rice and sunflower oils for the production of GAs (Negrete-Rodríguez 2002; Almanza-Rangel 2005) and have concluded that the acetyl-CoA is a central intermediary of oxidative metabolism in mitochondria. This is derived from the descarboxylation of the pyruvate obtained by the bioconversion of carbohydrates for a glycolysis pathway, and transmination of some amino acids together with the source of acetyl-CoA, corresponding β-oxidation of the fatty acids. In addition to the biochemical function of acetyl-CoA within the citric acid cycle, there are other biological functions of vital importance, emphasizing the metabolic source of all carbon atoms in the synthesis of fatty acids. Additionally, in certain living organisms, the acetyl-CoA along with acetatoacetil-CoA and water, condense through the action of enzyme hydroxymethylglutaryl-CoA synthase to form hydroxymethylglutaryl-CoA (Fig. 2), a precursor for GAs via the mevalonic acid pathway.
The main objective of this work was to study an alternative strategy for increasing GA3 production by G. fujikuroi, using a combination of glucose and vegetal oil as a carbon source during the fermentation process and a reduction in the activation time of the production of GA3 due to the immediate availability of fatty acids and glycerol for the microbial cell. An increase in the production of GA3 is therefore expected; a positive effect because of the acetyl-CoA occurring during the descarboxylation of pyruvate from the glycolysis that may be used for the production of secondary metabolites. It is important to note that until now neither item has been reported in any scientific research, which makes the project both interesting and ground-breaking.
Materials and methods
Gibberella fujikuroi strain CDBB H-984 maintained on potato dextrose agar slants at 4°C and sub-cultured every 2 months was used in the present work (Culture collection of the Department of Biotechnology and Bioengineering, CINVESTAV-IPN, Mexico). Fully developed mycelia materials from a slant were removed by adding an isotonic solution (0.9% NaCl). The removed mycelium (2 ml) was used to inoculate 250 ml of fresh culture medium contained in an Erlenmeyer flask. The flask was placed in a radial shaker (280 rev min−1) for 38 h at 29 ± 2°C. Subsequently, 200 ml contents of the flask were used to inoculate 3,800 ml of culture medium contained in the stirred tank bioreactor. The culture medium employed for the inoculums preparation is reported by Escamilla et al. (2000).
A Continuo Stirrer Tank Bioreactor (CSTB; Applikon, The Nederland, 7 l) was used, which operates mechanically mixing the air-sparged to the liquid medium and jacket type to recycle water for control of temperature. It is also equipped with pH and dissolved oxygen sensors to control these variables by a bio-controller. The Bio-expert software was used to record all the parameters throughout the fermentation time. Moreover it allows material to be fed or removed from the bioreactor employing peristaltic pumps.
The typical culture medium contained glucose or glucose-corn oil mixture as the carbon source on the basis of 40 g of carbon source, 2 g l−1 NH4Cl, 3 g l−1 KH2PO4, 1.5 g l−1 MgSO4·7H2O and 2 ml l−1 of trace elements. A stock solution of the trace elements used contained (g l−1) 1.0 FeSO4·7H2O, 1.5 Na2MoO4·2H2O, 0.2 MnSO4·H2O and 1.0 ZnSO4·7H2O. A pH value of 3.5, 29°C, 600 rev min−1 agitation and 1 vvm aeration were maintained and controlled with a bio-controller connected to the bioreactor, throughout the culture time. Samples were taken over a 240 h period and stored at 4°C prior to analysis.
Fungi were harvested by filtration (under reduced pressure) through pre-weighed oven-dried nitrocellulose membranes (0.45 μm pore size). After drying at 90°C to constant weight, the biomass was quantified by the dry weight method. Lipids were determined gravimetrically after the extraction with hexane and were rota-evaporate for hexane recuperation in a rota evaporator (Büchi Switzerland) and dried for 1 h in vacuum oven at 90°C and. Residual NH4 + in the fermentation broth was assayed by the Kjeldahl method (A.O.A.C. 1990), and residual glucose was measured by the DNS method (Miller 1959).
Determination of gibberellic acid
The quantitative determination of gibberellic acid (Shchegolev and Kucherov 2005; Ge et al. 2007) by different methods reported a wide variation of GA3 concentration between one method and another, but enabled a comparison to be made with bioassays and establish a correlation between them. In this paper we report the analysis by HPLC and in a subsequent paper we will report in particular the results obtained using different methods and the equivalences between them. The GA3 in the culture fluid was quantified by HPLC using a Varian 9012 Chromatograph after extracting 10 mL samples of culture filtrate before adjusting to pH 2.0 with HCl 0.1 M, with three 10 ml portions of ethyl acetate. The organic fractions were rotary evaporated and the residue taken up in 3 mL of methanol. GA3 was identified and quantified by reference to a GA3 standard (Sigma–Aldrich), Negrete-Rodríguez (2002).
Results and discussion
Different studies conducted in this work showed that gibberellins production begins when the source of nitrogen is exhausted. Comparisons made with a mixture of carbon sources; one that was easily assimilated and another that assimilated slowly, producing an inductive effect: reducing the time before gibberellins production begins, and increasing the concentration of gibberellins, especially gibberellic acid, as can be seen in the following results.
Figure 3 shows the relation between glucose uptake and gibberellic acid throughout the fermentation time. It can be observed for 96 h, the rate of glucose consumption over the exponential period, the fungus only uses the glucose for its growth and in a respiratory chain. At the end of this period the secondary metabolism with the gibberellins production began. Glucose uptake follows a first order kinetics and its concentration decreases as zero-order changes. This shows that glucose is used practically for the growth of microorganisms and stores up to 40% for maintenance and this is where the kinetics of fungus growth changes (Brückner and Blechschmidt 1991). At 240 h the maximal GA3 was reached, after this time the reserve of glucose was used by the fungus and the GA3 production began to decline.
Figure 4 shows the dynamics of consumption of nitrogen, phosphorus and its relationship with biomass production. It can be seen that in less than 46 h the nitrogen is depleted and it is at this point where gibberellins biosyntheses shoots up through the glutamine, which is what governs the metabolic flux. Moreover, the dynamics of the phosphorous consumption during the growth period of G. fujikuroi can be observed in this figure, a stage where it is used for the production of ATP to metabolize glucose during the first 46 h of fermentation, and after this time remains constant throughout the process. Interestingly, when nitrogen and glucose sources were exhausted, maximum biomass was achieved, between 48 and 60 h, whereas maximum production of gibberellins is achieved after 230 h, a fact which shows that gibberellins are not associated with microbial growth.
Figure 5 shows the kinetics of production of GA3 when using a mixture of glucose and corn oil as a carbon source. It illustrates how the glucose is depleted in an exponential way, indicating that the microorganism used it for their growth, whereas the corn oil hydrolyzes first, a fact demonstrated by the presence of a large concentration of lipases. Corn oil enabled the growth rate of the fungus to be regulated from 96 to 350 h, which is very important because the period of production of GA3 was linear from 96 to 240 h, achieving a maximum at around 280 h. A quickly metabolized substrate such as glucose and nitrogen may often achieve maximum cell growth rates as can be seen in Fig. 6, but is known to inhibit the production of many secondary metabolites. This “catabolite repression” is thought to be due to intermediates generated from the rapid catabolism of glucose interfering with enzymes in the secondary metabolism process (Marwick et al. 1999). Therefore, plant oils as a carbon source are not only inert for carbon catabolite repression but also make available a pool of acetyl-CoA and additionally might contain natural precursors for GA biosynthesis (Vass and Jefferys 1979; Tudzynski 1999). Fast-growing cells generally have secondary metabolism “switched off” until their growth rate slows, via feedback inhibition. This can lead to a fully biphasic fermentation profile, with no production during growth, only during the stationary phase (Marwick et al. 1999; Chávez-Parga et al. 2008). This fact makes it interesting to use of a combination of an easily assailable carbon source and another with slow assimilation as shown in this investigation. Jacklin et al. (2000) reported the study of the interaction between the biosynthesis of fatty acids and GAs by Fusarium moniliforme linked to providing a novel method to maximize the production of GA3, through lipid-to-gibberellins metabolic switching. Basically, the experiment aimed to inhibit cell growth by adding sesamol to the fermentation medium. This resulted in the primary metabolism being blocked without any involvement on the secondary metabolism, because sesamol inhibited growth by about 40% and lipid accumulation by 35%. With GA3 counterparts, accumulation was increased 20-fold, to a specific production of 63 mg g−1 biomass. A metabolic analysis of these biological phenomena indicated that the acetyl-CoA for the biosynthesis of fatty acids is switched to the synthesis of GA3. Therefore, on F. moniliforme, GAs and lipids are synthesized from a common precursor, acetyl-CoA. In accordance with our data we may assume that using a glucose- corn oil mixture as the carbon source the lipid requirements of cell functions could be satisfied by the catabolism of the corn oil, which minimizes the use of acetyl-CoA in the biosynthesis of fatty acids, and thus increases the flux of acetyl-CoA to GA3. In a similar way to carbon, the nitrogen source is understood to regulate secondary metabolism. The biosynthesis of GAs as secondary metabolites reduces its production in G. fujikuroi by regulating the source of nitrogen, beginning after the nitrogen source depletion (Rybakov and Bourd 1991; Sánchez-Fernández et al. 1997; Giordano et al. 1999). These data showed that GA3 production began after nitrogen exhaustion and Escamilla et al. (2000), found that in G. fujikuroi the exponential growth ceases when the nitrogen source is depleted in the culture medium and occurs within the idiophase. This is characterized by initiating the formation of GAS by the promotion of the biosynthesis of glutamine, which is the basis for the genes cluster, a set of synthetases. A group of these are six of the seven cloned genes under control of the positively acting general transcription factor AREA (Tudzynski 1999; Mihlan et al. 2003). High levels of preferred nitrogenous compounds can drastically reduce the expression of GA-biosynthetic genes and GA amounts produced. Phosphate, although essential for growth, can at certain concentrations suppress secondary metabolism, inhibiting, for example, phosphatases and oxygenases (Spizek and Tichy 1995; Chávez-Parga et al. 2007). In G. fujikuroi the GAs biosynthesis is not regulated by phosphate concentration or rate of growth (Giordano et al. 1999).
As shown in Fig. 7, the secondary metabolism began around 60 h of culture and a production of 380 mg GA3L−1 after 288 h of fermentation was obtained when the mixture glucose-corn oil was employed. This is in contrast to the 136 mg GA3L−1 at 264 h of culture when only glucose was used, and the GA3 production began around 96 h of fermentation as can see in Fig. 7. The results clearly indicate that the carbon source produced an inductive effect in the secondary metabolism and affected the fermentation time and the phytohormone production during the fermentation process of G. fujikuroi on the conditions studied (Fig. 8).
Conclusion
As for the assumption of the existence of a regulatory mechanism of glucose absorption from corn oil, the results clearly showed that both carbon sources are metabolized quickly, which means that glucose does not exert catabolic repression on assimilation corn oil, or some kind of metabolic control in the transport of fatty acids in the cells of G. fujikuroi. When using a mixture of carbon sources has been an increase in the production of gibberellic acid than when using only glucose and a decrease in time when production of GA3 is initiated.
References
Almanza-Rangel S (2005) MSc Thesis: Estudio del efecto de la relación C-N provenientes de harina y aceite de arroz sobre la producción de GA3. Instituto Tecnológico de Celaya
A.O.A.C. (1990) Official methods of analysis 15th edition of the association of official chemists. A.O.A.C., Washington, DC
Brückner B, Blechschmidt D (1991) The gibberellin fermentation. Crit Rev Biotechnol 11(2):163–192
Chávez-Parga MC, Gonzalez-Ortega O, Negrete-Rodriguez MLX, Medina-Torres L, Silva EME (2007) Hydrodynamics, mass transfer and rheological studies of gibberellic acid production in an airlift bioreactor. World J Microbiol Biotechnol 23:615–623
Chávez-Parga MC, Gonzalez-Ortega O, de la Luz X Negrete-Rodríguez Ma, Vallarino IG, Alatorre GG, Escamilla-Silva EM (2008) Kinetic modelling, airlift bioreactor, gibberellic acid, bikaverin, Gibberella fujikuroi. Process Biochem 43:855–860
Escamilla EM, Dendooven L, Magaña IP, Parra R, de la Torre M (2000) Optimization of gibberellic acid production by immobilized Gibberella fujikuroi mycelium in fluidized bioreactors. J Biotechnol 76:147–155
Escamilla-Silva EM, Dendooven L, Uscanga-José A, Monroy RAI, González-Alatorre G, de la Torre Martínez M (1999) Morphological development and gibberellin production by different strains of Gibberella fujikuroi in shake flasks and bioreactor. World J Microbiol Biotechnol 15:753–755
Gancheva V, Dimova T, Kamenov K, Foutekova M (1984) Biosynthesis of gibberellins III. Optimization of nutrient medium for biosynthesis of gibberellins upon using mathematical methods for planning the experiments. Acta Microbiol Bulgarica 14:80
Ge L, Peh CYC, Yong JWH, Tan SN, Lin H, Ong SE (2007) Analyses of gibberellins by capillary electrophoresis—mass spectrometry combined with solid-phase extraction. J Chromatogr A 1159:242–249
Giordano W, Avalos J, Cerdá-Olmedo E, Doménech CE (1999) Nitrogen availability and production of bikaverin and gibberellins in Gibberella fujikuroi. FEMS Microbiol Lett 173:389–393
Jacklin A, Ratledge C, Wynn JP (2000) Lipid-to-gibberellin metabolic switching in Fusarium moniliforme via the action of sesamol. Biotechnol Lett 22(24):1983–1986
Linnemannstönns P, Vob T, Hedden P, Gaskin P, Tudzynski B (1999) Deletions in the gibberellin biosynthesis gene cluster of Gibberella fujikuroi by restriction enzyme-mediated integration and conventional transformation-mediated mutagenesis. Appl Environ Microbiol 65(6):2558–2564
MacMillan J (1997) Biosynthesis of the gibberellin plant hormones. Nat Prod Rep 1:221–243
Marwick JD, Wright PC, Burgess JG (1999) Bioprocess intensification for production of novel marine bacterial antibiotics through bioreactor operation and design. Marin Biotechnol 1:495–507
Mihlan M, Homann V, Liu TWD, Tudzynski B (2003) AREA directly mediates nitrogen regulation of gibberellin biosynthesis in Gibberella fujikuroi, but its activity is not affected by NMR. Mol Microbiol 47:975–991
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Muromtsev GS, Agnistova VN (1984) In: Letunova SV (ed) Gibberellins. Nauka, Moscow (in Russian)
Negrete-Rodríguez X (2002) MSc Thesis: Producción de ácido giberélico en biorreactor de tanque agitado con Gibberella fujikuroi empleando aceite de maíz como fuente de carbono. Instituto Tecnológico de Celaya
Phinney BO (1983) The history of gibberellins. In: Crozier A (ed) The biochemistry and physiology of gibberellins, vol 1. Praeger, New York, pp 19–52
Rademacher W (1994) Gibberellin formation in microorganisms. Plant Growth Regul 15:303–314
Rojas MC, Hedden P, Gaskin P, Tudzynski B (2001) The P450-1 gene of Gibberella fujikuroi encodes a multifunctional enzyme in gibberellin biosynthesis. Microbiol 98(10):5838–5843
Rybakov YA, Bourd GI (1991) Nitrogen regulation of gibberellin biosynthesis enzyme complex in Fusarium moniliforme. J Biotechnol 21:219–228
Sánchez-Fernández R, Avalos J, Cerdá-Olmedo E (1997) Inhibition of gibberellin biosynthesis by nitrate in Gibberella fujikuroi. FEBS Lett 413:35–39
Shchegolev AA, Kucherov VF (2005) The use of gas-liquid chromatography for the analysis of gibberellins and related compounds. Russ Chem Bull 18(6):1117–1119
Shukla R, Srivastava AK, Chand S (2003) Bioprocess strategies and recovery processes in gibberellic acid fermentation. Biotechnol Bioproc Engin 8:269–278
Spizek J, Tichy P (1995) Some aspects of overproduction of secondary metabolites. Folia Microbiol 40:43–50
Tudzynski B (1999) Biosynthesis of gibberellins in Gibberella fujikuroi: biomolecular aspects. Appl Microbiol Biotechnol 52:298–310
Vass RC, Jefferys EG (1979) Gibberellic acid. Chapter 9. In: Rose AH (ed) Economic microbiology, vol 3. Academic Press, London
Yabuta T (1935) Biochemistry of the “bakanae” fungus of rice. Ag Hort 10:17–22
Acknowledgments
The research was funded by the Consejo Nacional de Ciencia y Tecnología (CONACyT) la Secretaría de. Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación Secretaría agropecuaria (SAGARPA) grant-2004-C01-77, Dirección General de Estudios Superiores Tecnológicos (DGEST) grant-2141.09-P and Ms Alison S. Williams for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rios-Iribe, E.Y., Flores-Cotera, L.B., Chávira, M.M.G. et al. Inductive effect produced by a mixture of carbon source in the production of gibberellic acid by Gibberella fujikuroi . World J Microbiol Biotechnol 27, 1499–1505 (2011). https://doi.org/10.1007/s11274-010-0603-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0603-4